Abstract
Kinase-catalyzed protein phosphorylation is an important post-translational modification that regulates a variety of cellular functions. Identification of the many substrates of a specific kinase is critical to fully characterize cell biology. Unfortunately, kinase–substrate interactions are often transient, which makes their identification challenging. Here, the transient kinase–substrate complex was stabilized by covalent crosslinking using γ-phosphate modified ATP analogs. Building upon prior use of an ATP-aryl azide photocrosslinking analog, we report here the creation of an ATP-benzophenone photocrosslinking analog. ATP-benzophenone displayed a higher conversion percentage but more diffuse crosslinking compared to the ATP-aryl azide analog. A docking study was also performed to rationalize the conversion and crosslinking data. In total, the photocrosslinking ATP analogs produced stable kinase–substrate complexes that are suitable for future applications characterizing cell signaling pathways.
Keywords: Kinase, Kinase substrates, Photocrosslinking, ATP analogs
1. Introduction
Phosphorylation is an important protein post-translational modification that regulates a variety of signaling cascades in a cell.2,3 Phosphorylation is catalyzed by kinases and occurs on the hydroxyl group of serine, threonine, and tyrosine. With over 500 known protein kinases and roughly 30% of the proteome phosphorylated at a given time, characterizing phosphorylation events is a critical aspect of cell biology.4 In particular, identifying the cellular kinase of a known substrate or the substrates of a specific kinase would be helpful to fully map signaling pathway in normal and diseased states.
A variety of methods have been used to identify kinase–substrate pairs. The consensus sequences for many protein kinases have been determined using combinatorial peptide libraries. Based on sequence preferences, kinase–substrate pairs are routinely revealed by consensus site searching.5–7 Unfortunately, the consensus sequences of all 500 human protein kinases are not yet available. Techniques to identify protein–protein interactions are also used, including yeast two-hybrid and pull-down assays.8,9 While these classical methods have shown success, the transient kinase–substrate interaction can be lost or overlooked during purification or gene expression.
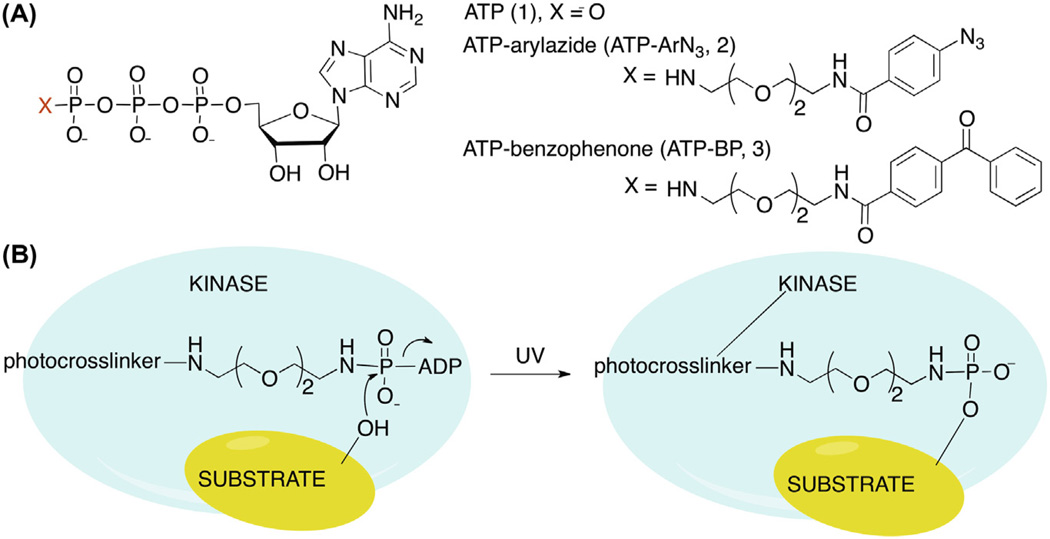
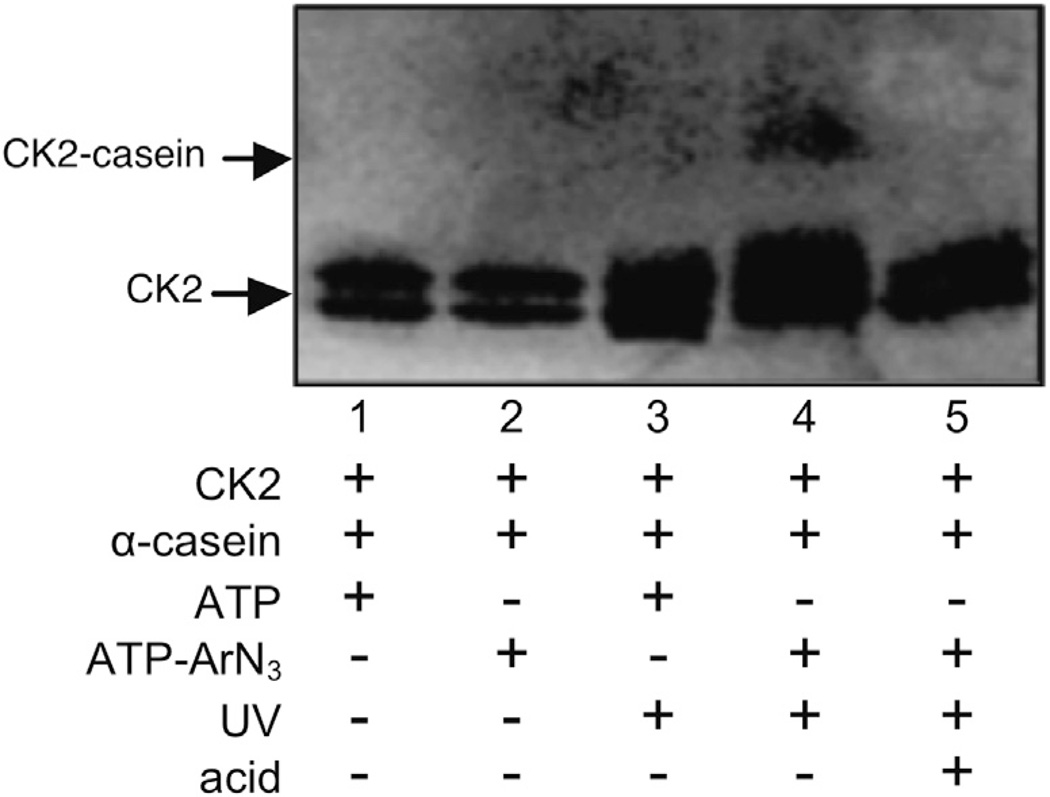
As alternatives, several chemical methods have been developed to convert the transient kinase–substrate interaction into a stable complex, which facilitates purification and identification.10–12 Building on the fact that adenosine-5′-triphosphate (ATP) (1, Fig. 1A) acts as a universal phosphoryl donor, we developed an ATP-based crosslinking reagent that requires phosphorylation for covalent kinase–substrate linkage. For phosphorylation-dependent crosslinking, ATP was modified at the γ phosphate with an aryl azide photocrosslinker (ATP–ArN3, 2, Fig. 1A) to covalently link kinase and substrate upon phosphorylation and UV irradiation (Fig. 1B).1 Specifically, crosslinking was observed between CK2 kinase and its substrate α-casein in a UV-dependent and ATP–ArN3-dependent manner (Fig. 2, lane 4 vs lanes 3 and 5). ATP–ArN3 crosslinking of kinase–substrate pairs is phosphorylation-dependent and uses native kinase and substrate, making it a powerful approach to mapping the phosphoproteome.
Figure 1.
(A) Chemical structures of ATP (1) and γ-phosphate modified ATP analogs, ATP–aryl azide (ATP–ArN3, 2) and ATP–benzophenone (ATP–BP, 3). (B) Cartoon depicting kinase-catalyzed protein phosphorylation along with UV-mediated crosslinking of kinase and substrate.
Figure 2.
Kinase-catalyzed labeling and subsequent photocrosslinking with ATP–ArN3.1 CK2 kinase was incubated with full length α-casein in the presence of ATP 1 or ATP–ArN3 2 before separation by SDS–PAGE and visualization with a CK2 antibody. Reactions were incubated either with or without exposure to 365 nm light (UV). Trifluoroacetic acid was added to a final concentration of 50% after crosslinking to cleave the phosphoramidate bond of the crosslink. The expected 68 kDa crosslinked complex is indicated as CK2-casein. The gel is representative of at least three independent trials.
In the present study, we aimed to identify optimized conditions for phosphorylation-dependent kinase–substrate crosslinking to facilitate future cell biology studies. We report here a new photocrosslinking analog, ATP–benzophenone (ATP–BP, 3, Fig. 1A), and compare its kinase–substrate crosslinking to ATP–ArN3. While ATP–BP was the more efficient kinase cosubstrate, ATP–ArN3 produced a single crosslinked band. To provide a structural rationale for the data, the ATP analogs were also docked into the active site of two kinases. In total, this study revealed that the ATP analogs were well suited for kinase–substrate pair identification with our model system.
2. Material and methods
2.1. Materials
ATP was obtained from Fisher. CK2 was purchased from New England Biolabs. CK2 peptide substrate (RRREEETEEE) was purchased from Promega. The 2,2′-ethylenedioxy bis-(ethylamine), alpha-cyano-4-hydroxy cinnamic acid, and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) were purchased from Acros. The 4-benzoyl benzoic acid, N-methyl morpholine (NMM), and 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) were obtained from Sigma–Aldrich. Tris-base and sodium chloride were obtained from JT Baker. D4-MeOH, D2O, and CDCl3 were purchased from Cambridge Isotope Lab. Inc. Flash chromatography was performed on silica gel, 200–400 mesh (Merck). A-25 sephadex was purchased from Aldrich. The anti-CK2 antibody was obtained from Millipore and Sigma–Aldrich. Goat anti-Mouse IgG H&L (HRP) secondary antibody was purchased from Abcam. SuperSignal West Dura Chemiluminescent Substrate was purchased from Thermo Scientific. Coomassie blue stain was purchased from NuSep.
2.2. Instruments
1H NMR, 13C NMR, 31P NMR (Varian Mercury-400) and ESI MS (Waters ZQ2000) were used to authenticate the compounds synthesized. The triethyl amine counter ion of ATP–BP 3 was observed at δ 1.18 and 2.8 in the 1H NMR. The peaks around δ 4.95 and 3.31 in the 1H NMR and δ 58.0 in 13C NMR were due to CD3OD solvent. Likewise, the peaks at δ 4.80 and 7.24 in the 1H NMR were due to D2O and CDCl3, respectively. The peak at δ 77.0 in 13C NMR was due to CDCl3. IR spectra were recorded on an FT/IR-460 plus (JASCO D Co. Ltd) spectrometer. Absorbance of intermediate compounds and the ATP-analog were measured using a UV–Vis spectrophotometer (HP 8452A Diode array spectrophotometer). High resolution mass spectra were obtained using LCT Premier XT (Waters). The concentration of the ATP analog was calculated using the UV–Vis spectrophotometer. Quantitative mass spectrometric analyses of phosphopeptides resulting from the kinase reactions were performed using a MALDI-TOF MS (Bruker Ultraflex). A Thermo Scientific-Pierce UVP 3UV Ultraviolet lamp was used for crosslinking.
2.3. Synthesis of the ATP–BP analog
2.3.1. Synthesis of N-(2-(2-(2-aminoethoxy)ethoxy)ethyl)-4-benzoylbenzamide 6
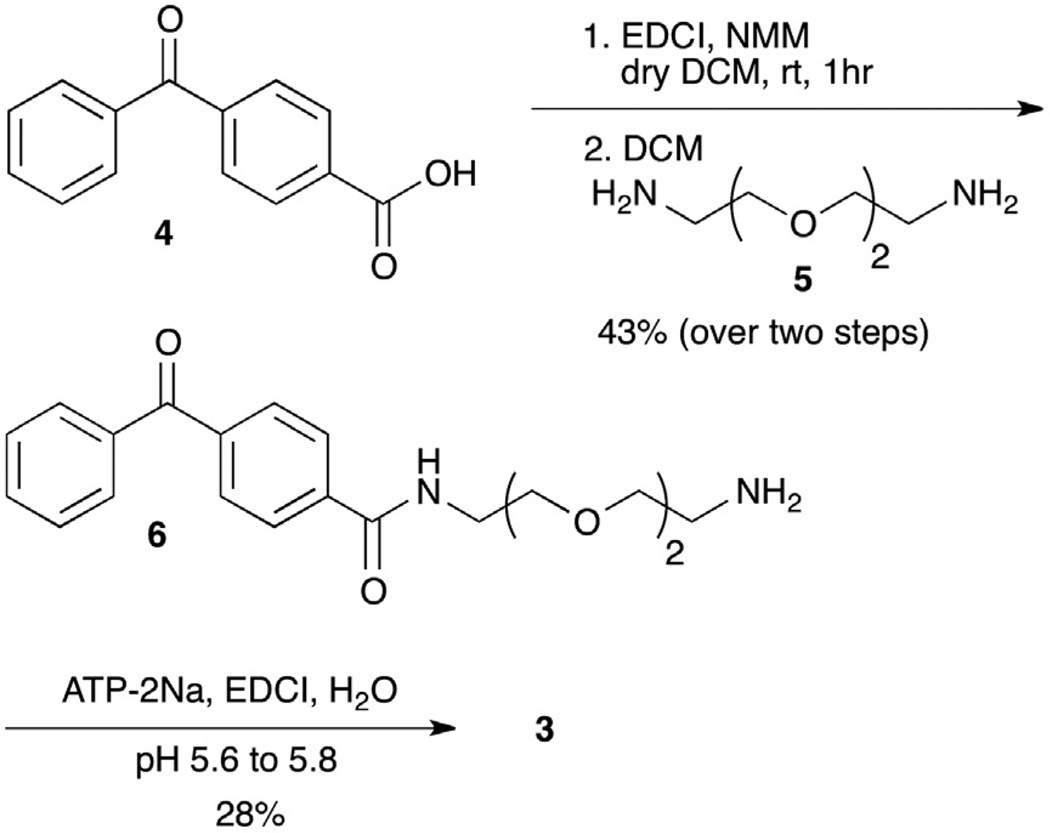
To a stirred solution of 4-benzoyl benzoic acid 4 (620 mg, 2.13 mmol) in dry DCM (10 mL) was added EDCI (713 mg, 3.73 mmol) and NMM (322 mg, 3.13 mmol) at room temperature. After stirring for 30 min, this solution was added dropwise to a cooled DCM solution (200 mL) of 2,2′-ethylenedioxy bis-(ethylamine) 5 (638 mg, 4.3 mmol) over a period of 30 min, followed by stirring for 2 h at room temperature. The solvent was evaporated to dryness and purified by flash chromatography using a stepwise solvent system, initially with DCM/EtOH (3:1) and later with DCM/EtOH/NH4OH (3:1:0.05) to yield benzophenone linker 6 (43%, 420 mg, 1.2 mmol). 1H NMR (400 MHz, CDCl3): δ 3.15 (b, 2H), 3.62–3.74 (m, 12H), 7.47 (t, 2H), 7.59 (t, 1H), 7.74 (m, 4H), 7.89 (b, 1H), 8.05 (d, 2H) 13C NMR (100 MHz, CDCl3): δ 40.1, 69.9, 70.1, 70.2, 127.5, 128.5, 129.9, 130.0, 132.9, 136.9, 137.5, 139.9, 167.0, 196.3. IR (neat, cm−1): 658, 716, 768, 864, 926, 1117, 1277, 1541, 1649, 2872. HRMS [M+H+] C20H25N2O4; calcd 357.1814 found: 357.1812, Figures S1–S4.
2.3.2. Synthesis of ATP-[γ]–N-(2-(2-(2-aminoethoxy)ethoxy)ethyl)-4-benzoylbenzamide 3
ATP-2Na (30 mg, 0.05 mmol) was dissolved in water (5 mL) and the pH was adjusted to 7.0 with 1 N aqueous HCl. EDCI (380 mg, 2 mmol) was added and the pH was adjusted to 5.6–5.8 with 1 N aqueous HCl followed by addition of water (1 mL). Benzophenone amine linker 6 (400 mg, 1.1 mmol) was dissolved in water (1 mL), added to the reaction mixture, and incubated for 2 h at room temperature while controlling the pH at 5.6–5.8. The reaction mixture was brought to pH 8.5 using 1 M triethylamine bicarbonate (TEAB) buffer and purified using an A-25 sephadex anion exchange column with a gradient of 0.1–1.0 MTEAB buffer solution (pH 8.5) as eluent. The purified product was lyophilized to dryness to obtain the TEA salt of ATP–BP 4 (28%, 11.83 mg, 0.014 mmol). The product was dissolved in 25 mM HEPES buffer pH 7.2–7.5 and stored at −80 °C for several months. With the aid of UV absorbance, the concentration of the final product was calculated. λ = 261 nm, ε = 21000. 1H NMR (400 MHz, D2O): δ 3.54–3.74 (m, 12H), 4.15 (m, 1H), 4.21 (m, 1H), 4.28 (m, 1H), 4.50 (m, 1H), 4.67 (m, 1H), 5.98 (d, 1H), 7.50 (t, 2H), 7.67 (m, 3H), 7.75 (d, 2H), 7.83 (d, 2H), 8.10 (s, 1H), 8.43 (s, 1H). 31P NMR (162 MHz, CD3OD): δ −1.53 (d, γ-P), −11.69 (d, α-P), −23.08 (t, β-P). IR (cm−1): 655, 715, 914, 991, 1060, 1222, 1647, 1707, 2366, 2981, 3275. HRMS: [M−H+] C30H31N7O16-P3; calcd 844.1510; found: 844.1505, Figures S5–S9.
2.4. Quantitative mass spectrometric analysis
Quantitative MALDI-TOF MS was performed using a previously described protocol.13 Briefly, each reaction mixture contained either ATP 1 or synthesized ATP–BP 3 (1.0 mM final concentration), CK2 substrate peptide (40 µM), and CK2 kinase (10 units/µL) in the manufacturer-supplied buffer (1×). The final volume of the reaction was 10 µL and the reaction mixtures were incubated for 2 h at 30 °C. The products formed after enzymatic reactions were esterified at all carboxylic acids by incubating with a solution of acetyl chloride (50 µL) and either d4-methanol (300 µL, for the ATP reaction) or d0-methanol (300 µL, for the ATP–BP reaction) at RT for 2 h. Under these conditions, the phosphoramidate bond of the ATP–BP product was cleaved to yield a phosphopeptide product. After esterification, the solvent was evaporated using a SpeedVac concentrator (ThermoScientific Savant) and phosphopeptides were separately resuspended in water (5 µL). For MS analysis, the two phosphopeptide samples were mixed (2 µL of each) with 10 µL of a matrix solution (saturated solution of alpha-cyano-4-hydroxy cinnamic acid in 70% acetonitrile, 30% water and 0.1% TFA). The mixture (1 µL) was spotted on a MALDI plate (Standard 384 MTP, Bruker) and the isotopically differentiated phosphopeptides were analyzed on MALDI-TOF MS instrument (Bruker Ultraflex). The mean of three independent trials is reported in the text (Figs. S10–S12).
2.5. Crosslinking experiment
Crosslinking experiments were performed as reported previously.1 Briefly, CK2 kinase (5 units/µL) was incubated with ATP 1 or ATP–BP 3 (2.5 mM) in CK2 buffer (20 mM HEPES, 50 mM KCl, 10 mM MgCl2) for 5 min on ice, followed by addition of α-casein (100 µM). The final reaction volume was 20 µL. The reaction mixtures were incubated for 2 h at 30 °C. Crosslinked protein was obtained by simultaneously irradiating the appropriate reaction tubes with a handheld UV lamp (365 nm). The presence of the phosphoramidate bond in the crosslinked product was confirmed by acid-mediated cleavage of the complex by adding 20 µL of trifluoroacetic acid (TFA) into the reaction mixture (final concentration of 50% TFA) and incubating at 30 °C for 1 h. The sample tube containing TFA was neutralized using 100 mM Tris base (50 µL) and the volume was reduced in vacuo. The reaction products were separated by SDS–PAGE and the proteins were visualized with coomassie stain, or Western blotting with an anti-CK2 antibody after electrotransfer onto PVDF membrane (Immobilon-PSQ).
2.6. Autodock analysis
The Autodock Vina program was designed by Dr. Oleg Trott of Molecular Graphics Lab at The Scripps Research Institute.14 It was downloaded free from the website-http://vina.scripps.edu/index.html. The Autodock Vina program was executed as per the tutorial instructions found on the website. The crystal structures were downloaded from the RCSB Protein Data Bank (1 ATP for PKA and 1DAW for CK2). The PyMOL program (Schrödinger Inc.) was used to delete ATP in the active site. The ATP analog structure was drawn in Chem 3D Pro (CambridgeSoft) and MM2 from Chem 3D pro was used for energy minimization. The output files of the different binding modes are shown in Tables S1–S6. Movies for each docked structure are also provided as Supplementary files.
3. Results and discussion
Photocrosslinking experiments require careful selection of photoreactive group. Factors that can influence the efficiency of crosslinking include size of the photoreactive group, excitation wavelength, half-life of active species, stability, and bioorthogonality.15 Due to their variable properties, several photoreactive groups have been widely used for crosslinking. Aryl azides lose N2 upon irradiation to form nitrene, a reactive species that can insert into the C–H and heteroatom–H bonds of a variety of biomolecules.16 Azides can be activated over a broad range of wavelength (254–400 nm) depending on the substitution.17,18 Similar to aryl azides, dialkyl diazirines requires 350 nm light to form a highly reactive carbene, which also broadly reacts with biomolecules through C/heteroatom–H bond insertions.19 A disadvantage common to both diazirines and aryl azides is rearrangement to long-lived electrophiles that participate in undesirable, nonspecific crosslinking reactions, which reduces crosslinking efficiency.20,21 Benzophenone overcomes some of these disadvantages by forming a diradical species with 350–360 nm light. The diradical can participate in crosslinking or relax back to benzophenone for subsequent reactivation, which results in high crosslinking efficiency.22 One disadvantage of benzophenone is its relatively large size and lipophilicity, which can preclude use in constrained systems. In addition, the diradical species requires prolonged irradiation times23 and displays crosslinking selectivity for methionine residues,24 which can limit use to only select protein targets.
Few reports have directly compared the crosslinking of nitrene and diradical groups under identical conditions.25,26 Given their strengths and weaknesses, we synthesized a new ATP analog containing a benzophenone to compare kinase compatibility and photocrosslinking with aryl azide.
3.1. Synthesis of ATP–BP
The ATP–BP analog was designed to include an ethylene glycol linker separating the γ-phosphate of ATP from the benzophenone group (Fig. 1A). This ethylene glycol linker is identical to that of ATP–ArN3 (Fig. 1A), which will allow direct comparison of the two photocrosslinking probes. ATP–BP 3 was synthesized in two steps (Scheme 1). Benzophenone amine linker 6 was prepared by activating 4-benzoyl benzoic acid 4 with EDCI, followed by reaction with the diamine linker 5. Amine 6 was then coupled to ATP using a reported procedure involving EDCI under controlled pH of 5.6–5.8, with subsequent purification by ion exchange chromatography.1,27
Scheme 1.
Synthesis of ATP–BP 3.
3.2. Kinase cosubstrate compatibility of ATP–BP
The compatibility of the ATP–BP analog in kinase-catalyzed labeling was tested using quantitative mass spectrometric (QMS) analysis with peptide substrates.13 CK2 kinase was tested due to its variable conversions with several ATP analogs,27 which make it an ideal model kinase. QMS data showed that ATP–BP was accepted by CK2. The CK2 peptide was modified with ATP–BP 3 and CK2 kinase with 71% conversion compared to ATP phosphorylation (100%, Figs. S10–S12). In earlier studies, ATP–ArN3 demonstrated a labeling efficiency of 51%.1 Therefore, ATP–BP demonstrated enhanced labeling efficiency compared to ATP–ArN3.
3.3. Phosphorylation-dependent kinase–substrate photocrosslinking
After confirming the kinase compatibility of ATP–BP, full-length protein crosslinking experiments were carried out with casein protein substrates and CK2 kinase. Casein was chosen as a model because it is widely used as substrates for CK2 kinase.28 Crosslinking experiments were carried out by incubating CK2 kinase and α-casein with ATP–BP under UV light. The reaction mixtures were separated on SDS–PAGE and visualized by Western blot analysis with a CK2 antibody.
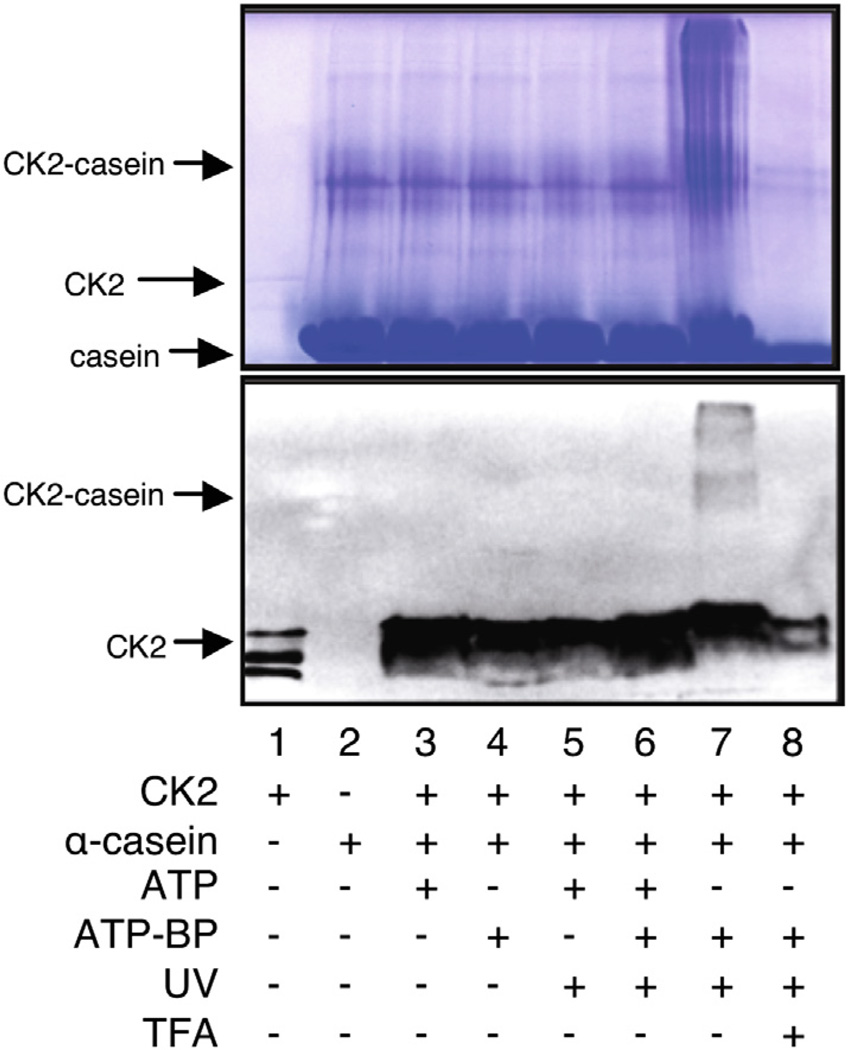
Immunoblotting analysis after UV-mediated crosslinking demonstrated the presence of a diffuse CK2-reactive band at 68 kDa, corresponding to the crosslinked catalytic α subunit of CK2 (45 kDa)29,30 and α-casein (23 kDa) (Fig. 3, lane 7). The crosslinked band was absent when the reaction mixture was incubated without UV light (Fig. 3, lane 4) or with ATP in the absence and presence of UV (Fig. 3, lanes 3 and 5). The crosslinked complex was also absent in the presence of competing ATP (Fig. 3, lane 6) or when the reaction mixture was treated with trifluoroacetic acid (TFA), due to acid-mediated cleavage of phosphoramidate bond (Fig. 3, lane 8). Unlike ATP–ArN3 crosslinking (Fig. 2, lane 4), higher molecular weight complexes were observed with ATP–BP, in addition to the expected 68 kDa crosslinked band (Fig. 3, lane 7). Prior work showed that genetically incorporated benzophenone was capable of producing multiply crosslinked protein complexes, suggesting that the higher molecular weight complexes are multimers.31–33 The experiments show that ATP–BP is capable of phosphorylation-dependent crosslinking, although with formation of higher molecular weight complexes, unlike the ATP–ArN3 analog.
Figure 3.
Kinase-catalyzed labeling and subsequent photocrosslinking with ATP–BP. CK2 kinase was incubated with α-casein in the presence of ATP or ATP–BP under UV for 2 h at 30 °C. The resulting mixtures were separated by SDS–PAGE and visualized by Coomassie blue staining (top) and Western blotting with anti-CK2 (bottom). The gels are representative of three independent trials.
3.4. Docking studies of photocrosslinking ATP analogs
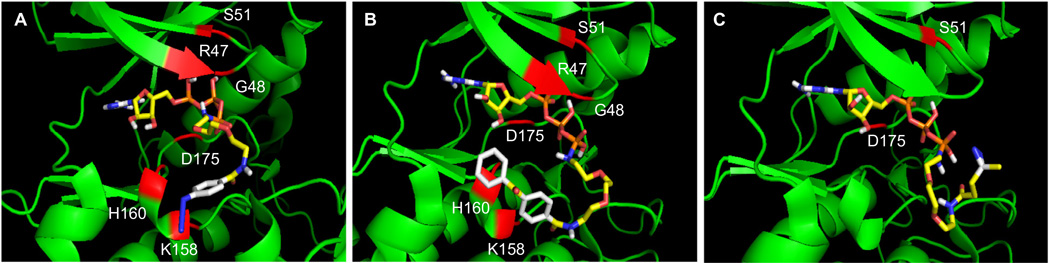
To understand the differences in kinase-catalyzed labeling and crosslinking efficiencies, we docked ATP–ArN3 and ATP–BP into the active site of CK2 kinase (GenBank ID: 7766821, pdb:1DAW) using the Autodock Vina program (http://vina.scripps.edu/).14 For comparison, we also docked an ATP analog displaying a diazirine photocrosslinking group (Fig. S13, ATP–DAz). ATP–ArN3 demonstrated the lowest binding energy amongst the three analogs (−8.1 kcal/mol, Table S1). The most favorable binding mode positioned the triphosphate within 2.4–2.9 Å of R47, G48, S51, and D175 of CK2, while the linker of ATP–ArN3 was located near K158 and H160 (Fig. 4A). The most favorable pose of ATP–BP maintained interactions similar to that of ATP–ArN3 (Fig. 4B), although with reduced binding energy of −7.3 kcal/mol (Table S2). In the case of ATP–DAz, the most favorable pose displayed an even higher binding energy of −6.5 kcal/mol (Table S3), perhaps due to the fewer interactions observed compared to the ATP–ArN3 and ATP–BP analogs (Fig. 4C). In the case of ATP–ArN3 and ATP–BP, the docking data poorly mimic the observed kinase conversion or crosslinking efficiencies (Figs. 2 and 3). One possible cause for the discrepancy is the lack of a bound substrate in the CK2 kinase structure, which could skew the analysis.
Figure 4.
Docking of ATP-analogs (ball and stick structures) into the active site of CK2 kinase (green, pdb:1DAW). The crosslinkers of ATP–ArN3 (A), ATP–BP (B), and ATP–DAz (C) protrude from the active site, but within close proximity to R47, G48, S51, K158, H160, and D175 (red). Images with the full kinase structure are shown in Figure S14. Video images are also available as Supplementary documents.
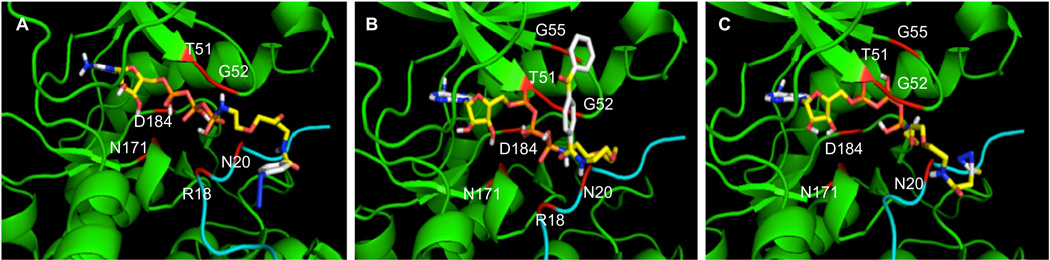
To study the influence of a bound substrate on ATP analog binding, we also performed docking studies with PKA kinase. The PKA structure contains a bound peptide inhibitor in the active site, in addition to an ATP mimic (Genbank ID 6755076, pdb: 1ATP). With PKA, ATP–BP demonstrated the lowest binding energy of the three analogs (−9.7 kcal/mol, Table S5). The most favorable binding mode positioned the triphosphate within 2.4–2.8 Å of T51, G52, R171, and N184 of PKA, and R18 and N20 of peptide inhibitor (Fig. 5B). Similar types of interactions were observed with ATP–ArN3 and ATP–DAz, but with higher energy binding (−7.2 kcal/mol for both analogs, Fig. 5A and C; Tables S4 and S6). In this case with PKA and its bound peptide inhibitor, the ATP analog binding energies show a similar trend to the labeling efficiency data observed with CK2, where ATP–BP demonstrates the highest kinase conversions (71%) and the lowest binding energies (−9.7 kcal/mol). The data strongly suggest that docking studies should utilize a kinase with a bound peptide to best reflect experimental results.
Figure 5.
Docking of ATP analogs (ball and stick structures) into the active site of PKA (green, pdb: 1ATP) in complex with peptide substrate inhibitor (cyan). The crosslinking groups of ATP–ArN3 (A), ATP–BP (B), and ATP–DAz (C) protrude from the active site, but within close proximity to T51, G52, N171 and D184 of PKA and R18 and N20 of the peptide (red). Images with the full kinase structure are shown in Figure S15. Video images are also available as Supplementary documents.
In addition to binding energies, another key observation from the docking analysis was the positioning of the photocrosslinkers relative to the kinase structure. In the case of ATP–ArN3, the aryl azide group was located within 3.2–4.4 Å of the protein complex in both the CK2 and PKA studies (Figs. 4A and 5A, S16 and S17). In contrast, the benzophenone group of ATP–BP was positioned within 4.0–4.4 Å of the CK2 kinase (Figs. 4B and S18), but 5.2–6.5 Å away from the PKA complex (Figs. 5B and S19). The distance analysis suggests that the large benzophenone group is positioned away from the kinase–substrate complex, which could result in reduced crosslinking efficiency. In contrast, the smaller aryl azide is located near the kinase active site for efficient crosslinking. Therefore, the docking studies point towards the size of the photocrosslinking group as a factor influencing the efficiency of kinase-mediated crosslinking.
4. Conclusion
We report a new photocrosslinking ATP analog, ATP–BP, for use in phosphorylation-dependent kinase–substrate crosslinking. Like ATP–ArN3, ATP–BP was compatible with kinase-catalyzed labeling and appropriate for crosslinking CK2 with casein substrates. Interestingly, while ATP–BP was the most efficient kinase cosubstrate (71%), ATP–ArN3 produced the most distinct crosslinked complexes (compare Figs. 2 and 3). Docking studies indicated that the size of the crosslinking group could influence its position in the active site, and consequently crosslinking efficiency. Combined, the study revealed that ATP–ArN3 is an excellent reagent for kinase-catalyzed photocrosslinking, possibly due to its small size and reasonable kinase compatibility. While ATP–BP was the best kinase cosubstrate, it produced higher order crosslinked complexes with CK2 and casein. Photocrosslinking ATP analogs will serve as useful tools in dissecting kinase substrate specificity due to their phosphorylation-dependent mechanism. Studies using crosslinking ATP analogs to identify substrate–kinase pairs are in progress and will be reported in due course.
Supplementary Material
Acknowledgments
We thank NIH (GM067657) and Wayne State University for funding, S. Suwal for technical support, and T. Anthony, T. Faner, and A. Fouda for comments on the manuscript.
Footnotes
Supplementary data (all compound characterization, QMS data, and docking parameters and figures) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2014.01.034.
References and notes
- 1.Suwal S, Pflum MH. Angew. Chem. Int., Ed. 2010;49:1627. doi: 10.1002/anie.200905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter T. Cell. 1995;80:225. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 3.Hunter T. Cell. 2000;100:113. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 4.Ubersax JA, Ferrell JE., Jr Nat. Rev. Mol. Cell Biol. 2007;8:530. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 5.Amanchy R, Periaswamy B, Mathivanan S, Reddy R, Tattikota SG, Pandey A. Nat. Biotechnol. 2007;25:285. doi: 10.1038/nbt0307-285. [DOI] [PubMed] [Google Scholar]
- 6.Pflum MKH, Tong JK, Lane WS, Schreiber SL. J. Biol. Chem. 2001;276:47733. doi: 10.1074/jbc.M105590200. [DOI] [PubMed] [Google Scholar]
- 7.Hennrich ML, Marino F, Groenewold V, Kops GJ, Mohammed S, Heck AJ. J. Proteome Res. 2013;12:2214. doi: 10.1021/pr400074f. [DOI] [PubMed] [Google Scholar]
- 8.Fields S, Song O. Nature. 1989;340:245. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook J, Russel D. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 10.Allen JJ, Lazerwith SE, Shokat KM. J. Am. Chem. Soc. 2005;127:5288. doi: 10.1021/ja050727t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maly DJ, Allen JA, Shokat KM. J. Am. Chem. Soc. 2004;126:9160. doi: 10.1021/ja048659i. [DOI] [PubMed] [Google Scholar]
- 12.Parang K, Kohn JA, Saldanha SA, Cole PA. FEBS Lett. 2002;520:156. doi: 10.1016/s0014-5793(02)02778-3. [DOI] [PubMed] [Google Scholar]
- 13.Senevirathne C, Green KD, Pflum MKH. Current Protocols in Chemical Biology. Vol. 4. John Wiley & Sons Inc; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trott O, Olson AJ. J. Comput. Chem. 2010;31:455. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Bond MR, Kohler JJ. Mol. Biosyst. 2008;4:473. doi: 10.1039/b803218a. [DOI] [PubMed] [Google Scholar]
- 16.Hall JH, Hill JW, Tsai HC. Tetrahedron Lett. 1965:2211. [Google Scholar]
- 17.Geiger MW, Elliot MM, Karacostas VD, Moricone TJ, Salmon JB, Sideli VL, Stonge MA. Photochem. Photobiol. 1984;40:545. [Google Scholar]
- 18.Reiser A, Willets FW, Terry GC, Williams V, Marley R. Trans. Faraday Soc. 1968;64:3265. [Google Scholar]
- 19.Blencowe A, Hayes W. Soft Matter. 2005;1:178. doi: 10.1039/b501989c. [DOI] [PubMed] [Google Scholar]
- 20.Knowles JR. Acc. Chem. Res. 1972;5:155. [Google Scholar]
- 21.Doering WVE, Buttery RG, Laughlin RG, Chaudhuri N. J. Am. Chem. Soc. 1956;78:3224. [Google Scholar]
- 22.Dorman G, Prestwich GD. Biochemistry. 1994;33:5661. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 23.Weber PJ, Beck-Sickinger AG. J. Pept. Res. 1997;49:375. doi: 10.1111/j.1399-3011.1997.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 24.Wittelsberger A, Thomas BE, Mierke DF, Rosenblatt M. FEBS Lett. 1872;2006:580. doi: 10.1016/j.febslet.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 25.Tate JJ, Persinger J, Bartholomew B. Nucleic Acids Res. 1998;26:1421. doi: 10.1093/nar/26.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegand M, Lindhorst TK. Eur. J. Org. Chem. 2006:4841. [Google Scholar]
- 27.Suwal S, Senevirathne C, Garre S, Pflum MK. Bioconjugate Chem. 2012;23:2386. doi: 10.1021/bc300404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackinlay AG, West DW, Manson W. Eur.. J. Biochem. 1977;76:233. doi: 10.1111/j.1432-1033.1977.tb11588.x. [DOI] [PubMed] [Google Scholar]
- 29.Litchfield DW. Biochem. J. 2003;369:1. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allende JE, Allende CC. FASEB J. 1995;9:313. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 31.Mahmood R, Yount RG. J. Biol. Chem. 1984;259:12956. [PubMed] [Google Scholar]
- 32.Farrell IS, Toroney R, Hazen JL, Mehl RA, Chin JW. Nat. Methods. 2005;2:377. doi: 10.1038/nmeth0505-377. [DOI] [PubMed] [Google Scholar]
- 33.Yan P, Wang T, Newton GJ, Knyushko TV, Xiong Y, Bigelow DJ, Squier TC, Mayer MU. Chem Bio Chem. 2009;10:1507. doi: 10.1002/cbic.200900029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.