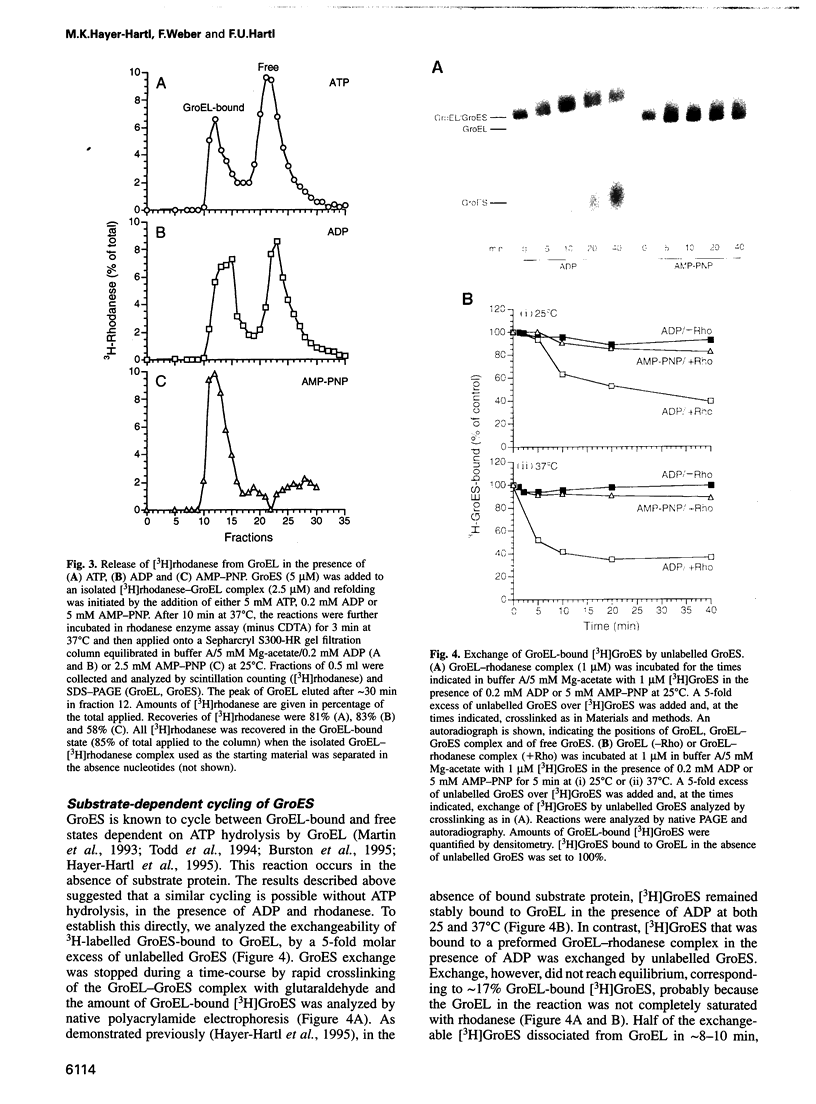
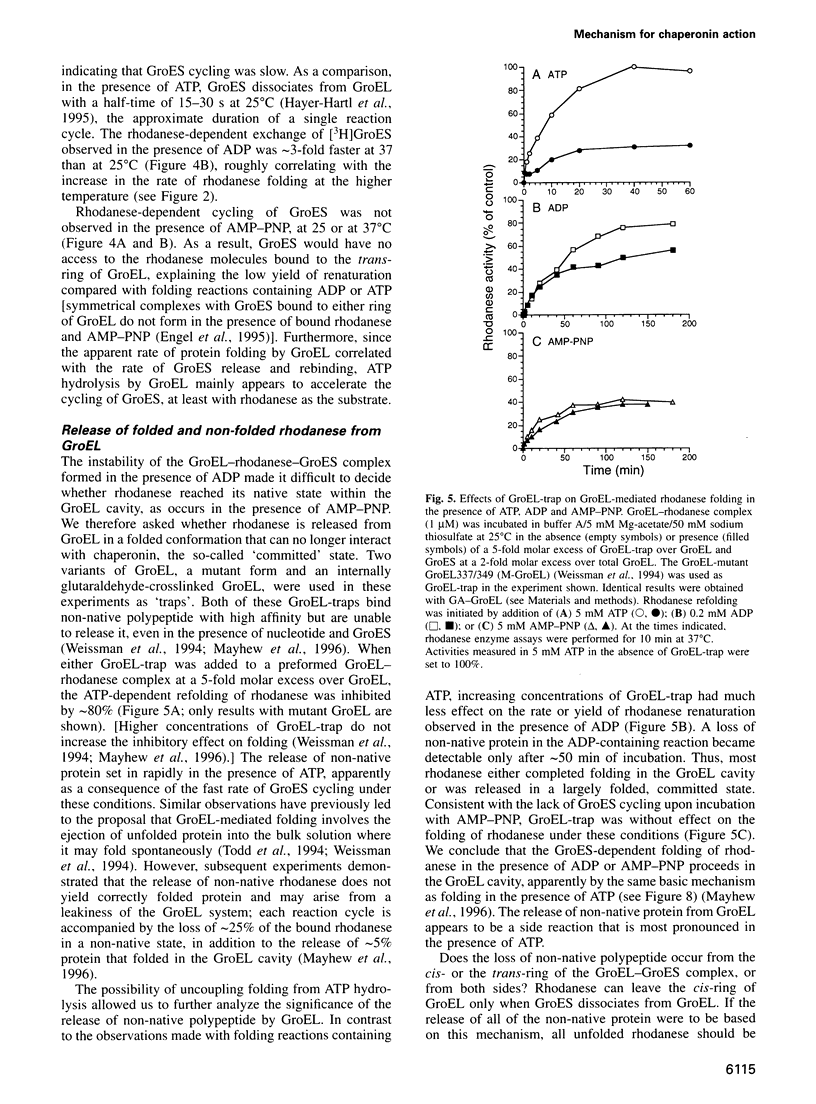
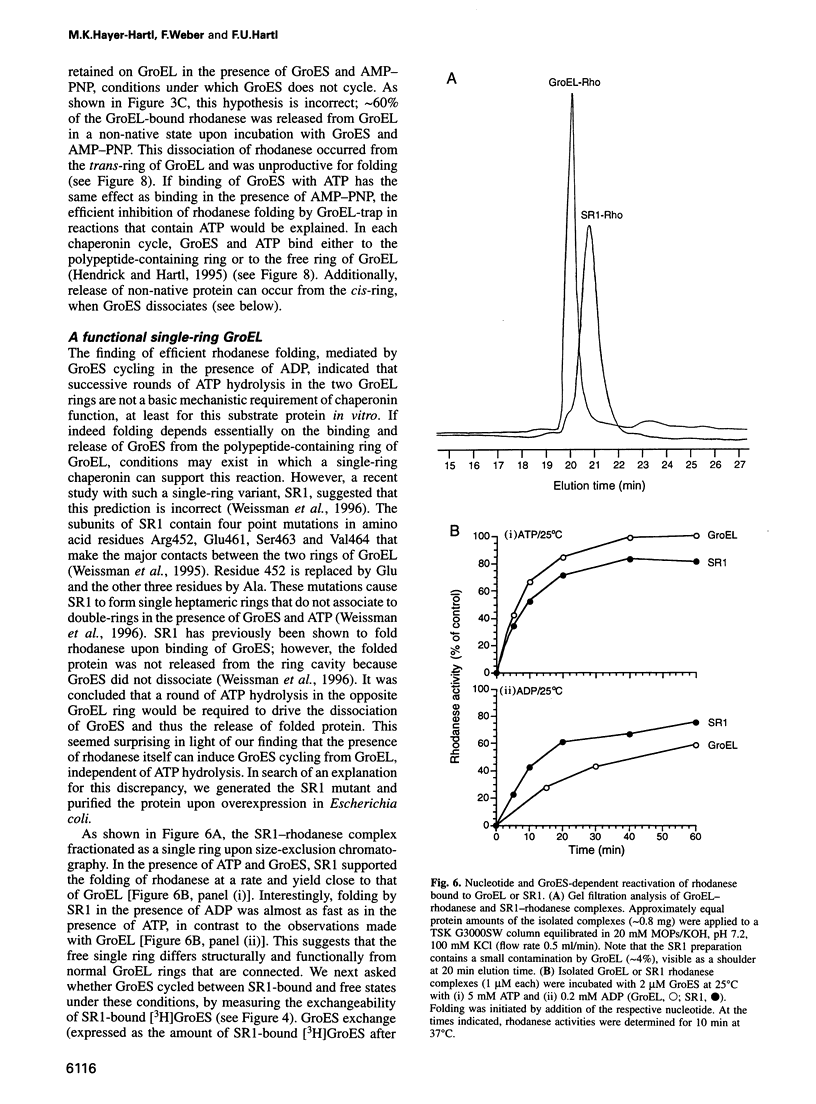
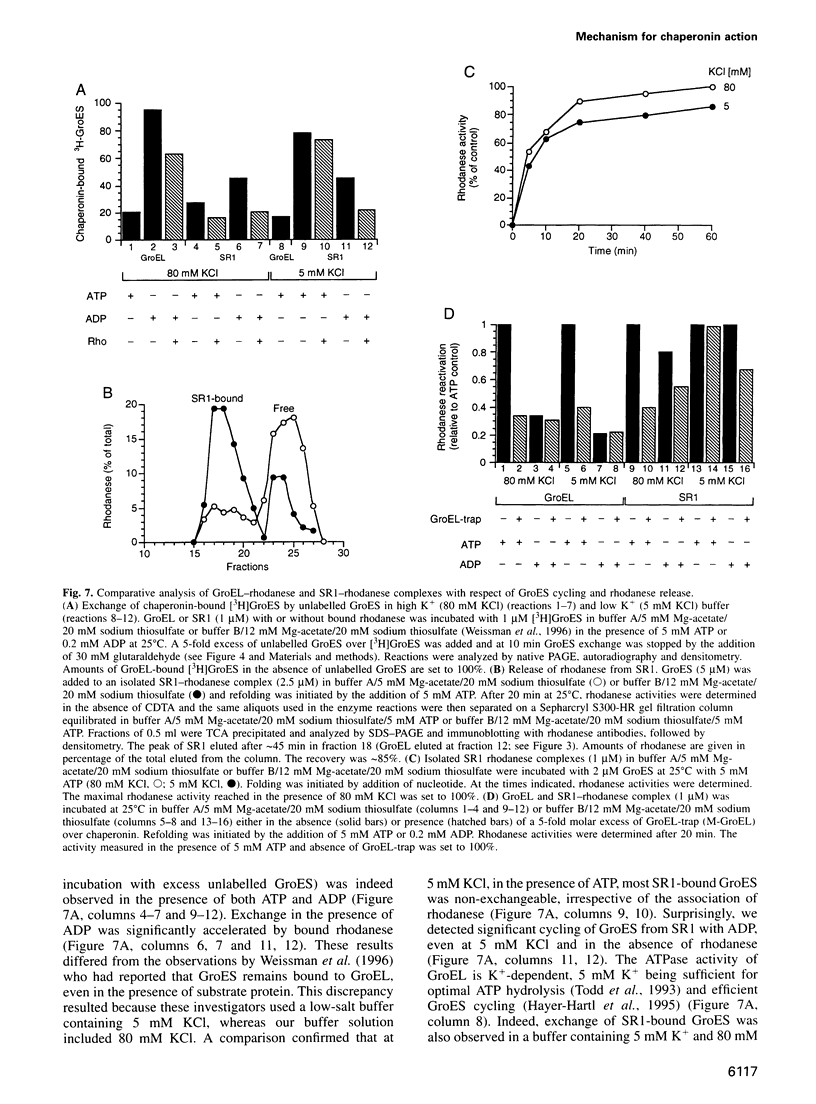
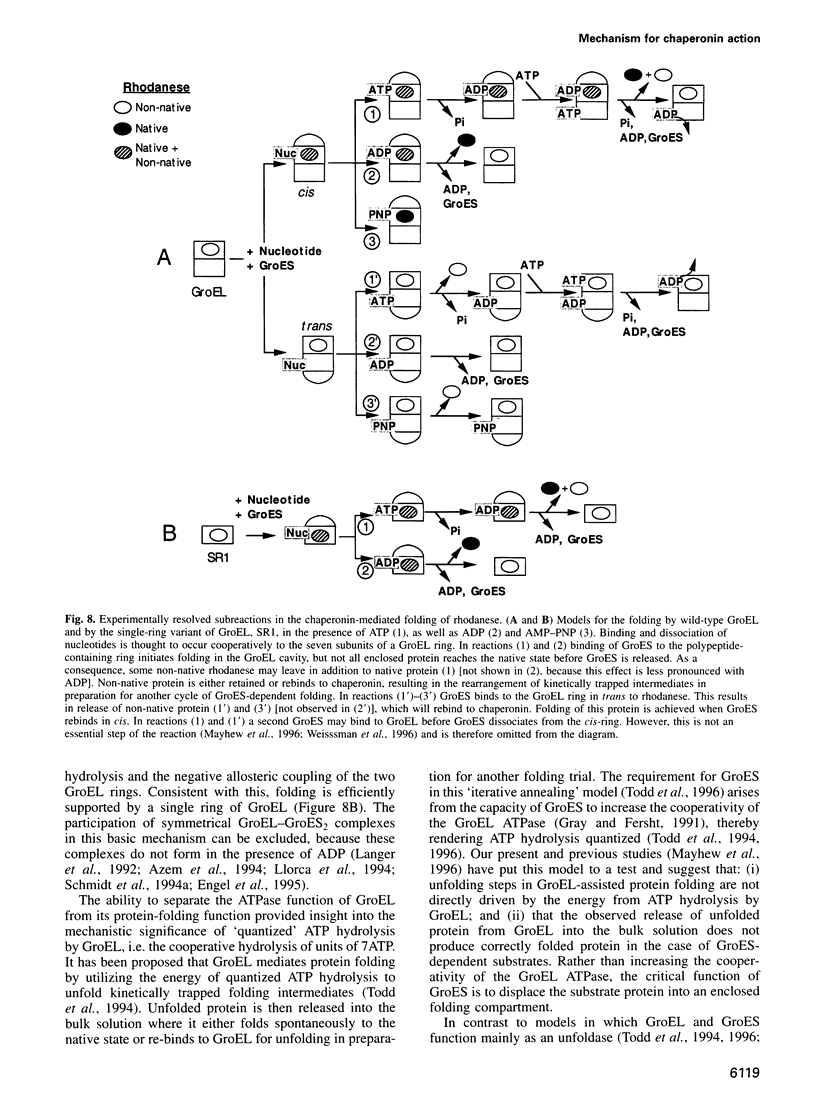
Abstract
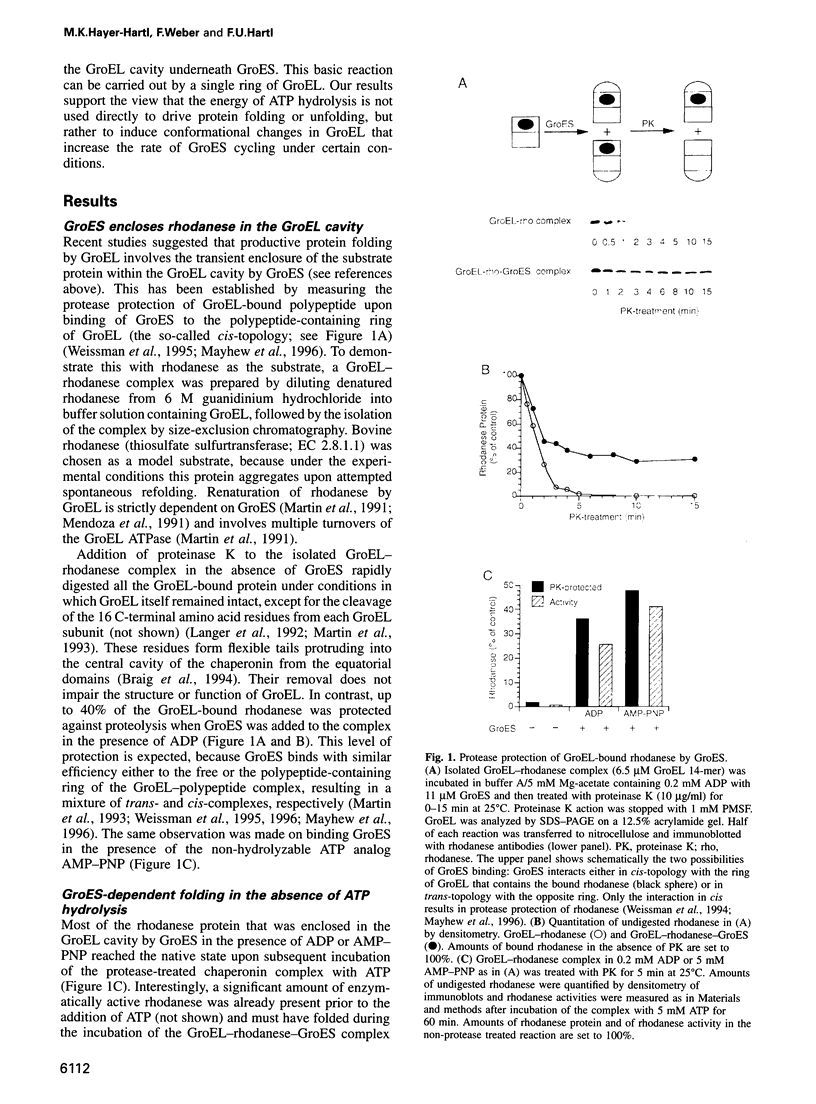
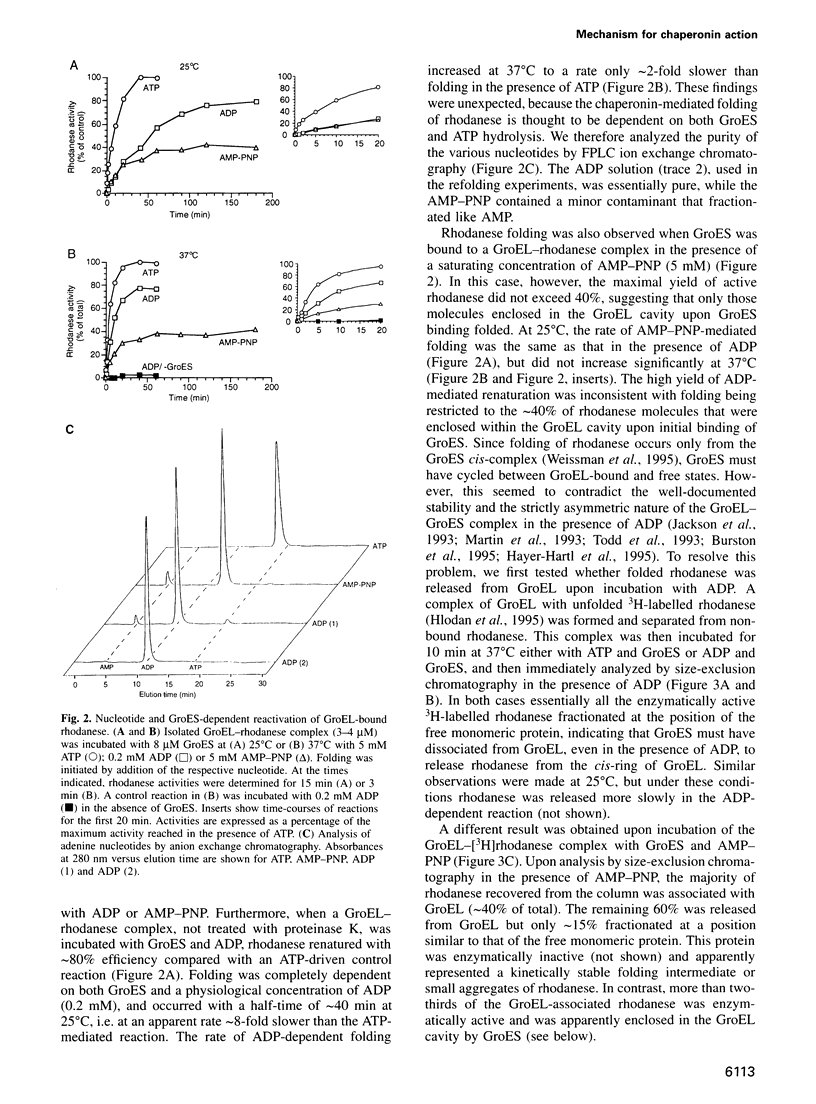
As a basic principle, assisted protein folding by GroEL has been proposed to involve the disruption of misfolded protein structures through ATP hydrolysis and interaction with the cofactor GroES. Here, we describe chaperonin subreactions that prompt a re-examination of this view. We find that GroEL-bound substrate polypeptide can induce GroES cycling on and off GroEL in the presence of ADP. This mechanism promotes efficient folding of the model protein rhodanese, although at a slower rate than in the presence of ATP. Folding occurs when GroES displaces the bound protein into the sequestered volume of the GroEL cavity. Resulting native protein leaves GroEL upon GroES release. A single-ring variant of GroEL is also fully functional in supporting this reaction cycle. We conclude that neither the energy of ATP hydrolysis nor the allosteric coupling of the two GroEL rings is directly required for GroEL/GroES-mediated protein folding. The minimal mechanism of the reaction is the binding and release of GroES to a polypeptide-containing ring of GroEL, thereby closing and opening the GroEL folding cage. The role of ATP hydrolysis is mainly to induce conformational changes in GroEL that result in GroES cycling at a physiologically relevant rate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. To fold or not to fold.... Science. 1993 Jun 25;260(5116):1903–1904. doi: 10.1126/science.8100365. [DOI] [PubMed] [Google Scholar]
- Azem A., Kessel M., Goloubinoff P. Characterization of a functional GroEL14(GroES7)2 chaperonin hetero-oligomer. Science. 1994 Jul 29;265(5172):653–656. doi: 10.1126/science.7913553. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braig K., Otwinowski Z., Hegde R., Boisvert D. C., Joachimiak A., Horwich A. L., Sigler P. B. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994 Oct 13;371(6498):578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Burston S. G., Ranson N. A., Clarke A. R. The origins and consequences of asymmetry in the chaperonin reaction cycle. J Mol Biol. 1995 May 26;249(1):138–152. doi: 10.1006/jmbi.1995.0285. [DOI] [PubMed] [Google Scholar]
- Chen S., Roseman A. M., Hunter A. S., Wood S. P., Burston S. G., Ranson N. A., Clarke A. R., Saibil H. R. Location of a folding protein and shape changes in GroEL-GroES complexes imaged by cryo-electron microscopy. Nature. 1994 Sep 15;371(6494):261–264. doi: 10.1038/371261a0. [DOI] [PubMed] [Google Scholar]
- Corrales F. J., Fersht A. R. The folding of GroEL-bound barnase as a model for chaperonin-mediated protein folding. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5326–5330. doi: 10.1073/pnas.92.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales F. J., Fersht A. R. Toward a mechanism for GroEL.GroES chaperone activity: an ATPase-gated and -pulsed folding and annealing cage. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4509–4512. doi: 10.1073/pnas.93.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Molecular chaperones. Unfolding protein folding. Nature. 1991 Jul 4;352(6330):17–18. doi: 10.1038/352017a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Hartl F. U. Protein folding in the cell: competing models of chaperonin function. FASEB J. 1996 Jan;10(1):20–26. doi: 10.1096/fasebj.10.1.8566542. [DOI] [PubMed] [Google Scholar]
- Ellis R. J. Molecular chaperones. Opening and closing the Anfinsen cage. Curr Biol. 1994 Jul 1;4(7):633–635. doi: 10.1016/s0960-9822(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Revisiting the Anfinsen cage. Fold Des. 1995;1(1):R9–R15. [PubMed] [Google Scholar]
- Engel A., Hayer-Hartl M. K., Goldie K. N., Pfeifer G., Hegerl R., Müller S., da Silva A. C., Baumeister W., Hartl F. U. Functional significance of symmetrical versus asymmetrical GroEL-GroES chaperonin complexes. Science. 1995 Aug 11;269(5225):832–836. doi: 10.1126/science.7638600. [DOI] [PubMed] [Google Scholar]
- Fayet O., Louarn J. M., Georgopoulos C. Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. Mol Gen Genet. 1986 Mar;202(3):435–445. doi: 10.1007/BF00333274. [DOI] [PubMed] [Google Scholar]
- Fenton W. A., Kashi Y., Furtak K., Horwich A. L. Residues in chaperonin GroEL required for polypeptide binding and release. Nature. 1994 Oct 13;371(6498):614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- Gray T. E., Fersht A. R. Cooperativity in ATP hydrolysis by GroEL is increased by GroES. FEBS Lett. 1991 Nov 4;292(1-2):254–258. doi: 10.1016/0014-5793(91)80878-7. [DOI] [PubMed] [Google Scholar]
- Gray T. E., Fersht A. R. Refolding of barnase in the presence of GroE. J Mol Biol. 1993 Aug 20;232(4):1197–1207. doi: 10.1006/jmbi.1993.1471. [DOI] [PubMed] [Google Scholar]
- Hartl F. U. Molecular chaperones in cellular protein folding. Nature. 1996 Jun 13;381(6583):571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hartl F. U. Protein folding. Secrets of a double-doughnut. Nature. 1994 Oct 13;371(6498):557–559. doi: 10.1038/371557a0. [DOI] [PubMed] [Google Scholar]
- Hayer-Hartl M. K., Ewbank J. J., Creighton T. E., Hartl F. U. Conformational specificity of the chaperonin GroEL for the compact folding intermediates of alpha-lactalbumin. EMBO J. 1994 Jul 1;13(13):3192–3202. doi: 10.1002/j.1460-2075.1994.tb06618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer-Hartl M. K., Martin J., Hartl F. U. Asymmetrical interaction of GroEL and GroES in the ATPase cycle of assisted protein folding. Science. 1995 Aug 11;269(5225):836–841. doi: 10.1126/science.7638601. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. The role of molecular chaperones in protein folding. FASEB J. 1995 Dec;9(15):1559–1569. doi: 10.1096/fasebj.9.15.8529835. [DOI] [PubMed] [Google Scholar]
- Hlodan R., Tempst P., Hartl F. U. Binding of defined regions of a polypeptide to GroEL and its implications for chaperonin-mediated protein folding. Nat Struct Biol. 1995 Jul;2(7):587–595. doi: 10.1038/nsb0795-587. [DOI] [PubMed] [Google Scholar]
- Horst M., Oppliger W., Feifel B., Schatz G., Glick B. S. The mitochondrial protein import motor: dissociation of mitochondrial hsp70 from its membrane anchor requires ATP binding rather than ATP hydrolysis. Protein Sci. 1996 Apr;5(4):759–767. doi: 10.1002/pro.5560050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. F., Weaver A. J., Landry S. J., Gierasch L., Deisenhofer J. The crystal structure of the GroES co-chaperonin at 2.8 A resolution. Nature. 1996 Jan 4;379(6560):37–45. doi: 10.1038/379037a0. [DOI] [PubMed] [Google Scholar]
- Jackson G. S., Staniforth R. A., Halsall D. J., Atkinson T., Holbrook J. J., Clarke A. R., Burston S. G. Binding and hydrolysis of nucleotides in the chaperonin catalytic cycle: implications for the mechanism of assisted protein folding. Biochemistry. 1993 Mar 16;32(10):2554–2563. doi: 10.1021/bi00061a013. [DOI] [PubMed] [Google Scholar]
- Langer T., Pfeifer G., Martin J., Baumeister W., Hartl F. U. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992 Dec;11(13):4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O., Marco S., Carrascosa J. L., Valpuesta J. M. The formation of symmetrical GroEL-GroES complexes in the presence of ATP. FEBS Lett. 1994 May 30;345(2-3):181–186. doi: 10.1016/0014-5793(94)00432-3. [DOI] [PubMed] [Google Scholar]
- Mande S. C., Mehra V., Bloom B. R., Hol W. G. Structure of the heat shock protein chaperonin-10 of Mycobacterium leprae. Science. 1996 Jan 12;271(5246):203–207. doi: 10.1126/science.271.5246.203. [DOI] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Martin J., Mayhew M., Langer T., Hartl F. U. The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature. 1993 Nov 18;366(6452):228–233. doi: 10.1038/366228a0. [DOI] [PubMed] [Google Scholar]
- Mayhew M., da Silva A. C., Martin J., Erdjument-Bromage H., Tempst P., Hartl F. U. Protein folding in the central cavity of the GroEL-GroES chaperonin complex. Nature. 1996 Feb 1;379(6564):420–426. doi: 10.1038/379420a0. [DOI] [PubMed] [Google Scholar]
- Mendoza J. A., Rogers E., Lorimer G. H., Horowitz P. M. Chaperonins facilitate the in vitro folding of monomeric mitochondrial rhodanese. J Biol Chem. 1991 Jul 15;266(20):13044–13049. [PubMed] [Google Scholar]
- Ranson N. A., Dunster N. J., Burston S. G., Clarke A. R. Chaperonins can catalyse the reversal of early aggregation steps when a protein misfolds. J Mol Biol. 1995 Jul 28;250(5):581–586. doi: 10.1006/jmbi.1995.0399. [DOI] [PubMed] [Google Scholar]
- Saibil H. R., Zheng D., Roseman A. M., Hunter A. S., Watson G. M., Chen S., Auf Der Mauer A., O'Hara B. P., Wood S. P., Mann N. H. ATP induces large quaternary rearrangements in a cage-like chaperonin structure. Curr Biol. 1993 May 1;3(5):265–273. doi: 10.1016/0960-9822(93)90176-o. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Buchner J., Todd M. J., Lorimer G. H., Viitanen P. V. On the role of groES in the chaperonin-assisted folding reaction. Three case studies. J Biol Chem. 1994 Apr 8;269(14):10304–10311. [PubMed] [Google Scholar]
- Schmidt M., Rutkat K., Rachel R., Pfeifer G., Jaenicke R., Viitanen P., Lorimer G., Buchner J. Symmetric complexes of GroE chaperonins as part of the functional cycle. Science. 1994 Jul 29;265(5172):656–659. doi: 10.1126/science.7913554. [DOI] [PubMed] [Google Scholar]
- Tandon S., Horowitz P. Detergent-assisted refolding of guanidinium chloride-denatured rhodanese. The effect of lauryl maltoside. J Biol Chem. 1986 Nov 25;261(33):15615–15618. [PubMed] [Google Scholar]
- Todd M. J., Lorimer G. H., Thirumalai D. Chaperonin-facilitated protein folding: optimization of rate and yield by an iterative annealing mechanism. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4030–4035. doi: 10.1073/pnas.93.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994 Jul 29;265(5172):659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Hydrolysis of adenosine 5'-triphosphate by Escherichia coli GroEL: effects of GroES and potassium ion. Biochemistry. 1993 Aug 24;32(33):8560–8567. doi: 10.1021/bi00084a024. [DOI] [PubMed] [Google Scholar]
- Vachereau A. Luminescent immunodetection of western-blotted proteins from coomassie-stained polyacrylamide gel. Anal Biochem. 1989 May 15;179(1):206–208. doi: 10.1016/0003-2697(89)90227-3. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Hohl C. M., Kovalenko O., Kashi Y., Chen S., Braig K., Saibil H. R., Fenton W. A., Horwich A. L. Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell. 1995 Nov 17;83(4):577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kashi Y., Fenton W. A., Horwich A. L. GroEL-mediated protein folding proceeds by multiple rounds of binding and release of nonnative forms. Cell. 1994 Aug 26;78(4):693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Rye H. S., Fenton W. A., Beechem J. M., Horwich A. L. Characterization of the active intermediate of a GroEL-GroES-mediated protein folding reaction. Cell. 1996 Feb 9;84(3):481–490. doi: 10.1016/s0092-8674(00)81293-3. [DOI] [PubMed] [Google Scholar]