Abstract
Background:
Progression of systemic lupus erythematosus (SLE) could be due to oxidative stress especially through reactive oxygen species (ROS). Detoxification of ROS is largely performed by Glutathione S-transferases (GSTs), therefore polymorphisms of GSTM1, GSTT1 and GSTP1 genes which decrease enzymes activity could affect SLE susceptibility. The aim of this study was to determine the effects of GSTM1 (deletion), GSTT1 (deletion) and GSTP1 (Ile105Val) polymorphisms on SLE susceptibility.
Methods:
Genomic DNA was extracted from blood samples of 163 SLE patients and 180 age, sex and ethnically matched controls. GSTs genotypes were determined by polymerase chain reaction (PCR)-multiplex procedure or polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis.
Results:
GSTT1 null genotype frequency was higher in SLE patients than controls. NO association observed between GSTM1 null genotype or GSTP1 Ile105Val polymorphism with SLE. Nevertheless combination of GSTT1 null/ GSTM1 null genotypes showed 2.8-fold increase in risk of SLE. Moreover the combination of GSTT1 null/ GSTM1 null/GSTP1 Ile/Val and Val/Val genotypes increased the SLE risk about 8 fold.
Conclusion:
Present data suggest that GSTT1 null/ GSTM1 null/GSTP1 Ile/Val and Val/Val genotypes might largely contribute to the pathogenesis of SLE.
Keywords: Systemic lupus erythematosus, Glutathione S-transferase, Gene, Polymorphism
Introduction
Systemic lupus erythematosus (SLE) is a multisystemic disorder with diverse incidence and prevalence between different populations. This complication is characterized by mal-regulation of the immune system, expression of impaired T cell responses, hyperactive B cells which synthesize excessive amounts of different autoantibodies and formation of immune complexes against various nuclear antigens. SLE could affect several organs and display a complex spectrum of clinical and immunologic manifestations such as arthritis, vasculitis and nephritis (1, 2). Although the exact pathophysiology of SLE is unclear, environmental and genetic risk factors may play an important role in susceptibility to this multi-etiologic disease (2, 3).
Environmental stimulators such as ultraviolet radiation and xenobiotic compounds have critical roles in the onset and progression of SLE (4, 5). Environmental carcinogens including smoking, air contamination and occupational exposures have strong influences on individual factors (6, 7).
There are several known enzymes involved in metabolic activation and detoxification of carcinogens including polycyclic aromatic hydrocarbons (PAH) and aromatic amines. Therefore inter-individual differences in ability to activate and detoxify carcinogens might affect the risk of developing SLE (8).
Glutathione S-transferases (GSTs) are a superfamily of dimeric phase II metabolic enzymes which are divided into four major subfamilies designated as GSTα (GSTA1), GSTμ (GSTM1), GSTθ (GSTT1) and GST π (GSTP1). These enzymes catalyze detoxification of a wide variety of potentially toxic and carcinogenic electrophiles in human environment, by conjugating to glutathione (GSH) (8, 9).
Recently, GSTM1, GSTT1 and GSTP1 have been extensively studied for their potential modulating role in individual susceptibility to environmentally induced diseases, including cancer (10).
GSTM1 and GSTP1 gene products could detoxify polycyclic aromatic hydrocarbons (PAHs) while GSTT1 detoxifies smaller hydrocarbons. Moreover they might play a role in scavenging reactive oxygen species (ROS) such as superoxide radicals and hydrogen peroxide (11) and play a crucial role in protecting DNA from oxidative damage (12).
GSTM1 gene, organized in a gene cluster on chromosome 1p13.3, has been shown to be polymorphic and it is absent in 35–60% of individuals (13, 14). GSTT1 gene, located on chromosome 22q11.2, is also polymorphic and it is absent in 10–65% of different populations (15, 16). There are two common deletion polymorphisms in GSTM1 and GSTT1 genes, which consequently results in virtual absence of enzyme activity, particularly in subjects with both deletions (17, 18).
GSTP1 gene product plays an important role in biotransformation and bio activation of cigarette smoke carcinogens (19). Inactivated or down-regulated GSTP1 gene could increase genomic damage when individuals were exposed to carcinogens (20–22). A313G polymorphism (Ile105Val, rs1695) in GSTP1 gene, located in the enzyme’s active site, could alter the enzymatic activity of the protein, decrease its detoxification ability for environmental mutagens and increase DNA damage. Therefore A313G polymorphism is an important risk factor for developing different diseases (23, 24).
GSTs polymorphisms have been associated with SLE and its manifestations (25, 26), whereas other studies did not observe any relation between these variants and susceptibility to SLE (27–29). These functional polymorphisms vary by race and ethnicity (30–33).
Present research was carried out in south east of Iran, to determine the effect of GSTM1, GSTT1 and GSTP1 genes polymorphisms, alone and in combination, on susceptibility of SLE.
Materials and Methods
Study population
This case-control study conducted on one hundred sixty three SLE patients (13 males and 150 females, average age 32.6 ± 8.6 years) who were referred to Rheumatology Clinic of Ali-Ebn Abitaleb Hospital in Zahedan from 2011 to 2012. The study was approved by the Ethics Committee of Zahedan University of Medical Sciences.
The control group consisted of one hundred eighty age, sex and ethnically matched volunteers (14 males and 166 females, average age 32.1 ± 11.7 years) with negative ANA test that had no systemic disease and family relation with SLE patients. SLE patients have been diagnosed with systemic lupus erythematosus according to ACR 1998 criteria (American Rheumatology Association).
Determination of Genetic Polymorphisms
Whole blood was collected in EDTA containing tubes. Genomic DNA was isolated from peripheral blood leukocytes by using the commercial available kit (Roche, Germany) in accordance with the manufacturer's instructions. The extracted DNA was stored at −20 °C until analyzed.
Analysis of GSTM1 and GSTT1 polymorphisms
Genetic polymorphism analysis for the GSTM1 and GSTT1 genes were conducted by the multiplex PCR (34). The β-globin gene was co-amplified as an internal positive control and GSTM1 and GSTT1 genotypes were not scored unless the PCR product from the internal reference gene (β-globin) was evident. PCR was performed in a total volume of 25 μl containing 200 ng genomic DNA, 25 pM of each primer, 2.5 mM deoxyribonucleo-side triphosphates (dNTPs) (Fermentas, Lithuania), 1.5 mM MgCl2, and 1U thermostable Taq DNA polymerase (Fermentas, Lithuania) using My Cycler Thermal cycler, BIO-RAD PCR system (BIO-RAD Co., U.S.A.). The amplification conditions were initial denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 63°C for 30 s, extension at 72°C for 1 min, and final extension at 72°C for 10 min. The amplified product was visualized in an ethidium bromide stained 2% agarose gel. The absence of amplified GSTM1 and GSTT1 products (in the presence of control PCR product) indicated the respective null genotypes.
Analysis of GSTP1 Ile105val polymorphism
GSTP1 Ile105val (A313G) polymorphism was genotyped using a polymerase chain reaction (PCR) amplification refractory mutation system (ARMS). Based on the flanking region of GSTP1 Ile105val polymorphism, following primers was designed for tetra-ARMS-PCR according to the published sequence for GSTP1 gene obtained from GenBank: Forward outer primer 5′-AGGTTACGTAGTTTGCCCAAGGTC-3′, Reverse outer primer 5′-CGTTACTTGGCTGGTTGATGTCC-3′, Forward inner primer 5′-GAGGACCTCCGCTGCAAATTCG-3′ and Reverse inner primer 5′- CATAGTTGGTGTAGATGAGGGAGCT-3′. PCR reaction mixture (25 μl) contained 5 ϱM of each primer, 200 ng of genomic DNA, 1.5mM MgCl2, 2.5mM each dNTPs (Fermentas, Lithuania) and 1 U Taq polymerase (Fermentas, Lithuania). Amplification was performed with an initial denaturation at 94°C for 5 min followed by 35 cycles at 94°C for 30 s, 64°C for 30 s, and 72°C for 1 min. After a final extension at 72°C for 10 minutes, PCR products were separated on 2% agarose gel and stained with ethidium bromide. The primer design for this polymorphism amplify a control product of 563 base pairs (bp), while the PCR products of 360 bp and 260 bp would identify Ile and Val variants, respectively (Fig. 1).
Fig. 1:
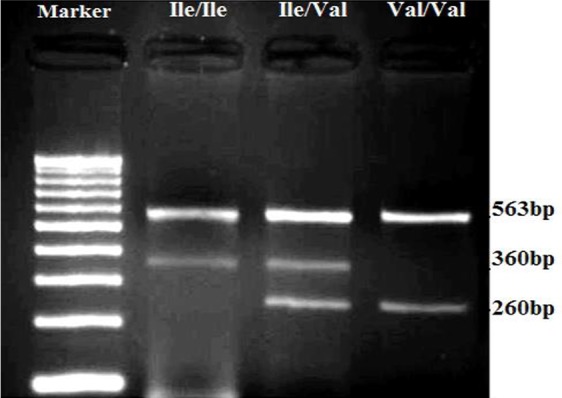
Electrophoresis pattern of tetra-ARMS-PCR for detection of GSTP1 Ile105val polymorphism on Agarose gel
All of the samples were analyzed by PCR restriction fragment length polymorphism (PCRRFLP) using 10U ALW 261 restriction enzyme (Fermentas, Lithuania) (35) for quality control.
Statistical analysis
All statistical analysis was performed with SPSS V.15. Demographic differences between groups were examined by χ2 test or an independent Student’s t-test whenever appropriate. Allele frequencies were estimated by the gene counting method. Frequencies of the alleles and genotypes were compared between patients and control groups by the χ2 test or Fisher’s exact test. The odds ratio (OR) and 95% confidence intervals (CI) were also estimated by Binary Logistic regression. The χ2 test was used for deviation of genotype distribution from Hardy-Weinberg equilibrium. All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Demographic characteristics of SLE patients and control group are showed in Table 1. This study included 163 SLE patients and 180 age, gender - and ethnicity matched control subjects. Dermo-mucus manifestations developed in 83% of patients during the course of their disease. Arthritis was found in 87%, whereas neuropsychiatric manifestations were found in 14% of SLE patients. Lupus nephritis was advanced with raised serum creatinine in 22% of patients.
Table 1:
Demographic characteristics of SLE patients and controls
| Parameter | SLE n=163 | Controls n=180 | P-Value | x2 |
|---|---|---|---|---|
| Age (yr) | 32.6±8.6 | 32.1±11.7 | 0.68 | 0.04 |
| Sex (male/female) | 13/150 | 14/166 | 0.6 | 0.04 |
| Race n (%) | 0.36 | 0.27 | ||
| Persian | 82 (50) | 86 (48) | ||
| Balouch | 81 (50) | 94 (52) |
GSTM1 and GSTT1 gene polymorphisms
The frequency of GSTM1 null genotype was 53.4% and 44% in SLE patients and controls respectively, which was not statistically significant (OR, 1.4[95% CI, 1 to 2.2]; P=0.095). Moreover the frequency of GSTT1 null genotype was significantly higher in SLE patients (25.2%) compared to healthy controls (15.1%) and risk of SLE was 1.9 fold higher in individuals with null mutation of GSTT1 after adjusting for age, gender and ethnicity (OR = 1.9, 95% CI = 1.1 to 3.2, P = 0.02) (Table 2).
Table 2:
Association of GSTM1 and GSTT1 polymorphisms and SLE risk
| Polymorphism | SLE n=163 | Control n=180 | P-value | Adjusted OR* (95% CI) |
|---|---|---|---|---|
| GSTM1 | ||||
| Present, n (%) | 76 (46.6) | 100 (56) | 1 | |
| Null, n (%) | 87 (53.4) | 79 (44) | 0.095 | 1.4 (1 – 2.2) |
| GSTT1 | ||||
| Present, n (%) | 122 (74.8) | 152 (84.9) | 1 | |
| Null, n (%) | 41 (25.2) | 27 (15.1) | 0.02 | 1.9 (1.1–3.2) |
| GSTM1/GSTT1 | ||||
| Present/present, n (%) | 54 (33.1) | 81 (45.3) | ||
| Null/ Null, n (%) | 20 (12.3) | 11 (6.1) | 0.014 | 2.8 (1.2–6.4) |
OR = odds ratio; CI = confidence interval./
Adjusted OR for age, ethnicity and gender
Analysis of the combined effects of GSTM1, GSTT1 null genotypes on risk of SLE after adjusting for age, gender and ethnicity revealed that the null/null genotype for GSTT1 and GSTM1 polymorphisms increase 2.8 fold the risk of SLE compared to present/present genotype (OR, 2.8 [95% CI, 1.2 to 6.4]; P=0.014) (Table 2).
GSTP1 gene Ile105Val polymorphism
The frequency of Ile105Val polymorphism geno-types and alleles has shown in Table 3. No significant differences in genotypes and alleles frequency of Ile105Val polymorphism were found between SLE patients and healthy controls.
Table 3:
Association of GSTP1 Ile105Val polymorphism and SLE risk
| SLE N=163 | Control N=180 | P-value | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| GSTP1 Genotype | ||||
| Ile/Ile, n (%) | 88 (54) | 112 (62.2) | 1 | |
| Ile/Val, n (%) | 69 (42.3) | 60 (33.3) | 0.09 | 1.5 (0.9–2.3) |
| Val/Val, n (%) | 6 (3.7) | 8 (4.8) | 0.95 | 1 (0.3–2.9) |
| Allele | ||||
| Ile | 245 (75) | 284 (79) | 1 | |
| Val | 81 (25) | 76 (21) | 0.3 | 1.2 (0.9–1.8) |
OR = odds ratio; CI = confidence interval.
Adjusted OR for age, ethnicity and gender
Combination effect of GSTs genotypes
In order to assess the existence of any interaction between the polymorphisms of GSTM1, GSTT1 and GSTP1 genes, we calculated the frequencies of the simultaneous presence of the putative ‘high-risk’ genotypes. Individuals carrying all three presumptive low-risk genotypes, GSTM1 and GSTT1 non-deleted (present) and GSTP1 Ile/Ile geno-types were used as the reference group. Table 4 shows the results of the association between combined genotypes and SLE. A highly significant difference in the combination of GSTM1, GSTT1 null mutation with GSTP1 Ile/Val, Val/Val geno-types were found between the SLE patients (8%) and controls (1.6%), with 8 fold increase in risk of SLE [OR of 8.1, (95% CI= 2.1 to 30.6, P= 0.003)], while other combination genotypes did not show significant association with risk of SLE (Table 4). There were no significant differences in GSTs polymorphisms between different ethnic groups. Moreover no association was found between GSTs genotypes and SLE manifestations.
Table 4:
The combination effect of GSTs polymorphisms and Risk of SLE
| GSTs polymorphisms | Lupus N=163 n (%) | Control N=180 n (%) | P-value | Adjusted OR(95%CI)* | ||
|---|---|---|---|---|---|---|
| GSTT1 | GSTM1 | GSTP1 | ||||
| Present | Present | Ile/Ile | 27 (17) | 50 (15) | - | 1 |
| Null | Present | Ile/Ile | 12 (7.4) | 9 (5) | 0.08 | 2.5 (0.9–6.5) |
| Present | Null | Ile/Ile | 42 (26) | 44 (24.4) | 0.09 | 1.7 (0.9–3.3) |
| Null | Null | Ile/Ile | 7 (4.3) | 8 (4.4) | 0.35 | 1.7 (0.6–5.5) |
| Null | Present | Ile /Val, Val/Val | 8 (5) | 7 (3.9) | 0.16 | 2.2 (0.7–6.9) |
| Present | Null | Ile /Val, Val/Val | 26 (16) | 24 (13.3) | 0.05 | 2.1 (1–4.5) |
| Null | Null | Ile /Val, Val/Val | 13 (8) | 3 (1.6) | 0.003 | 8.1 (2.1–31.6) |
Adjusted OR for age, ethnicity and sex
Discussion
The present study was conducted to investigate the relation between the GSTT1, GSTM1 and GSTP1 polymorphisms and SLE susceptibility in an Iranian population. The contribution of the GST supergene family to oxidative stress resistance is well established (19), therefore the absence of GST enzymes could increase ROS-mediated damage. Since the lack of detoxification might be a risk factor for SLE development, analysis of GSTs genes status, particularly detection of GSTM1 and GSTT1 null mutation, and GSTP1 polymorphisms could have prognostic and pathologic importance. GSTT1, GSTM1 and GSTP1 genes are three major phase II xenobiotic bio-transforming enzymes that are known to be involved in the metabolic activation and generation of ROS. Although a few studies have been performed on the relation between GSTs polymorphisms and SLE, the association between these polymorphisms and SLE has not been clearly demonstrated (25–28). Furthermore a few studies showed a relation between GSTs polymorphisms and clinical manifestations of SLE, but not with SLE susceptibility (29–32).
In the current study, a significant difference in the GSTT1 null genotype frequency was observed between SLE patients and control group. However no association observed between GSTM1 null genotype and GSTP1 Ile105Val polymorphism with SLE. Moreover combination of GSTT1 null/GSTM1 null genotypes showed 2.8-fold increase in SLE risk and the combination of GSTT1 null/GSTM1 null/GSTP1 Ile/Val and Val/Val geno-types increased the SLE risk about 8.1-fold.
Our data strongly suggest that GSTT1 null/GSTM1 null/GSTP1 Ile/Val and Val/Val geno-types might substantially contribute to the pathogenesis of SLE.
Similarly Kang et al. in Korea observed no association between GSTM1 and GSTP1 genotypes and risk of SLE (25), however in present study, a relation between GSTT1 null genotype and SLE was found. Moreover Kang et al. observed an association between GSTM1 null genotype and lower frequency of hematological disorders, as well as lower frequency of discoid rash and nephritis in individuals with GSTT1 null genotype (25). Kiohara et al. observed increased risk of SLE in smokers with combined CYP1A1 rs4646903/GSTM1 null polymorphisms. (OR 17.5, 95% CI 3.20–95.9) (26).
Moreover Fraser et al. in United States reported no relation between GSTs polymorphisms and SLE, but they observed 3 fold increase in risk of SLE in individuals with ≥ 24 months' occupational sun exposure and GSTM1 null genotype in Caucasians (27).
In contrast to our results, Zhang et.al, in China observed an association between GSTM1 null (P =0.003, OR 1.66 [95% CI 1.19–2.32]), but not GSTT1 null (P =0.119, OR 0.77 [95% CI 0.56–1.07]) genotypes and SLE. Also the analysis of genes in combination for double-null deletion of both GSTT1 and GSTM1 showed no significant difference (P =0.863, OR 1.03 [95% CI 0.70–1.52]) (28).
In present study there was no significant difference in the frequency of the GSTM1 null geno-type between patients and controls (55% versus 47%) which is in agreement with previously published studies(25, 27). However current data was in contrast with Zhang et. al. study in Chinese population (28).
There were limited studies which examined the GSTM1 null genotype in combination with CYP1A1 or TNF receptor type II (TNF-RII) geno-types and SLE susceptibility (29). GSTM1 null genotype was not associated with SLE risk, nevertheless combination of CYP1A1 3801C/GSTM1 null polymorphisms was strongly associated with an increased risk of SLE in Japanese (OR = 4.35; 95% CI = 1.76, 10.73) (29). Moreover the authors analyzed the combined effect of TNF-RII 196M/CYP1A1 3801C/GSTM1 null genotype and observed significant association with SLE (OR = 5.83; 95% CI = 2, 17.04) (29).
There was no previously published report about the association between combined GSTs geno-types and SLE. In current investigation we concluded that the combination of GSTM1 and GSTT1 null mutation with GSTP1 Val/Ile, Ile/Ile genotypes increased the risk of SLE about 8 fold. However, combined studies including various genetic polymorphisms in various genes/enzymes are required to clarify genetic etiology of SLE.
There were some limitations in present study, including low sample size, environmental conditions and different ethnic groups (Fars and Balouch) existing in South East of Iran. Therefore further investigations using a larger sample size and different ethnic groups are necessary to confirm the present results.
Conclusion
GSTT1 null genotype alone and in combination with GSTM1 and also combination of GSTM1, GSTT1 null mutation with GSTP1 Val/Ile, Ile/Ile genotype could have a significant relation with SLE and might be associated with increased risk in Iranian patients.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This article was extracted from the MS thesis (registered number 2160) at Zahedan University of Medical Sciences. The authors thank Zahedan Deputy of Research Affairs for funding this project. The authors declare that they have no conflicts of interest.
References
- 1. Yasutomo K. (2003). Pathological lymphocyte activation by defective clearance of self-ligands in systemic lupus erythematosus. Rheumatology (Oxford), 42: 214–22. [DOI] [PubMed] [Google Scholar]
- 2. Wakeland EK, Liu K, Graham RR, Behrens TW. (2001). Delineating the genetic basis of systemic lupus erythematosus. Immunity, 15: 397–408. [DOI] [PubMed] [Google Scholar]
- 3. Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. (2004). Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol, 31: 1928–33. [PubMed] [Google Scholar]
- 4. Rider JR, Ollier WE, Lock RJ, Brookes ST, Pamphilon DH. (1997). Human cytomegalovirus infection and systemic lupus erythematosus. Clin Exp Rheumatol, 15: 405–9. [PubMed] [Google Scholar]
- 5. Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Parks CG, Gilkeson GS. (1998). Hormonal, environmental, and infectious risk factors for developing systemic lupus erythematosus. Arthritis Rheum, 41: 1714–24. [DOI] [PubMed] [Google Scholar]
- 6. Kilburn KH, Warshaw RH. (1992). Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ Res, 57: 1–9. [DOI] [PubMed] [Google Scholar]
- 7. Washio M, Horiuchi T, Kiyohara C, et al. (2006). Smoking, drinking, sleeping habits, and other lifestyle factors and the risk of systemic lupus erythematosus in Japanese females: findings from the KYSS study. Mod Rheumatol, 16: 143–50. [DOI] [PubMed] [Google Scholar]
- 8. Mannervik B. (1985). The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol, 57: 357–417. [DOI] [PubMed] [Google Scholar]
- 9. Mannervik B, Awasthi YC, Board PG, et al. (1992). Nomenclature for human glutathione transferases. Biochem J, 282 ( Pt 1): 305–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falck GC, Hirvonen A, Scarpato R, Saarikoski ST, Migliore L, Norppa H. (1999). Micronuclei in blood lymphocytes and genetic polymorphism for GSTM1, GSTT1 and NAT2 in pesticide-exposed greenhouse workers. Mutat Res, 441: 225–37. [DOI] [PubMed] [Google Scholar]
- 11. Barnes PJ. (1990). Reactive oxygen species and airway inflammation. Free Radic Biol Med, 9: 235–43. [DOI] [PubMed] [Google Scholar]
- 12. Ryberg D, Skaug V, Hewer A, et al. (1997). Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. Carcinogenesis, 18: 1285–9. [DOI] [PubMed] [Google Scholar]
- 13. Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW. (1993). Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst, 85: 1159–64. [DOI] [PubMed] [Google Scholar]
- 14. Katoh T, Inatomi H, Nagaoka A, Sugita A. (1995). Cytochrome P4501A1 gene polymorphism and homozygous deletion of the glutathione S-transferase M1 gene in urothelial cancer patients. Carcinogenesis, 16: 655–7. [DOI] [PubMed] [Google Scholar]
- 15. Nelson HH, Wiencke JK, Christiani DC, et al. (1995). Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis, 16: 1243–5. [DOI] [PubMed] [Google Scholar]
- 16. Chenevix-Trench G, Young J, Coggan M, Board P. (1995). Glutathione S-transferase M1 and T1 polymorphisms: susceptibility to colon cancer and age of onset. Carcinogenesis, 16: 1655–7. [DOI] [PubMed] [Google Scholar]
- 17. Zhong S, Howie AF, Ketterer B, et al. (1991). Glutathione S-transferase mu locus: use of genotyping and phenotyping assays to assess association with lung cancer susceptibility. Carcinogenesis, 12: 1533–7. [DOI] [PubMed] [Google Scholar]
- 18. Bruhn C, Brockmoller J, Kerb R, Roots I, Borchert HH. (1998). Concordance between enzyme activity and genotype of glutathione S-transferase theta (GSTT1). Biochem Pharmacol, 56: 1189–93. [DOI] [PubMed] [Google Scholar]
- 19. Hayes JD, Pulford DJ. (1995). The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol, 30: 445–600. [DOI] [PubMed] [Google Scholar]
- 20. Chasseaud LF. (1979). The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res, 29: 175–274. [DOI] [PubMed] [Google Scholar]
- 21. Zhang YJ, Chen Y, Ahsan H, et al. (2005). Silencing of glutathione S-transferase P1 by promoter hypermethylation and its relationship to environmental chemical carcinogens in hepatocellular carcinoma. Cancer Lett, 221: 135–43. [DOI] [PubMed] [Google Scholar]
- 22. Autrup H. (2000). Genetic polymorphisms in human xenobiotica metabolizing enzymes as susceptibility factors in toxic response. Mutat Res, 464: 65–76. [DOI] [PubMed] [Google Scholar]
- 23. Lacerda LL, Serrano SV, Mathes A, Rey JA, Bello MJ, Casartelli C. (2005). An intronic variant in the TP53 gene in a Brazilian woman with breast cancer. Cancer Genet Cytogenet, 160: 160–3. [DOI] [PubMed] [Google Scholar]
- 24. Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. (1997). Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem, 272: 10004–12. [DOI] [PubMed] [Google Scholar]
- 25. Kang TY, El-Sohemy A, Comelis MC, Eny KM, Bae SC. (2005). Glutathione S-transferase genotype and risk of systemic lupus erythematosus in Koreans. Lupus, 14: 381–4. [DOI] [PubMed] [Google Scholar]
- 26. Kiyohara C, Washio M, Horiuchi T, et al. (2012). Risk modification by CYP1A1 and GSTM1 polymorphisms in the association of cigarette smoking and systemic lupus erythematosus in a Japanese population. Scand J Rheumatol, 41: 103–9. [DOI] [PubMed] [Google Scholar]
- 27. Fraser PA, Ding WZ, Mohseni M, et al. (2003). Glutathione S-transferase M null homozygosity and risk of systemic lupus erythematosus associated with sun exposure: a possible gene-environment interaction for autoimmunity. J Rheumatol, 30: 276–82. [PubMed] [Google Scholar]
- 28. Zhang J, Deng J, Zhang C, et al. (2010). Association of GSTT1, GSTM1 and CYP1A1 polymorphisms with susceptibility to systemic lupus erythematosus in the Chinese population. Clin Chim Acta, 411: 878–81. [DOI] [PubMed] [Google Scholar]
- 29. Horiuchi T, Washio M, Kiyohara C, et al. (2009). Combination of TNF-RII, CYP1A1 and GSTM1 polymorphisms and the risk of Japanese SLE: findings from the KYSS study. Rheumatology (Oxford), 48: 1045–9. [DOI] [PubMed] [Google Scholar]
- 30. Karlson EW, Watts J, Signorovitch J, et al. (2007). Effect of glutathione S-transferase polymorphisms and proximity to hazardous waste sites on time to systemic lupus erythematosus diagnosis: results from the Roxbury lupus project. Arthritis Rheum, 56: 244–54. [DOI] [PubMed] [Google Scholar]
- 31. Kelsey KT, Spitz MR, Zuo ZF, Wiencke JK. (1997). Polymorphisms in the glutathione S-transferase class mu and theta genes interact and increase susceptibility to lung cancer in minority populations (Texas, United States). Cancer Causes Control, 8: 554–9. [DOI] [PubMed] [Google Scholar]
- 32. Bailey LR, Roodi N, Verrier CS, Yee CJ, Dupont WD, Parl FF. (1998). Breast cancer and CYPIA1, GSTM1, and GSTT1 polymorphisms: evidence of a lack of association in Caucasians and African Americans. Cancer Res, 58: 65–70. [PubMed] [Google Scholar]
- 33. Park LY, Muscat JE, Kaur T, et al. (2000). Comparison of GSTM polymorphisms and risk for oral cancer between African-Americans and Caucasians. Pharmacogenetics, 10: 123–31. [DOI] [PubMed] [Google Scholar]
- 34. Arand M, Muhlbauer R, Hengstler J, et al. (1996). A multiplex polymerase chain reaction protocol for the simultaneous analysis of the glutathione S-transferase GSTM1 and GSTT1 polymorphisms. Anal Biochem, 236: 184–6. [DOI] [PubMed] [Google Scholar]
- 35. Harries LW, Stubbins MJ, Forman D, Howard GC, Wolf CR. (1997). Identification of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis, 18: 641–4. [DOI] [PubMed] [Google Scholar]


