Abstract
A major impediment to economical, worldwide vaccine distribution is the requirement for a “cold chain” to preserve antigenicity. We addressed this problem using a model human papillomavirus (HPV) vaccine stabilized by immobilizing HPV16 L1 capsomeres, i.e., pentameric subunits of the virus capsid, within organic glasses formed by lyophilization. Lyophilized glass and liquid vaccine formulations were incubated at 50°C for 12 weeks, and then analyzed for retention of capsomere conformational integrity and the ability to elicit neutralizing antibody responses after immunization of BALB/c mice. Capsomeres in glassy-state vaccines retained tertiary and quaternary structure, and critical conformational epitopes. Moreover, glassy formulations adjuvanted with aluminum hydroxide or aluminum hydroxide and glycopyranoside lipid A were not only as immunogenic as the commercially available HPV vaccine Cervarix®, but also retained complete neutralizing immunogenicity after high-temperature storage. The thermal stability of such adjuvanted vaccine powder preparations may thus eliminate the need for the cold chain.
Keywords: Human Papillomavirus, vaccine, lyophilization, stability, aluminum hydroxide, glycopyranoside lipid A
Graphical Abstract
1. Introduction
Cervical cancer is the third most common cancer in women worldwide [1]. The majority of cervical cancer occurs in women in less developed countries [1] where the availabilities of vaccines and preventative screenings such as Pap smears are limited [2]. Infection with high-risk types of human papillomavirus (HPV) is the primary etiologic event associated with cervical cancer [3], and therefore affordable, stable vaccines to prevent HPV infection could significantly reduce the disease prevalence in resource-poor regions of the world.
To prevent loss of efficacy, vaccines typically must be refrigerated during transport and storage. The logistical requirements associated with maintenance of carefully controlled cold chains make vaccine delivery in many regions challenging [4], and adds substantial costs. For the commercially available HPV vaccines Gardasil® and Cervarix®, cold chain requirements (storage at 2-8°C without freezing (http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093830.htm)) and cost (currently $360 for a three-dose series in the United States) are impediments for widespread use in many regions of the world [5].
The recommended temperature ranges for vaccine transport through the cold chain are narrow [6]. If liquid vaccine formulations freeze, or if they are exposed to elevated temperatures, loss of efficacy or undesirable side effects may result [7-11]. Liquid vaccines that contain microparticulate adjuvants such as aluminum hydroxide may be particularly prone to damage, in part because of the tendency of these adjuvants to agglomerate during freezing [8, 12-16].
Lyophilization may be used to immobilize vaccine antigens and adjuvants within glassy organic matrices [17], wherein the combination of low molecular mobility and low moisture content inhibits antigen degradation. Moreover, by utilizing high concentrations of glass-forming excipients and rapid freezing rates, agglomeration of microparticulate adjuvants can be avoided or minimized during the lyophilization process [17]. Reconstituted lyophilized vaccines adjuvanted with aluminum hydroxide have been reported to be equally immunogenic as their liquid counterparts [10, 18-20].
To generate adequate protective immune responses to vaccines that are based on purified protein antigens, microparticulate adjuvants typically must be added to formulations. Currently, aluminum salts such as aluminum hydroxide are present in the majority of commercially-available adjuvanted vaccines. Aluminum salt adjuvants primarily provoke a humoral response. To produce a more robust cellular immune response, other adjuvants typically also must be added [21]. One such adjuvant, monophosphoryl lipid A (MPL), is a non-toxic derivative of lipopolysaccharide that can stimulate a cellular immune response through its Toll-like receptor-4 agonist activity [21]. MPL adsorbed to aluminum hydroxide is used to adjuvant Cervarix® HPV vaccines. Glycopyranoside lipid A (GLA) is a synthetic variant of MPL that is more homogeneous in acyl chain properties and active than MPL [21]. At present there are no commercial vaccines that combine both an aluminum hydroxide adjuvant and cellular immunity stimulants such as MPL or GLA in a lyophilized formulation presumably due at least in part to the tendency of alum particles to aggregate during freezing.
Commercial HPV vaccines contain virus-like particles (VLPs) as the vaccine antigens [2], but subunit capsomere protein preparations also have shown promise as alternative vaccine antigens [22-26]. Capsomeres are comprised of L1 protein monomers assembled into pentamers to form the basic subunit of the HPV capsid. Although in some formulations capsomeres may be less immunogenic than their VLP counterparts, formulations with MPL appear equally immunogenic [27]. Capsomeres purified after expression in E. coli may offer reduced production costs with respect to currently marketed VLP-based vaccines, which are produced in S. cerevisiae (Gardasil®) or baculovirus-infected insect cells (Cervarix®).
We hypothesized that embedding HPV16 L1 capsomeres within glassy matrices formed during lyophilization would yield a dry powder vaccine formulation with enhanced thermal stability. To test this hypothesis, we prepared HPV16 L1 capsomeres in formulations that contained trehalose as a glass-forming excipient. Relatively high concentrations of trehalose (9.5% wt/vol) were used to promote rapid glass formation during the freezing step of the lyophilization process. Trehalose also served as a tonicity modifier, and hence we used only minimal additional buffer salts (54 mM histidine). In addition, we tested formulations that contained aluminum hydroxide or both aluminum hydroxide and GLA as adjuvants. The formulations were processed using controlled rapid freezing rates [10, 17] to avoid agglomeration of aluminum hydroxide microparticles, followed by drying under vacuum to form glassy matrices. The lyophilized formulations were reconstituted either immediately after lyophilization, or after 12 weeks of incubation at 50°C, and tested for retention of native capsomere structure using transmission electron microscopy, size exclusion chromatography, fluorescence spectroscopy, epitope binding assays, and immunoassays. The immunogenicities of the formulations were tested in BALB/c mice, and compared to the immunogenicities of commercially available Cervarix® HPV vaccines subjected to similar storage conditions.
2. Material and Methods
2.1 Materials
High purity α,α-trehalose dihydrate and H2SO4 were purchased from Mallinckrodt Baker (Phillipsburg, NJ). L-Histidine monohydrochloride monohydrate, triethanolamine, ethylene glycol tetraacetic acid (EDTA), Triton™ X-100, Benzonase® nuclease, Optiprep™ density gradient medium and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO). Two percent Alhydrogel® (aluminum hydroxide adjuvant) was obtained from Accurate Chemicals and Scientific Corp (Westbury, NY). Lyophilized synthetic monophosphoryl lipid A (glycopyranoside Lipid A (GLA) adjuvant) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Three mL 13 mm glass lyophilization vials, caps and seals were from West Pharmaceutical Services (Lititz, PA). Concentrated 10X phosphate buffered saline (PBS), Tween 20, ammonium sulfate, glycerol, acrylamide, tris(hydroxymethyl)aminomethane (Tris), and NaCl were from Fischer Scientific (Fair Lawn, NJ). Water for injection was purchased from Baxter Healthcare Corporation (Deerfield, IL). Dry powdered milk was purchased though Safeway Inc. (Pleasanton, CA). Peroxidase-conjugated affinipure donkey anti-mouse IgG (H+L) was from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). 3,3’,5,5’-tetramethylbenzidine (Ultra TMB and Turbo TMB) was from Thermo Scientific (Rockford, IL). Lipofectamine was from Invitrogen (Carlsbad, CA). Plasmid-safe DNase was from Epicentre (Madison, WI).
2.2 HPV16 L1 capsomere protein purification
In brief, HPV16 L1 protein was expressed in an untagged form in HMS174 E. coli using the vector HPV16-p3 which expresses a non-GST fusion HPV 16 L1 protein which has deletions at its amino and carboxy termini. Cells were resuspended in 200 mM NaCl Tris buffer pH 8.1 and lysed by two passages through a GEA Niro Soavi Panda homogenizer (Bedford, NH) at 800-1000 bar. The soluble fraction was collected after centrifugation of the cell lysate. This fraction was then chromatographed on a Q Fast Flow column (GE Healthcare, Piscataway NJ). The L1 protein eluted in the flow-through, and was then precipitated using ammonium sulfate at 30% saturation. The ammonium sulfate precipitate was solubilized in a 25 mM NaCl Tris, pH 8.5 buffer and chromatographed on a Q sepharose anion exchange column (GE Healthcare, Piscataway, NJ). L1 eluted as pentamers from the sepharose column using a sodium chloride gradient. A second purification was done of the Q sepharose fractions containing the L1 protein on a second Q sepharose column, again eluting with a sodium chloride gradient. L1 spontaneously pentamerizes post translation, and was not subjected to denaturing conditions during purification. A final purity of >95% was estimated by SDS-PAGE. Protein concentration was determined by the Bradford assay. HPV16 capsomere formulations were tested for endotoxin using QCL 1000TM Limulus Amebocyte Lysate test kit (LONZA, Basel, Switzerland), and found to contain <1EU/ml. More detailed information about HPV16 capsomeres can be found in previously published work [28-30]. Before formulation, fractions containing L1 were exchanged into a 100 mM histidine buffer pH 7.1 by size exclusion chromatography.
2.3 Vaccine formulation
Vaccines were formulated to contain 0.1 mg/mL HPV16 L1 capsomeres in 54 mM histidine HCl pH 7.1 with 9.5 w/v% trehalose for isotonicity. Additionally, some formulations contained 0.5 mg/mL aluminum from Alhydrogel®, or 0.5 mg/mL aluminum from Alhydrogel® and 0.05 mg/mL GLA. GLA was prepared at 1 mg/mL by suspending lyophilized GLA in a 0.5% triethanolamine pH 7 solution using probe sonication [31]. To create the vaccine formulations containing GLA, suspended GLA was added to Alhydrogel suspensions, vortexed for 5 seconds and then rotated end over end for 30 minutes at 4 °C. HPV L1 protein was then added and formulations were rotated end over end at 8 rpm in 2 mL polypropylene microcentrifuge tubes at 4°C for 1 hr to allow adsorption of capsomeres to adjuvant. HPV L1 protein has a pI of 6.2 and aluminum hydroxide has a PZC of approximately 11, making the antigen and adjuvant oppositely charged at the formulation pH, promoting essentially complete adsorption of protein to adjuvant.
2.4 Lyophilization
One mL aliquots of vaccine formulations at 4°C were filled into 3 mL lyophilization vials and placed on lyophilizer shelves that had been pre-cooled to −10°C (FTS Systems Lyophilizer, Warminster, PA). Vials containing vaccine formulation were surrounded by dummy vials filled with DI water to minimize radiative heat transfer effects for vials near the edge of the lyophilizer shelves. The shelf temperature was decreased at a rate of 0.5 °C/min to −40°C and then held at −40°C for 1 hr to allow the samples to freeze completely. Primary drying was initiated by decreasing the chamber pressure to 60 mTorr and then increasing the shelf temperature to −20°C at a rate of 2°C/min. Shelf temperatures were held at −20°C for 20 hr. Secondary drying was conducted at a pressure of 60 mTorr by increasing the shelf temperature to 0°C at a rate of 0.2°C/min, followed by increasing to 30°C at a rate of 0.5°C/min and holding the shelf temperature at 30°C for 5 hr. Finally, the shelf temperature was returned to 25°C and the chamber was back-filled with nitrogen until atmospheric pressure was reached. Chlorobutyl rubber stoppers were then inserted into vials under a nitrogen atmosphere, and the vials were sealed with aluminum caps and transferred to a freezer for storage at −80°C. The lyophilization cycle was optimized in previous studies [10, 17]. Vaccine formulations were reconstituted with 1 mL of water for injections prior to use.
2.5 Differential scanning calorimetry (DSC)
Onset glass transition temperatures of placebo lyophilized formulations were obtained using differential scanning calorimetry (Diamond DSC, Perkin Elmer, Waltham, MA). Triplicate samples were prepared inside an aluminum pan under dry nitrogen. Pans were cycled twice between 25°C and 150°C at a scan rate of 100°C/min. The second heating scan was used to determine the onset glass transition temperature.
2.6 Particle size analysis
The particle size distributions in liquid formulations prior to lyophilization or after lyophilization and reconstitution were determined by microflow analysis using a FlowCAM instrument (Fluid Imaging Technologies, Yarmouth, ME). The FlowCAM measures the size and concentration of particles of sizes greater than 2 μm. A 100 micron path-length flow cell was used at a flow rate of 0.08 mL/min with images taken at a rate of 10 frames per second. A 10X objective and collimator were used. Light and dark settings of 17 and 15, respectively, were used to capture particles. To avoid capturing overlapping particles, formulations were diluted ten times for placebo formulations, and 100 times for formulations containing capsomeres. A total sample volume of 0.35 mL of each diluted sample was analyzed.
2.7 Transmission electron microscopy (TEM)
Vaccine formulations were adsorbed to formvar/carbon-coated, glow-discharged 400 mesh copper TEM grids. After sample adsorption, grids were washed with 5 mM EDTA and stained with 1-2% uranyl acetate. Images were collected using a Philips CM10 transmission electron microscope operating at 80 kV equipped with a Gatan Bioscan2 digital camera.
2.8 Size exclusion high performance liquid chromatography (SE-HPLC) analysis of capsomere integrity
Reconstituted lyophilized HPV16 L1 capsomeres were analyzed using a Beckman Coulter Gold HPLC system (Fullerton, CA) with a TSK-gel G3000SWXL column (TOSOH Bioscience, Montgomeryville, PA) and a running buffer containing 50 mM Tris, 350 mM NaCl, 10% glycerol, pH 8.1 at a flow rate of 0.6 mL/min. UV absorbance at 280 nm was used for detection. Each sample was analyzed in duplicate.
2.9 SDS-PAGE
Capsomere proteins stored in the liquid or lyophilized states were analyzed on a 4-20% Mini-PROTEAN BioRad polyacrylamide gel at 0.5 μg/well under non-reducing and reducing (100 mM dithiothreitol) conditions in a Tris-glycine running buffer. The gels were stained with Coomassie Blue.
2.10 Fluorescence spectroscopic analysis of thermally-induced denaturation of HPV16 LI capsomeres
The intrinsic fluorescence of HPV16 L1 capsomeres was monitored as a function of temperature to determine the capsomere melting temperature. Fluorescence was excited at 295 nm, and spectra were collected from 305 to 400 nm on a fluorimeter (SLM Instruments Inc. Urbana, IL). Spectra were recorded every 5°C from 20 to 90°C, after an equilibration time of 10 min at each temperature. The wavelength center of spectral mass was calculated at each temperature, and the apparent melting temperature of the capsomeres was determined as the onset temperature of the thermal transition that was observed around 60°C.
2.11 Front-face mode fluorescence analysis of capsomere tertiary structure
For front-face mode spectroscopic measurements, 3 mL aliquots of vaccine formulations were pipetted into quartz cuvettes and placed in a fluorimeter with a holder that maintained the angle of incidence at 53°C. Samples were excited at 295 nm and emission spectra were collected from 310 nm to 400 nm. 10 μL aliquots of a 5 M acrylamide quencher solution was added to the sample and the peak intensity at 331 nm for capsomeres and 340 nm for 8 M urea unfolded capsomeres were monitored after each acrylamide addition. The ratios of fluorescence intensity without any quencher present to the fluorescence intensity with acrylamide present were plotted against the acrylamide concentration and linear regression was used to determine the Stern-Volmer quenching constant, Ksv as the slope of the resulting line. At the low acrylamide concentrations used, inner filter effects were negligible.
2.12 L1 and V5 epitope binding assay
An ELISA-based assay was used to monitor the presence of L1 and V5 capsomere epitopes. L1 is a polyclonal antibody that detects multiple epitopes on L1, whereas V5 binds a neutralizing epitope specific to conformationally intact capsomeres. Vaccine formulations with or without aluminum hydroxide adjuvant were diluted in PBS, and 0.125 μg/well of HPV16 L1 capsomere protein was coated on 96-well Nunc flat bottom PolySorp Immuno plates and incubated overnight at 4°C. Plates were washed three times with 0.05% Tween 20 in PBS at 300 μL/well. Plates were blocked with 100 μL/well of blocking buffer (5% dry milk, 0.05% Tween 20 in PBS) for 1 hr at 37°C. After blocking, blocking buffer was removed and primary antibodies, either L1 or V5 at a dilution of 1:1000 in blocking buffer, were added 50 μL/well and incubated at 37 °C for 1 hr. After washing three times, secondary antibody diluted 1:5,000 in wash buffer (0.05% Tween 20 in PBS) was added 50 μL/well and incubated at 37 °C for 1 hr. The secondary antibodies against L1 and V5 were a goat anti-rabbit and a goat anti-mouse HRP conjugated IgG antibody, respectively. After washing five times, 50 μL/well of Turbo TMB was added and plates were incubated at room temperature for 5 min. The reaction was quenched with 50 μL/well 1 M H2SO4 and plates were read for absorbance at 450 nm on a Molecular Devices Kinetic Microplate Reader (Sunnyvale, CA).
2.13 Vaccine immunogenicity
2.13.1 Dose-dependency of immune response to lyophilized and reconstituted vaccines
Murine studies were conducted under the University of Colorado at Boulder Institutional Animal Care and Use Committee (IACUC) protocol #1209.02. Female BALB/c mice from Taconic (Hudson, NY) were allowed to acclimate at least one week before use and were 10 to 11 weeks old at the start of the immunization study. Blood samples were collected through the retro orbital cavity under isofluorane anesthesia on days 0, 21, and 36, and mice were injected intramuscularly on days 0 and 21 with reconstituted lyophilized capsomeres, capsomeres with aluminum hydroxide, capsomeres with aluminum hydroxide and GLA or liquid Cervarix® vaccines. Reconstituted lyophilized vaccines were injected at doses of 1, 3, 5, or 8 μg of HPV16 L1 capsomeres and Cervarix® was injected in doses containing 1, 2, 3, or 4 μg of HPV16 VLP's. Serum was separated by centrifugation at 9,400×g for 14 min at 4°C and stored at −80°C until use.
2.13.1 Thermal stability of lyophilized HPV16 L1 vaccines
To test their ability to withstand high-temperature excursions from cold-chain conditions, liquid and lyophilized vaccine formulations were incubated at 50°C for 12 weeks. After incubation the lyophilized vaccines were reconstituted with water for injection, and these formulations as well as the liquid formulations were compared to formulations that had not been subjected to incubation at elevated temperatures. Lyophilized HPV16 L1 vaccines were reconstituted and injected into mice at either 1 or 5 μg/dose, and Cervarix® was injected at either 1 or 4 μg/dose. Two doses of the lyophilized HPV16 L1 and Cervarix® vaccines were administered. Serum samples were collected and processed as described above.
2.14 Total antibody enzyme linked immunosorbent assay (ELISA)
Nunc MaxiSorb 96 well plates (Thermo Fischer Scientific, Rochester, NY) were coated with 50 μL/well of 1 μg HPV16 L1 capsomere/mL diluted in PBS and incubated at 2-8°C overnight. Plates were washed 3 times with PBS containing 0.05% Tween 20. Plates were blocked with 300 μ L/well of PBS with 1% BSA, incubated at room temperature for 2 hr, and washed again. Serum was initially diluted in PBS with 1% BSA, 0.05% Tween 20, 100-fold for serum collected on days 0, 500-fold for serum collected on day 14, and 1,000 or 5,000-fold for serum collected on day 28 for mice injected without and with adjuvant respectively. A series of in-plate 2-fold dilutions were made for each sample. Plates were incubated for 1.5 hr at room temperature and washed. 40 μL of HRP-conjugated donkey anti-mouse antibody diluted 10,000 times was added to each well and incubated for 1.5 hr at room temperature with shaking, followed by washing. 40 μL Ultra TMB was added to each well and incubated for 15 min, followed by quenching with 40 μL of 1 M H2SO4. Absorbances of samples in the wells of the plates were measured at 450 nm on a Molecular Devices Kinetic Microplate Reader (Sunnyvale, CA).
To determine titers, average OD 450 values as a function of dilution were fit to a 4-parameter logistic equation using SigmaPlot 12 (Systat Software, San Jose, CA) software. The constraints 0 < min < 0.15 and max < 3.3 were used. A cutoff value of 0.5 was used. Groups with normally distributed antibody titers were compared with a t-test and groups without normal distributions were compared with the nonparametric Mann-Whitney Rank Sum Test.
2.15 Pseudovirus production
For a detailed protocol see (http://home.ccr.cancer.gov/lco/pseudovirusproduction.htm). In brief, 293TT cells were transfected using lipofectamine with DNA plasmids expressing secreted alkaline phosphatase (SEAP), HPV16 L1 and HPV16 L2 capsid proteins. Cells were lysed 2 to 3 days after transfection using Triton™ X-100, Benzonase®, Plasmid-safe™, and ammonium sulfate. The pseudovirions were salt extracted, and isolated from the clarified cell lysate by sedimentation in an Optiprep™ gradient. Fractions were collected from the bottom of the gradient tube and assayed for DNA and protein content by PicoGreen assays and BCA assay, respectively.
2.16 Neutralizing antibodies
One hundred μL/well of a suspension containing 3×105 293TT cells/mL were plated in 96 well tissue culture plates and incubated at 37°C for 2-5 hr. HPV16 pseudovirus was added to dilutions of mouse serum and incubated on ice for 1 hr. 100 μL of pseudovirus/mouse serum solution was added to plated cells and incubated at 37°C for 3 days. The negative control was an anti-bovine papillomavirus (BPV) antibody, and the positive control was heparin, which inhibits infection. After incubation, supernatant was collected from cells. The Great Escape SEAP Chemiluminescence test kit (Clontech, Mountainview, CA) was used for detection of SEAP. Plates were read on a multifunctional BioTek plate luminometer at a set glow-endpoint of 0.20 seconds/well. For a detailed protocol, see (http://home.ccr.cancer.gov/lco/neutralizationassay.htm).
The neutralization titer was defined as the dilution of mouse serum that neutralized 50% of the pseudovirus as determined by SEAP colorimetric measurement. The fractional neutralization was defined as the difference between the anti-BPV values and diluted mouse serum value, divided by the difference between the anti-BPV value and the heparin value. Percent neutralization was then determined as 100 × the fractional neutralization value. Neutralization values were fit to a 4-parameter logistic equation using SigmaPlot™ 12. Neutralization values could not be fit for serum samples that did not exhibit a sufficient decrease in neutralization under the dilution conditions tested; these samples were assigned a titer value of 300,000. Groups with normally distributed neutralization titers were compared with a t-test, and groups without normal distributions were compared with the Mann-Whitney Rank Sum Test.
3. RESULTS
3.1 Choice of an incubation temperature for accelerated stability studies
Ideally, accelerated degradation studies for protein antigens are carried out at sufficiently high temperatures so that measurable damage occurs over the course the accelerated stability study, but at temperatures low enough to avoid phase changes within the formulation or gross denaturation of the protein antigen. Thus, we first determined a temperature that was below both the glass transition temperature (Tg) of the lyophilized formulation and the thermal melting point of capsomeres. The onset glass transition temperatures for lyophilized placebo formulations were found to be 97.2 ± 3.4°C and 102.6 ± 5.2°C in the absence and presence of aluminum hydroxide microparticles, respectively. At the low capsomere and GLA concentrations used in our studies, addition of capsomeres and/or GLA to these formulations would not be expected to affect the glass transition temperature significantly. Since the glass transition temperature was high and similar lyophilization conditions were used as previous studies [10], we expect that the moisture content of these formulations is less than 1%. We determined the onset melting temperature of the HPV16 L1 capsomere at approximately 60°C (melting curve not shown). An incubation temperature of 50°C was therefore chosen to evaluate stability.
3.2 HPV16 L1 capsomere vaccine characterization
HPV16 LI vaccine formulations that had been lyophilized and immediately reconstituted or lyophilized and stored for 12 weeks at 50°C prior to reconstitution were analyzed by transmission electron microscopy (TEM) for capsomere structural appearance, size exclusion-high performance liquid chromatography (SE-HPLC) for capsomere size, front-face fluorescence for tertiary structure, V5 and L1 antibody immunoassays for conformational epitope reactivity, and FlowCAM® flow microscopy for particle size and concentration. After 12 weeks of incubation within the liquid formulations at 50°C, capsomeres were degraded sufficiently such that they could not be reliably detected by SDS-PAGE (Figure 1e), and thus further characterization was not conducted.
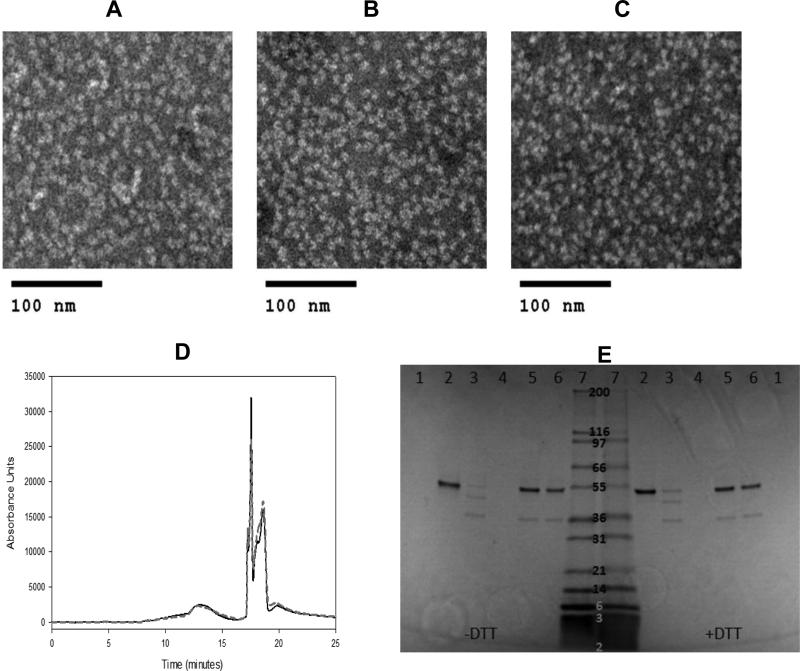
Figure 1. Preservation of HPV16 capsomere structure after lyophilization and incubation in the glassy state.
TEM images of HPV16 L1 capsomere formulations without adjuvant, before lyophilization (a), immediately after lyophilization and reconstitution (b), and after incubation at 50°C for 12 weeks in the lyophilized state and reconstitution (c) showed no change in capsomere appearance with lyophilization or incubation in the lyophilized state. SE-HPLC retention times (d) for HPV16 L1 capsomeres measured after lyophilization and immediate reconstitution (solid black line) were unchanged following lyophilization and incubation at 50°C for 12 weeks prior to reconstitution (gray dashed line). The capsomere peak appeared at approximately 13 mins; peaks eluting after 17 mins were due to buffer components. Analysis of capsomeres by SDS-PAGE (e) is shown under non-reducing (−dithiothreitol, DTT) and reducing conditions (+DTT). Sample lanes contained buffer (1), liquid HPV16 L1 capsomere vaccines prior to incubation (2), liquid HPV16 L1 capsomere vaccines after 20 weeks incubation at 4°C (3), liquid HPV16 L1 capsomere vaccines after 12 weeks at 50°C (4), reconstituted lyophilized HPV16 L1 capsomeres prior to incubation (5), reconstituted lyophilized HPV16 L1 capsomeres after 12 weeks of incubation at 50°C (6) or molecular weight markers (7). L1 subunits were intact after lyophilization and incubation in the glassy state at 50°C for 12 weeks, but some loss of L1 subunits was observed when liquid formulations were stored at 4°C. Incubation of liquid vaccine formulations at 50°C resulted in complete loss of intact L1 protein.
TEM was used to visualize HPV16 L1 capsomeres. Before lyophilization, HPV16 capsomeres were uniform in appearance with a diameter of approximately 9 to 10 nm (Figure 1a). After lyophilization and reconstitution, they appeared similar to capsomeres in the initial preparation (Figure 1b). Storing the lyophilized vaccine for up to 12 weeks at 50°C did not affect capsomere appearance (Figure 1c). Capsomere structure could not be analyzed by TEM in vaccine formulations that contained adjuvants.
The chromatographic retention time of the HPV16 L1 capsomeres was monitored by SE-HPLC, and did not to change after incubation for 12 weeks at 50°C in the lyophilized state (Figure 1d). Additionally, the subunit L1 protein remained intact after lyophilization and high temperature incubation, as shown by SDS-PAGE (Figure 1e).
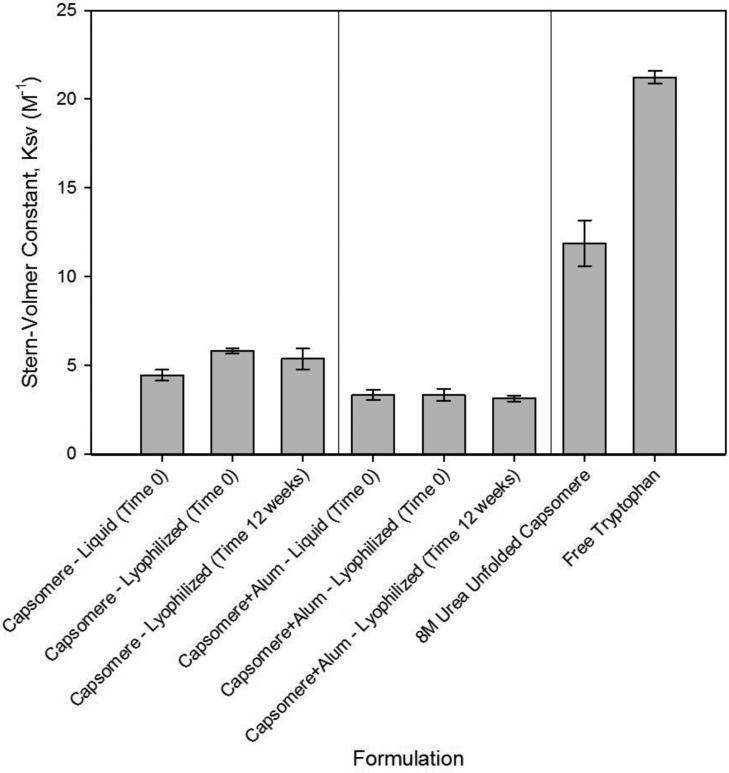
Stern-Volmer constants (KSV) determined from front-face mode fluorescence acrylamide quenching studies were used to gain insight on the effect of formulation and processing on the tertiary structure of capsomeres. High KSV values reflect facile solvent access to (normally buried) tryptophan residues within the capsomeres, whereas lower Stern-Volmer constants are typically associated with folded proteins wherein quenchers such as acrylamide have limited access to buried tryptophan residues [32]. Changes in KSV thus may reflect an overall alteration in the accessibility of tryptophan residues and folding of the protein [32]. KSV values for formulations that had been lyophilized and reconstituted immediately were unchanged from those of capsomeres in the initial liquid formulation. Likewise, KSV values did not change when the lyophilized capsomere or capsomere and aluminum hydroxide formulations were incubated for 12 weeks at 50°C prior to reconstitution, as shown in Figure 2. KSV values were slightly lower in the formulations containing aluminum hydroxide microparticles, which may be due to increased steric hindrance as capsomeres adsorb to aluminum hydroxide.
Figure 2. Capsomere tertiary structure is maintained after incubation for 12 weeks at 50°C as measured by intrinsic fluorescence.
Stern-Volmer fluorescence quenching constants (KSV) measured after reconstitution of lyophilized capsomere formulations (prepared either with or without aluminum hydroxide adjuvant) were the same as those observed in liquid formulations prior to lyophilization, and did not change after 12 weeks incubation at 50°C in the glassy state. KSV values for capsomeres unfolded in 8 M urea or for free tryptophan were much larger, indicative of greater solvent accessibility to normally buried tryptophan residues. KSV values and associated error bars represent mean ± standard deviation, n=3.
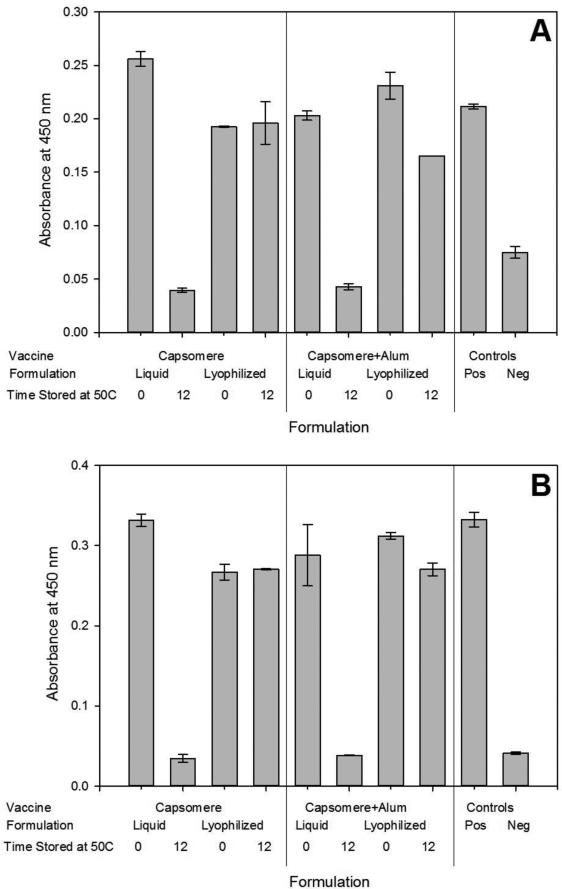
Binding of the antibodies L1 and V5 to HPV16 L1 capsomeres was measured to determine the effect of processing and storage on retention of HPV16 L1 capsomere structure. L1 is a polyclonal antibody that detects multiple epitopes on L1, whereas V5 binds a neutralizing epitope specific to conformationally intact capsomeres or VLPs [33-35]. Antibody binding to capsomeres was retained in each formulation following lyophilization and reconstitution, as well as after 12 weeks of incubation at 50°C in the lyophilized, glassy state (Figure 3). The positive control was a fresh sample of the HPV16 L1 capsomeres and the negative control was a polyomavirus capsid protein, VP1, a structural equivalent to L1[37-38].
Figure 3. Retention of critical epitopes in capsomeres after lyophilization and incubation inthe glassy state.
Lyophilized capsomere formulations with or without aluminum hydroxide adjuvant retained antibody reactivity with L1 (a) and V5 (b) measured by ELISA-based assays, even when incubated at 50°C for 12 weeks. In contrast, liquid formulations lost reactivity after incubation. Positive controls were freshly prepared HPV16 L1 samples and negative controls were samples containing the polyomavirus structural protein VP1. Results are shown as the mean ± standard deviation, n=2.
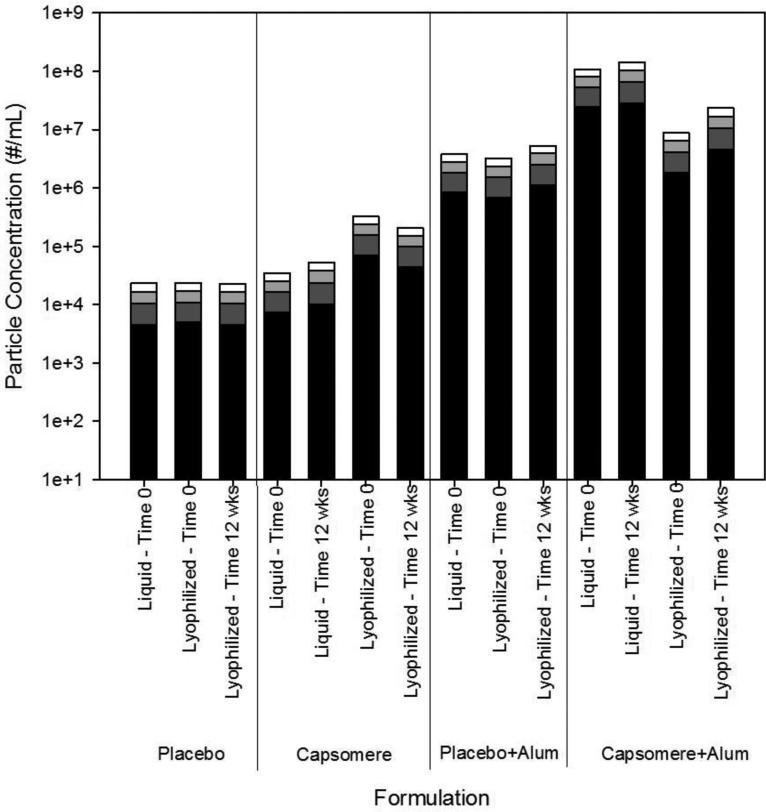
Microflow imaging analysis showed that placebo formulations containing only trehalose and buffer salts contained low (ca. 104/ml) levels of particles of size greater than 2 μm, and no changes in this background level were detected after lyophilization and reconstitution (Figure 4, panel 1). The concentration of particles >2μm was slightly larger following lyophilization and reconstitution of adjuvant-free formulations of capsomeres, presumably due to a small degree of capsomere aggregation (Figure 4, panel 2). Addition of suspensions of aluminum hydroxide adjuvant particles to placebo formulations increased the background concentration of particles, but lyophilization had no effect on the size distribution of these particles (Figure 4, panel 3). In comparison, when suspensions of aluminum hydroxide adjuvant particles were added to formulations containing capsomeres (Figure 4, panel 4), the concentration of particles >2μm increased by approximately an order of magnitude, likely because capsomeres induced agglomeration of smaller aluminum hydroxide particles via “bridging” interactions. Lyophilization of samples containing both capsomeres and aluminum hydroxide slightly decreased the concentration of particles >2μm. For all lyophilized samples, particle size distributions were essentially unaffected by 12 weeks of incubation at 50°C (Figure 4).
Figure 4. Particle size distributions in heat-treated vaccine formulations.
The particle size and concentration of particles of size greater than 2 μm in placebo, capsomere, placebo and aluminum hydroxide, and capsomere and aluminum hydroxide formulations did not change after lyophilization or after incubation at 50°C for 12 weeks in the glassy state. Particle concentrations are shown for particles 2 to 5 μm (black), 5 to 10 μm (dark gray), 10 to 20 μm (light gray), and >20 μm (white). Particles size distributions are reported as the mean of three measurements for each sample. Compared with particle size distributions observed in initial liquid samples, lyophilization and reconstitution induced only minor changes in particle size distributions. In addition, particle size distributions in lyophilized samples were largely unaffected by 12 weeks of incubation at 50°C.
3.3 Immunogenicity of HPV16 L1 vaccines
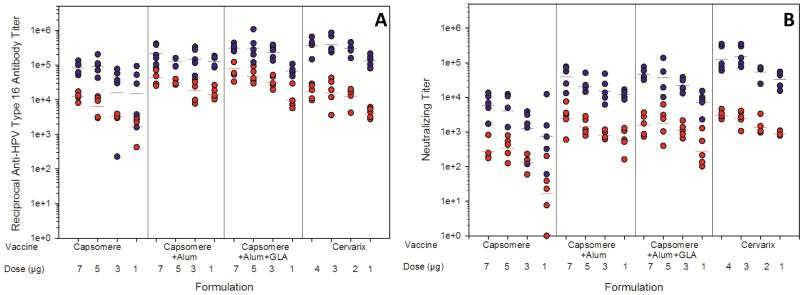
Immunogenicities of HPV16 L1 vaccine formulations were quantified and compared against immunogenicities of commercial Cervarix® HPV VLP-based vaccines by measuring total anti-HPV16 L1 capsomere antibody titers by ELISA, and determining neutralizing antibody titers with a pseudovirus neutralization assay. A dose-dependent response was seen for reconstituted, lyophilized HPV16 L1 vaccines following administration of 7, 5, 3, or 1 μg doses of capsomeres, and for Cervarix® following administration of 4, 3, 2, or 1 μg doses of HPV16 VLP's, as shown in Figure 5. All of the doses administered were in the linear range for the murine model.
Figure 5. Antibody responses to vaccine formulations after immunization of BALB/c mice.
Total anti-HPV16 antibody titers (a) were measured by ELISA; neutralizing antibody titers (b) were measured by the pseudovirus neutralization assay. Responses were measured following administration of one (red circles) or two (blue circles) injections. Horizontal bars represent the geometric mean for each group (n=5). From left to right, vaccine formulations contained capsomeres, capsomeres with aluminum hydroxide, capsomeres with aluminum hydroxide and GLA, or Cervarix®. After each dose, responses to both of the capsomere formulations that contained adjuvants (aluminum hydroxide or aluminum hydroxide and GLA) were similar those resulting from administration of Cervarix®. Un-adjuvanted capsomere vaccines yielded lower antibody responses (and, in particular, neutralizing antibody responses) than did both of the adjuvanted capsomere vaccines and Cervarix®.
Lyophilized vaccine formulations containing HPV16 L1 capsomeres without adjuvants elicited anti-HPV16 L1 antibody titers of 103-104, and 105 after one and two doses, respectively (Figure 5A). Addition of aluminum hydroxide increased immune responses after both one and two injections (p≤0.05), except for the 5 μg dose after two injections (p=0.46). The addition of GLA to formulations already containing aluminum hydroxide did not significantly increase the antibody titers (p>0.05) observed after either one or two injections. Neutralizing titers (Figure 5B) were approximately one order of magnitude lower than the anti-HPV16 L1 titers, but followed a similar pattern. At the highest doses tested, the lyophilized vaccines containing adjuvants generated immune responses equivalent to the commercially available vaccine, based on levels of total anti-HPV16 L1 IgG and neutralizing antibody titers produced in immunized animals. The neutralization titers give an indication of how the antibodies produced by immunization with the vaccine formulations immunization would neutralize the virus in vivo. Recent human clinical studies using the pseudovirus neutralizing assay to evaluate immune responses to commercial HPV vaccines suggest that the assay is more sensitive than competitive Luminex immunoassays, and likely more specific than total IgG Luminex (TIgG) immunoassays [38]. Since the lyophilized vaccines preformed equally as well as the commercially available vaccine, Cervarix, the lyophilized vaccines show promise for potential neutralization of HPV16 in humans.
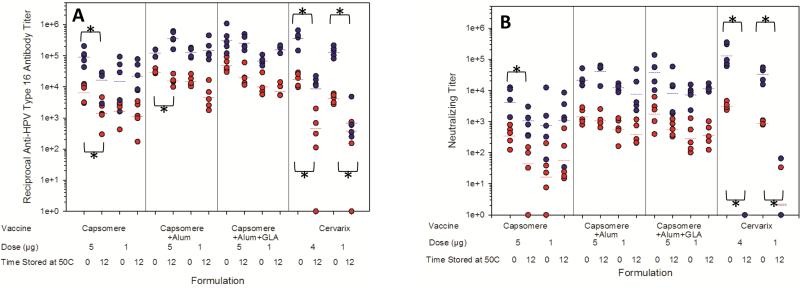
After incubation for 12 weeks at 50°C, lyophilized HPV16 L1 vaccines adjuvanted with aluminum hydroxide or with aluminum hydroxide and GLA produced anti-HPV16 L1 capsomere antibody titers in BALB/c mice similar to those elicited by their non-incubated counterparts (Figure 6). The 5 μg dose of vaccines containing capsomeres and aluminum hydroxide produced lower titers than non-incubated controls (p=0.008), but responses following the second dose were equivalent to those produced by non-incubated controls. Compared to non-incubated controls, lyophilized, adjuvant-free capsomere vaccines subjected to high-temperature incubation produced decreased anti-HPV16 L1 capsomere antibody titers when administered in 5 μg doses, but equivalent responses at all other doses. Neutralizing antibody titers elicited in response to lyophilized HPV16-L1 capsomere vaccines were unaffected by incubation at 50°C for 12 weeks, except for those generated in response to the second 5 μg dose of adjuvant-free capsomere vaccines (p=0.032). In contrast, incubation of Cervarix® at 50°C for 12 weeks resulted in dramatic reductions in anti-HPV16 LI antibody titers (p<0.02) and nearly complete loss of neutralizing titers (p<0.033).
Figure 6. Immunogenicity of HPV16 vaccine formulations after incubation for 12 weeks at 50°C.
Total anti-HPV16 antibody titers measured by ELISA (a) and neutralizing antibody titers measured by the pseudovirus neutralization assay (b) following one injection (red circles) or two injections (blue circles) of various HPV16 vaccines. Horizontal bars represent geometric mean titer values (n=5). Vaccines with significantly (p<0.05) reduced antibody titers after high-temperature incubation (based on t-test for normally distributed groups or Mann-Whitney Rank Sums test for non-normal groups) are noted with an *. From left to right, vaccine formulations contained capsomeres, capsomeres with aluminum hydroxide, capsomeres with aluminum hydroxide and GLA, or Cervarix®. Anti-HPV16 antibody titers and neutralizing antibody titers produced in response to Cervarix® were decreased when the vaccines were incubated for 12 weeks at 50°C. In contrast, anti-HPV16 antibody titers and neutralizing antibody titers produced in response to lyophilized, adjuvanted capsomere vaccines were unchanged after high-temperature incubation.
4. DISCUSSION
Highly effective HPV vaccines are commercially available, but their high cost and cold-chain requirements are barriers for their use in low resource regions of the world where the need is great [5]. HPV L1 capsomere-based vaccines may provide a lower cost alternative. The HPV VLP antigens in Cervarix® are produced in baculovirus-infected insect cells. In contrast, the ability to purify capsomeres after expression of L1 in E. coli may confer cost advantages in the manufacturing process.
HPV vaccines envisioned for use in low resource settings also must be highly efficacious. HPV16 L1 capsomere vaccines have been shown to protect mice from vaginal challenge with HPV16 [26] and, in the current study, HPV16 L1 capsomere vaccines adjuvanted with either aluminum hydroxide or both aluminum hydroxide and GLA elicited both anti-HPV16 and neutralizing responses that were similar to those resulting from administration of the commercially-available HPV vaccine Cervarix® (see Figure 5).
Development of stabilizing formulations for the storage and delivery of therapeutic proteins [39] and protein-based vaccines [40] remains a challenging endeavor. Lyophilization is widely used to stabilize therapeutic proteins [41], but lyophilization is not used for any currently marketed vaccine that contains adjuvants, likely because vaccines containing aluminum salt adjuvants are typically thought to be susceptible to loss of immunogenicity caused by freeze-thawing. However, using the combination of controlled, rapid freezing rates and high concentrations of the glass-forming excipient trehalose, we produced lyophilized glassy vaccine formulations of HPV16 L1 capsomeres without any detectable degradation of capsomere protein. Once dried, the formulations exhibited glass transition temperatures near 100°C. Below this temperature, viscosities in the glassy state are more than 1015 times the viscosity of water [42], preventing reactions that might otherwise cause protein degradation and loss of vaccine efficacy. Lyophilized vaccine formulations appeared identical to initial liquid formulations based on TEM, front-face mode fluorescence quenching, and antibody epitope reactivity, and yielded anti-HPV16 and neutralizing antibody responses equivalent to those generated by Cervarix® when tested in BALB/c mice.
The immunogenicity of Cervarix® was drastically reduced after 12 weeks of incubation at 50°C, even though previous work showed that minor interruptions in the cold chain do not affect its immunogenicity [43]. The mechanism(s) underlying the loss of activity of Cervarix® during high temperature storage are beyond the scope of the current manuscript, but they could potentially be related to chemical or conformational changes in the antigen, as suggested by an earlier study showing substantial reductions in deamidation of a protein antigen in a candidate anthrax vaccine that was lyophilized in a formulation similar to those in the current manuscript [20]. In contrast to the Cervarix® formulations, glassy-state lyophilized adjuvanted HPV16 L1 capsomere vaccines demonstrated remarkable thermal stability. Even after incubation for 12 weeks at 50°C, the immunogenicities of the HPV16 L1 capsomere vaccines were unchanged, lyophilized HPV16 L1 capsomere vaccines showed no alterations in capsomere structure and the multimeric protein complex of L1 subunits remained intact. The exceptional thermal stability of these vaccines might entirely obviate the need for refrigerated transport and storage conditions, in turn greatly reducing costs for future HPV vaccine programs.
ACKNOWLEDGEMENTS
Funding for this project was provided by a University of Colorado Seed Grant, and the SPORE in cervical cancer P50 CA098252. We thank Kim Erickson for help with TEM images of the HPV16 L1 capsomeres and Dennis Macejak for assistance with capsomere purification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sanjose S, Serrano B, Castellsagué X, Brotons M, Muñoz J, Bruni L, Bosch FX. Human papillomavirus (HPV) and related cancers in the global alliance for vaccines and immunization (GAVI) countries: A WHO/ICO HPV information centre report. Vaccine. 2012;30(Suppl 4) doi: 10.1016/S0264-410X(12)01435-1. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012;10:681–692. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Kane MA, Sherris J, Coursaget P, Aguado T, Cutts F. Chapter 15: HPV vaccine use in the developing world. Vaccine. 2006;24:S3/132–S3/139. doi: 10.1016/j.vaccine.2006.05.128. [DOI] [PubMed] [Google Scholar]
- 5.Wang JW, Roden RBS. Virus-like particles for the prevention of human Papillomavirus-associated malignancies. Expert Rev. Vaccines. 2013;12:129–141. doi: 10.1586/erv.12.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milstien J, Kartoglu U, Zaffran M, Galazka A. Temperature sensitivity of vaccine. World Health Organization. 2006 [Google Scholar]
- 7.Sokhey J, Gupta CK, Sharma B, Singh H. Stability of oral polio vaccine at different temperatures. Vaccine. 1988;6:12–13. doi: 10.1016/0264-410x(88)90006-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Tyagi A, Carpenter J, Perkins S, Sylvester D, Guy M, Kristensen DD, Braun LJ. Characterization of the freeze sensitivity of a hepatitis B vaccine. Hum. Vaccines. 2009;5:26–32. doi: 10.4161/hv.5.1.6494. [DOI] [PubMed] [Google Scholar]
- 9.Braun LJ, Tyagi A, Perkins S, Carpenter J, Sylvester D, Guy M, Kristensen D, Chen D. Development of a freeze-stable formulation for vaccines containing adjuvants. Vaccine. 2009;27:72–79. doi: 10.1016/j.vaccine.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Hassett KJ, Cousins MC, Rabia LA, Chadwick CM, O'Hara JM, Nandi P, Brey RN, Mantis NJ, Carpenter JF, Randolph TW. Stabilization of a recombinant ricin toxin A subunit vaccine through lyophilization. Eur. J. Pharm. Biopharm. 2013;85:279–286. doi: 10.1016/j.ejpb.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner L, Verma A, Meade BD, Reiter K, Narum DL, Brady RA, Little SF, Burns DL. Structural and immunological analysis of anthrax recombinant protective antigen adsorbed to aluminum hydroxide adjuvant. Clin. Vaccine Immunol. 2012;19:1465–1473. doi: 10.1128/CVI.00174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zapata MI, Feldkamp JR, Peck GE, White JL, Hem SL. Mechanism of freeze-thaw instability of aluminum hydroxycarbonate and magnesium hydroxide gels. J. Pharm. Sci. 1984;73:3–8. doi: 10.1002/jps.2600730103. [DOI] [PubMed] [Google Scholar]
- 13.Solanki VA, Jain NK, Roy I. Stabilization of tetanus toxoid formulation containing aluminum hydroxide adjuvant against freeze-thawing. Int. J. Pharm. 2011;414:140–147. doi: 10.1016/j.ijpharm.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Salnikova MS, Davis H, Mensch C, Celano L, Thiriot DS. Influence of formulation pH and suspension state on freezing-induced agglomeration of aluminum adjuvants. J. Pharm. Sci. 2012;101:1050–1062. doi: 10.1002/jps.22815. [DOI] [PubMed] [Google Scholar]
- 15.Kurzątkowski W, Kartoğlu Ü , Staniszewska M, Górska P, Krause A, Wysocki MJ. Structural damages in adsorbed vaccines affected by freezing. Biologicals. 2013;41:71–76. doi: 10.1016/j.biologicals.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Tonnis WF, Amorij JP, Vreeman MA, Frijlink HW, Kersten GF, Hinrichs WLJ. Improved storage stability and immunogenicity of hepatitis B vaccine after spray-freeze drying in presence of sugars. Eur. J. Pharm. Sci. 2014;55:36–45. doi: 10.1016/j.ejps.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Clausi AL, Merkley SA, Carpenter JF, Randolph TW. Inhibition of aggregation of aluminum hydroxide adjuvant during freezing and drying. J. Pharm. Sci. 2008;97:2049–2061. doi: 10.1002/jps.21143. [DOI] [PubMed] [Google Scholar]
- 18.Clausi A, Cummiskey J, Merkley S, Carpenter JF, Braun LJ, Randolph TW. Influence of particle size and antigen binding on effectiveness of aluminum salt adjuvants in a model lysozyme vaccine. J. Pharm. Sci. 2008;97:5252–5262. doi: 10.1002/jps.21390. [DOI] [PubMed] [Google Scholar]
- 19.Clausi AL, Morin A, Carpenter JF, Randolph TW. Influence of protein conformation and adjuvant aggregation on the effectiveness of aluminum hydroxide adjuvant in a model alkaline phosphatase vaccine. J. Pharm. Sci. 2009;98:114–121. doi: 10.1002/jps.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassett KJ, Vance DJ, Jain NK, Sahni N, Rabia LA, Cousins MC, Joshi S, Volkin DB, Middaugh CR, Mantis NJ, Carpenter JF, Randolph TW. Glassy-state stabilization of a dominant negative inhibitor anthrax vaccine containing aluminum hydroxide and glycopyranoside lipid A adjuvants. J Pharm Sci. 2015;104(2):627–639. doi: 10.1002/jps.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PloS One. 2011;6:1–12. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose RC, White WI, Li M, Suzich JA, Lane C, Garcea RL. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J. Virol. 1998;72:6151–6154. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan H, Estes PA, Chen Y, Newsome J, Olcese VA, Garcea RL, Schlegel R. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J. Virol. 2011;75:7848–7853. doi: 10.1128/JVI.75.17.7848-7853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fligge C, Giroglou T, Streeck RE, Sapp M. Induction of type-specific neutralizing antibodies by capsomeres of human papillomavirus type 33. Virology. 283:353–357. doi: 10.1006/viro.2000.0875. [DOI] [PubMed] [Google Scholar]
- 25.Jagu S, Kwak K, Garcea R, Roden RBS. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine. 2010;28:4478–4486. doi: 10.1016/j.vaccine.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Gersch E, Kwak K, Jagu S, Karanam B, Huh WK, Garcea RL, Roden RBS. Capsomer vaccines protect mice from vaginal challenge with human papillomavirus. Plos One. 2011;6:1–8. doi: 10.1371/journal.pone.0027141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thönes N, Herreiner A, Schädlich L, Piuko K, Müller M. A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J. Virol. 2008;82:5472–5485. doi: 10.1128/JVI.02482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Casini G, Harrison SC, Garcea RL. Papillomavirus capsid protein expression in Escherichia coli: Purification and assembly of HPV11 and HPV16 L1. J. Mol. Bio. 2001;307:173–182. doi: 10.1006/jmbi.2000.4464. [DOI] [PubMed] [Google Scholar]
- 29.Bishop B, Dasgupta J, Klein M, Garcea RL, Christensen ND, Zhao R, Chen XS. Atomic structures of four HPV L1 capsid proteins: Understanding the specificity of neutralizing monoclonal antibodies. J. Biol. Chem. 2007;282:31803–31811. doi: 10.1074/jbc.M706380200. [DOI] [PubMed] [Google Scholar]
- 30.Wu W-H, Gersch E, Kwak K, Jagu S, Karanam B, Huh WK, Garcea RL, Roden RBS. Capsomer vaccines protect mice from vaginal challenge with human papillomavirus. PLoS One. 2011;6(11):e27141. doi: 10.1371/journal.pone.0027141. PMID:22069498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 32.Eftink MR, Ghiron CA. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976;15:672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- 33.White WI, Wilson SD, Palmer-Hill FJ, Woods RM, Ghim S, Hewitt LA, Goldman DM, Burke SJ, Jenson AB, Koenig S, Suzich JA. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 1999;73:4882–4889. doi: 10.1128/jvi.73.6.4882-4889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryding J, Dahlberg L, Wallen-Öhman M, Dilner J. Deletion of a major neutralizing epitope of human Papillomavirus type 16 virus-like particles. J. Gen. Virol. 2007;88:792–802. doi: 10.1099/vir.0.82449-0. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Christensen N, Schiller JT, Dillner J. A monoclonal antibody against intact human papillomavirus type 16 capsids blocks the serological reactivity of most human sera. J. Gen. Virol. 1997;78:2209–2215. doi: 10.1099/0022-1317-78-9-2209. [DOI] [PubMed] [Google Scholar]
- 36.Rayment I, Baker TS, Caspar DLD, Murakami WT. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982;295:110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salunke DM, Caspar DL, Garcea RL. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986;46:895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- 38.Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, McNeil S, Money D, Dionne M, Palefsky J, Karunakaran K, Kollmann T, Ogilvie G, Petric M, Dobson S. Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine. 2014;32(5):624–630. doi: 10.1016/j.vaccine.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Frokjaer S, Otzen DE. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discovery. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen D, Chen D, Cummings R. Vaccine stabilization: Research, commercialization, and potential impact. Vaccine. 2011;29:7122–7124. doi: 10.1016/j.vaccine.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: Some practical advice. Pharm. Res. 1997;14:969–975. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 42.Angell CA. Formation of glasses from liquids and biopolymers. Science. 1995;267:1924–1935. doi: 10.1126/science.267.5206.1924. [DOI] [PubMed] [Google Scholar]
- 43.Le Tallec D, Doucet D, Elouahabi A, Harvengt P, Deschuyteneer M, Deschamps M. Cervarix, the GSK HPV-16/HPV-18 AS04-adjuvanted cervical cancer vaccine, demonstrates stability upon long-term storage and under simulated cold chain break conditions. Hum. Vaccines. 2009;5:467–474. doi: 10.4161/hv.8485. [DOI] [PubMed] [Google Scholar]