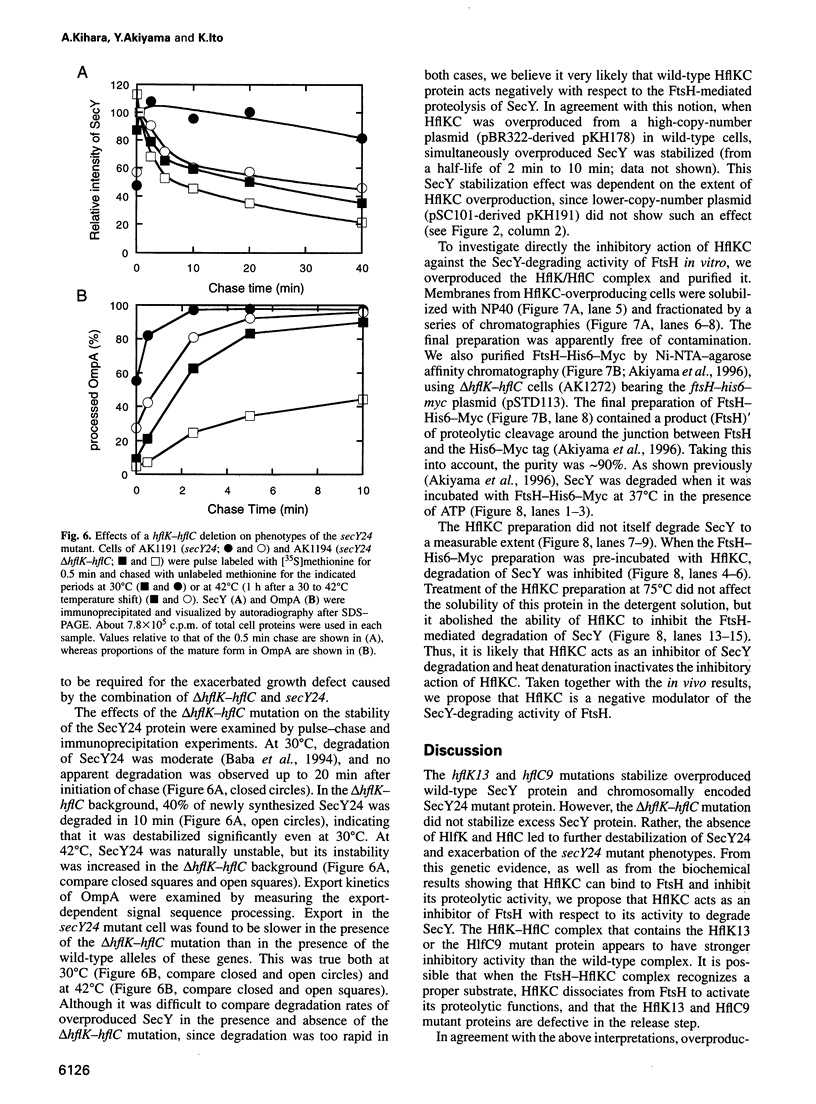
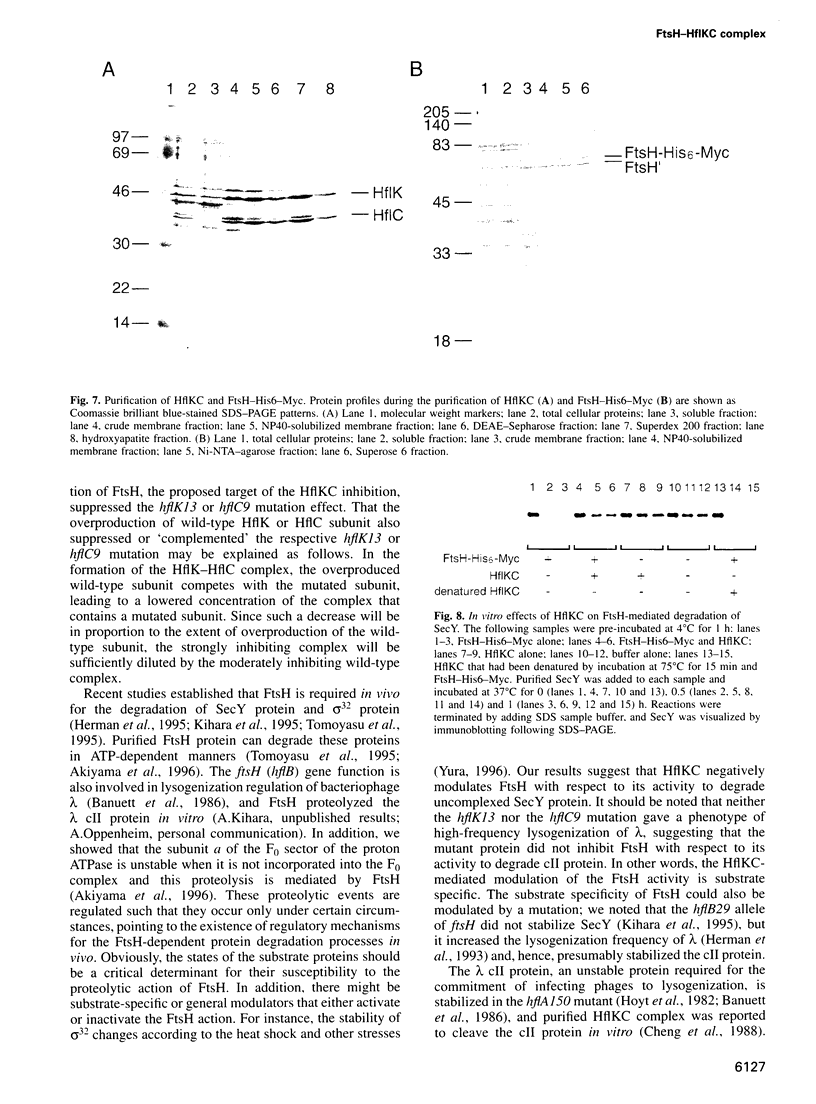
Abstract
Escherichia coli FtsH (HflB), a membrane-bound ATPase is required for proteolytic degradation of uncomplexed forms of the protein translocase SecY subunit. We have now isolated SecY-stabilizing mutations that cause an amino acid substitution in the HflK-HflC membrane protein complex. Although HflKC protein was believed to have a proteolytic activity against lambda cII protein, deletion of hflK-hflC did not stabilize SecY. Instead, the mutant alleles were partially dominant and overexpression of ftsH suppressed the mutational effects, suggesting that the mutant proteins antagonized the degradation of SecY. These results raise the possibility that even the wild-type HflKC protein acts to antagonize FtsH. Consistent with this notion, the hflkC null mutation accelerated degradation of the SecY24 protein. Furthermore cross-linking, co-immunoprecipitation, histidine-tagging and gel filtration experiments all indicated that FtsH and HflKC form a complex in vivo and in vitro. Finally, purified HflKC protein inhibited the SecY-degrading activity of purified FtsH protein in vitro. These results indicate that the proteolytic activity of FtsH is modulated negatively by its association with HflKC.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimaru J., Matsuyama S., Tokuda H., Mizushima S. Reconstitution of a protein translocation system containing purified SecY, SecE, and SecA from Escherichia coli. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6545–6549. doi: 10.1073/pnas.88.15.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. A new Escherichia coli gene, fdrA, identified by suppression analysis of dominant negative FtsH mutations. Mol Gen Genet. 1995 Nov 15;249(2):202–208. doi: 10.1007/BF00290367. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. SecY protein, a membrane-embedded secretion factor of E. coli, is cleaved by the ompT protease in vitro. Biochem Biophys Res Commun. 1990 Mar 16;167(2):711–715. doi: 10.1016/0006-291x(90)92083-c. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Ogura T., Ito K. Involvement of FtsH in protein assembly into and through the membrane. I. Mutations that reduce retention efficiency of a cytoplasmic reporter. J Biol Chem. 1994 Feb 18;269(7):5218–5224. [PubMed] [Google Scholar]
- Akiyama Y., Yoshihisa T., Ito K. FtsH, a membrane-bound ATPase, forms a complex in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1995 Oct 6;270(40):23485–23490. doi: 10.1074/jbc.270.40.23485. [DOI] [PubMed] [Google Scholar]
- Arlt H., Tauer R., Feldmann H., Neupert W., Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996 Jun 14;85(6):875–885. doi: 10.1016/s0092-8674(00)81271-4. [DOI] [PubMed] [Google Scholar]
- Baba T., Jacq A., Brickman E., Beckwith J., Taura T., Ueguchi C., Akiyama Y., Ito K. Characterization of cold-sensitive secY mutants of Escherichia coli. J Bacteriol. 1990 Dec;172(12):7005–7010. doi: 10.1128/jb.172.12.7005-7010.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T., Taura T., Shimoike T., Akiyama Y., Yoshihisa T., Ito K. A cytoplasmic domain is important for the formation of a SecY-SecE translocator complex. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4539–4543. doi: 10.1073/pnas.91.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F., Hoyt M. A., McFarlane L., Echols H., Herskowitz I. hflB, a new Escherichia coli locus regulating lysogeny and the level of bacteriophage lambda cII protein. J Mol Biol. 1986 Jan 20;187(2):213–224. doi: 10.1016/0022-2836(86)90229-9. [DOI] [PubMed] [Google Scholar]
- Begg K. J., Tomoyasu T., Donachie W. D., Khattar M., Niki H., Yamanaka K., Hiraga S., Ogura T. Escherichia coli mutant Y16 is a double mutant carrying thermosensitive ftsH and ftsI mutations. J Bacteriol. 1992 Apr;174(7):2416–2417. doi: 10.1128/jb.174.7.2416-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage L., Hendrick J. P., Schiebel E., Driessen A. J., Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990 Aug 24;62(4):649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cheng H. H., Muhlrad P. J., Hoyt M. A., Echols H. Cleavage of the cII protein of phage lambda by purified HflA protease: control of the switch between lysis and lysogeny. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7882–7886. doi: 10.1073/pnas.85.21.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confalonieri F., Duguet M. A 200-amino acid ATPase module in search of a basic function. Bioessays. 1995 Jul;17(7):639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- Douville K., Leonard M., Brundage L., Nishiyama K., Tokuda H., Mizushima S., Wickner W. Band 1 subunit of Escherichia coli preportein translocase and integral membrane export factor P12 are the same protein. J Biol Chem. 1994 Jul 22;269(29):18705–18707. [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L. M., Belin D., Carson M. J., Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995 Jul;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C., Ogura T., Tomoyasu T., Hiraga S., Akiyama Y., Ito K., Thomas R., D'Ari R., Bouloc P. Cell growth and lambda phage development controlled by the same essential Escherichia coli gene, ftsH/hflB. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10861–10865. doi: 10.1073/pnas.90.22.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C., Thévenet D., D'Ari R., Bouloc P. Degradation of sigma 32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma T., Yoshihisa T., Kihara A., Akiyama Y., Ito K. Intracellular stability of alpha fragments of beta-galactosidase: effects of amino-terminally fused polypeptides. Biochem Biophys Res Commun. 1995 Oct 13;215(2):452–458. doi: 10.1006/bbrc.1995.2486. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Ito K., Akiyama Y. In vivo analysis of integration of membrane proteins in Escherichia coli. Mol Microbiol. 1991 Sep;5(9):2243–2253. doi: 10.1111/j.1365-2958.1991.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Ito K. The major pathways of protein translocation across membranes. Genes Cells. 1996 Apr;1(4):337–346. doi: 10.1046/j.1365-2443.1996.34034.x. [DOI] [PubMed] [Google Scholar]
- Ito K., Wittekind M., Nomura M., Shiba K., Yura T., Miura A., Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983 Mar;32(3):789–797. doi: 10.1016/0092-8674(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Kihara A., Akiyama Y., Ito K. FtsH is required for proteolytic elimination of uncomplexed forms of SecY, an essential protein translocase subunit. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4532–4536. doi: 10.1073/pnas.92.10.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
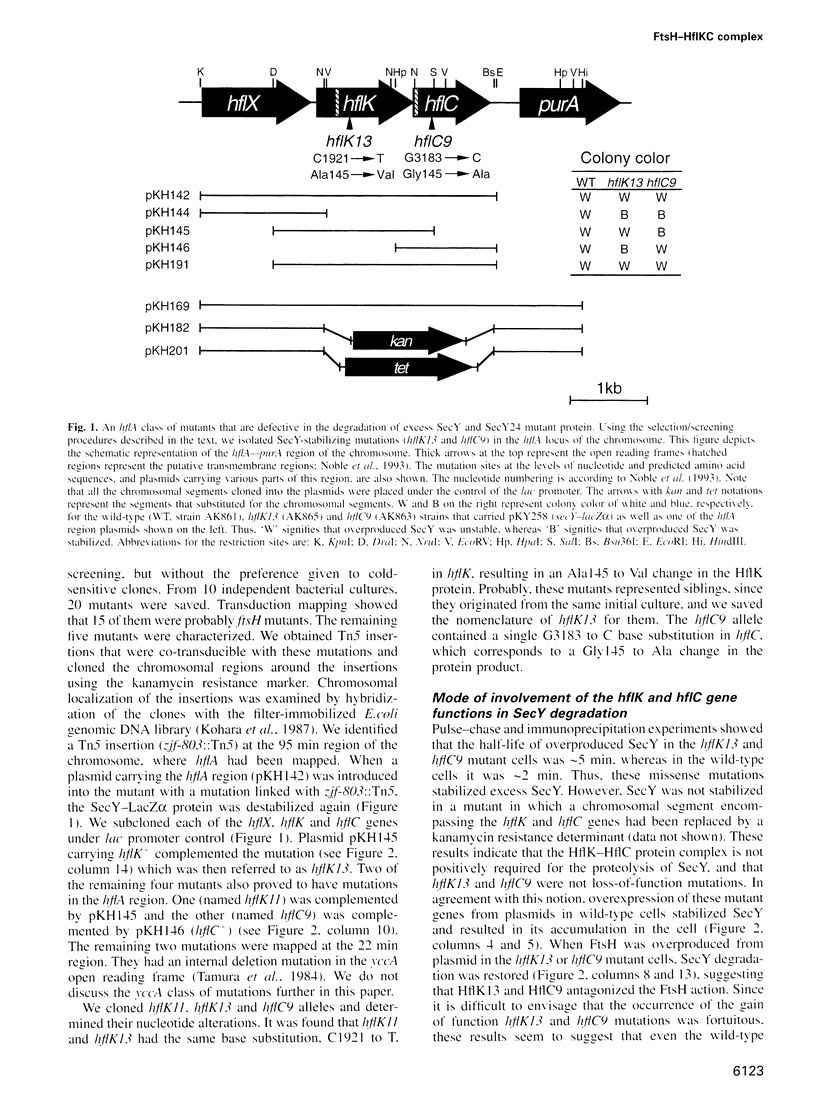
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Akimaru J., Mizushima S. SecE-dependent overproduction of SecY in Escherichia coli. Evidence for interaction between two components of the secretory machinery. FEBS Lett. 1990 Aug 20;269(1):96–100. doi: 10.1016/0014-5793(90)81128-b. [DOI] [PubMed] [Google Scholar]
- Noble J. A., Innis M. A., Koonin E. V., Rudd K. E., Banuett F., Herskowitz I. The Escherichia coli hflA locus encodes a putative GTP-binding protein and two membrane proteins, one of which contains a protease-like domain. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10866–10870. doi: 10.1073/pnas.90.22.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Mapping of a D-cycloserine resistance locus in escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):622–624. doi: 10.1128/jb.111.2.622-624.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D., De Almeida D. F. Isolation and characterization of a new temperature-sensitive cell division mutant of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1502–1507. doi: 10.1128/jb.124.3.1502-1507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T. Suppressors of the secY24 mutation: identification and characterization of additional ssy genes in Escherichia coli. J Bacteriol. 1986 Jun;166(3):849–856. doi: 10.1128/jb.166.3.849-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoike T., Taura T., Kihara A., Yoshihisa T., Akiyama Y., Cannon K., Ito K. Product of a new gene, syd, functionally interacts with SecY when overproduced in Escherichia coli. J Biol Chem. 1995 Mar 10;270(10):5519–5526. doi: 10.1074/jbc.270.10.5519. [DOI] [PubMed] [Google Scholar]
- Shirai Y., Akiyama Y., Ito K. Suppression of ftsH mutant phenotypes by overproduction of molecular chaperones. J Bacteriol. 1996 Feb;178(4):1141–1145. doi: 10.1128/jb.178.4.1141-1145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Kobayashi I., Thaler D., Stahl M. M. Direction of travel of RecBC recombinase through bacteriophage lambda DNA. Genetics. 1986 Jun;113(2):215–227. doi: 10.1093/genetics/113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura F., Nishimura S., Ohki M. The E. coli divE mutation, which differentially inhibits synthesis of certain proteins, is in tRNASer1. EMBO J. 1984 May;3(5):1103–1107. doi: 10.1002/j.1460-2075.1984.tb01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura T., Baba T., Akiyama Y., Ito K. Determinants of the quantity of the stable SecY complex in the Escherichia coli cell. J Bacteriol. 1993 Dec;175(24):7771–7775. doi: 10.1128/jb.175.24.7771-7775.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu T., Gamer J., Bukau B., Kanemori M., Mori H., Rutman A. J., Oppenheim A. B., Yura T., Yamanaka K., Niki H. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor sigma 32. EMBO J. 1995 Jun 1;14(11):2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu T., Yamanaka K., Murata K., Suzaki T., Bouloc P., Kato A., Niki H., Hiraga S., Ogura T. Topology and subcellular localization of FtsH protein in Escherichia coli. J Bacteriol. 1993 Mar;175(5):1352–1357. doi: 10.1128/jb.175.5.1352-1357.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu T., Yuki T., Morimura S., Mori H., Yamanaka K., Niki H., Hiraga S., Ogura T. The Escherichia coli FtsH protein is a prokaryotic member of a protein family of putative ATPases involved in membrane functions, cell cycle control, and gene expression. J Bacteriol. 1993 Mar;175(5):1344–1351. doi: 10.1128/jb.175.5.1344-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yura T. Regulation and conservation of the heat-shock transcription factor sigma32. Genes Cells. 1996 Mar;1(3):277–284. doi: 10.1046/j.1365-2443.1996.28028.x. [DOI] [PubMed] [Google Scholar]
- Zorick T. S., Echols H. Membrane localization of the HflA regulatory protease of Escherichia coli by immunoelectron microscopy. J Bacteriol. 1991 Oct;173(19):6307–6310. doi: 10.1128/jb.173.19.6307-6310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]