Abstract
It is known that ischemia/reperfusion induces neurodegeneration in the hippocampus in a sub-region dependent manner. The present study investigated the mechanism of selective resistance/vulnerability to oxygen glucose deprivation (OGD) using mouse organotypic hippocampal cultures. Analysis of propidium iodide uptake showed that OGD induced duration- and sub-region-dependent neuronal injury. As compared to the CA1-3 sub-regions, dentate neuronal survival was more sensitive to inhibition of PI3K/Akt signaling under basal conditions. Dentate neuronal sensitivity to PI3K/Akt signaling activation was inversely related to its vulnerability to OGD-induced injury; Insulin/IGF pretreatment conferred neuroprotection to dentate neurons via activation of PI3K/Akt signaling. In contrast, CA1 and CA3 neurons were less sensitive to disruptions of endogenous PI3K/Akt signaling and protective effects of insulin/IGF-1, but more vulnerable to OGD. OGD-induced injury in CA1 was reduced by inhibition of NMDA receptor or MAPK signaling, and was prevented by blocking NMDA receptor in the presence of insulin. The CA2 sub-region was distinctive in its response to glutamate, OGD, and insulin, compared to other CA sub-regions. CA2 neurons were sensitive to the protective effects of insulin against OGD-induced injury, but more resistant to glutamate. Distinctive distribution of insulin receptor β and basal phospho-Akt was detected in our slice cultures. Our results suggest a role for insulin signaling in sub-regional resistance/vulnerability to cerebral ischemia.
Keywords: Akt, dentate gyrus, insulin, insulin-like growth factor-1, oxygen-glucose deprivation, PI3K
Ischemic stroke leads to a complex cascade of cellular events resulting in neuronal death. Studies using in vivo ischemic animal models showed that there is differential neuronal vulnerability in the brain (Lipton, 1999). In hippocampus, short periods of anoxia/ischemia resulted in loss of CA1 pyramidal cells while dentate neurons were spared (Pulsinelli, 1988). Glutamate NMDA receptor mediated-excitotoxicity has been well accepted as a mechanism of neuronal death. However, the role of NMDA in the selective vulnerability pattern was questioned due to the fact that the resistant dentate neurons are also rich in NMDA receptors. Furthermore, treatment with NMDA antagonists has not been clearly shown to be of therapeutic benefit. On the other hand, some studies have indicated a significant amount of neuronal death in dentate granule cells via an apoptotic mechanism after ischemia (Wang et al. 1999; Sirén et al. 2002; Kirino and Sano 1980). Thus studying the differential responses between these distinct cell populations offers the potential for understanding factors that are critical in neuronal death and survival.
It is known that diabetes increases the frequency of ischemic stroke incidence and ischemic stroke-induced mortality, and adversely affects postischemic stroke outcomes. In the rodent model of type 1 diabetes, neurogenesis, synaptic plasticity, and learning potential are compromised (Zhang et al. 2008; Stranahan et al. 2008). Recently it was shown that neurogenesis is severely impaired in the adult type-2 diabetic GK rats, due to a decreased survival of neural progenitors in the neurogenic regions, although proliferation was increased (Lang et al. 2009). Since neuronal progenitor cells are found only in the subgranular zone of the dentate in hippocampus, characterizing ischemia neuropathology of dentate neurons offers the opportunity for experimental examination of the process of injury-induced neurogenesis. Dentate neurons, which reside in the neurogenic niche, are submitted to various regulatory factors that influence cell proliferation, maturation, fate determination, and survival. Insulin and the insulin receptor are found in specific brain regions including hippocampus where they show evidence of region-specific functions, including learning and memory, through mechanisms that are different from its direct glucose regulation in the periphery (Bravata et al. 2005; Zhao et al. 2004). Insulin has a high affinity for, and can signal via insulin-like growth factor 1 (IGF-1) receptors. Insulin/IGF signaling has been shown to be a potent regulator of adult hippocampal neurogenesis (Aberg et al. 2000; Lichtenwalner et al. 2001; Trejo et al. 2008). Focal ischemia is known to significantly induce IGF-1 expression as well as neural progenitor proliferation (Gluckman et al. 1992; Yan et al. 2006; Dempsey et al. 2003). IGF-induced increase in neurogenesis involves Akt (Kalluri et al. 2007).
In the present study, we hypothesized that insulin/IGF signaling plays a role in conferring the relative resistance of dentate neurons to ischemia/reperfusion-induced neurodegeneration. We used mouse organotypic hippocampal slice cultures exposed to an in vitro model of ischemia using oxygen-glucose deprivation (OGD). Slice cultures retain the cell architecture and connectivity of different anatomical sub-regions, as well as cell to cell functional interactions. In addition, this in vitro model offers easier pharmacological manipulations for the study of mechanisms of sub-regional resistance/vulnerability to ischemia. We investigated sub-regional sensitivity to OGD insult and insulin/IGF-mediated neuroprotection and found that the relative resistance of dentate neurons to OGD is mediated by signaling pathways involving PI3K/Akt.
Materials and methods
Organotypic hippocampal slice cultures
Animals were handled in accordance with local guidelines for ethical use of animals in research at the University of California San Diego, which is in accordance with international guidelines. All possible efforts were made to minimize animal suffering and the number of animals used. Mouse hippocampal slice cultures were prepared and grown on semiporous membranes, according to the standard interface method (Stoppini et al. 1991) as previously described (Yao et al. 2007) with some modifications. In brief, 8 day old CD1 mice (Charles River Laboratories, Raleigh, NC, USA) were decapitated and the dorsal hippocampi quickly isolated under aseptic conditions. The hippocampi were cut into 400µm thick, transverse slices by a McIlwain tissue chopper, and the slices transferred to Gey’s balanced salt solution (GBSS, Gibco, Paisley, Scotland) for separation and trimming of the tissue slices. Tissue slices from the middle part of the dorsal hippocampi were obtained and transferred to membrane inserts (Millicell, Bedford, MA, USA) placed in 35 mm culture dishes with 1.2 ml of serum-containing culture medium composed of Basal Medium Eagle Medium (50%), Earle’s Balanced Salt Solution (25%), horse serum (25%), and l-glutamine (1 mmol/L), supplemented with 50 U/mL penicillin/streptomycin and 10 mmol/L glucose. Cultures were maintained in a 5% CO2, 37°C incubator for 12–14 days before experiments were performed. Culture medium was half-replaced the second day after plating and twice a week thereafter until the day of treatment.
Oxygen–glucose deprivation
Oxygen-glucose deprivation (OGD) was used as an in vitro model of cerebral ischemia. The inserts with slice cultures were placed into 1 ml of sucrose-containing ACSF solution composed of (in mmol/L): NaCl 129, KCl 5, CaCl2 1.3, MgCl2 1.5, NaHCO3 21, sucrose 10 (315 mOsm, pH 7.4) following three washes using the same solution. The cultures were then placed into an airtight chamber (Billups and Rothenberg, CA, USA) and exposed to 5 min of 95% N2/5% CO2 gas flow. After that, the chamber was sealed for 25–60 min at 37°C. Control cultures were maintained for the same time under normoxic atmosphere in glucose-containing ACSF solution. After OGD, slice cultures were returned to their original culture conditions for 48–72 h.
Propidium iodide (PI) uptake and Quantification of cell death
Cell damage was monitored and quantified by densitometric measurements of the cellular uptake of propidium iodide (PI, Sigma, St. Louis, MO), a fluorescent dye that intercalates into the DNA of necrotic cells that have lost plasma membrane integrity and does not penetrate living or early apoptotic cell membranes. 5 µg/mL PI was added into the culture medium 24 h before treatments and kept at the same concentration throughout the experiment. The PI uptake was recorded before any pharmacological treatments and OGD, and then at different time points after treatments. All cells were killed after experiments by submerging the cultures in sucrose (10 mM) containing ACSF solution at 4°C for 24 h, and the maximum PI uptake served as positive controls for each slice. The death of most of neurons was confirmed in our preliminary experiments by using high glutamate (up to 20 mM), or in each experiment by extending exposure time up to 72 h until no further PI uptake in the neuronal layers were detected.
The PI uptake was quantified densitometrically using Axionvision Image software, delineating the hippocampal subfields CA1, CA2, CA3 and dentate gyrus (DG). From the PI uptake in these subfields in each culture, measured at the different time points, the basal PI uptake recorded in the same subfields before treatments was subtracted. Maximum PI uptake after all the cells were killed served as positive controls. Cell injury was expressed as either means of PI uptake for each sub-regions or percentage cell death (PI uptake/maximum PI uptake). For detection of PI intensity, slices were excited with a 510–560-nm light and the emitted fluorescence was acquired at 610 nm using a rhodamine filter on an inverted fluorescence microscope (Zeiss Axiovert 200 M microscope, Zeiss, Yena, Germany). Images were taken using a CCD camera and analyzed on a PC with Axiovison imaging-processing software (Zeiss). The fluorescence intensity was measured offline and damage was given as the integral density of PI intensity in CA1, CA2, CA3 and DG regions.
Pharmacological agents and glutamate treatments
In the interface culture method used in the present study, glial cells redistribute into a thin layer at the bottom of the culture. This layer of glial cells forms an interface between tissue and culture medium (Buchs and Muller 1993), and may function as a diffusion barrier and prevent access of some pharmacological agents from getting to neurons. To facilitate access of glutamate to neurons, slice cultures were treated by submersion. Other agents were treated by applying a thin layer of drug-containing solutions above the top of slice cultures and letting it diffuse through the slice culture. Control cultures were treated in the same manner with drug-free solutions, to prove that the procedure itself was harmless. Doses used in the present study were chosen according to the literature, and also based on our preliminary studies. Insulin, IGF-1 and signaling pathway blockers were applied 24 h before and during OGD exposure, and glutamate receptor blockers were applied 1 h before and during OGD.
Immunofluorescence staining in slice cultures
The slice cultures (at days in vitro 12–14) were fixed in 4% paraformaldehyde for 6 h at 4°C and then permeabilized with 1% triton X-100 in PBS overnight at 4°C. The slices were incubated in 10% goat serum for 1 h before being treated for 3 days with either a rabbit anti-insulin receptor β subunit (IRβ, C-terminal) polyclonal antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or a rabbit anti-phospho-Akt (Ser473) monoclonal antibody (1:100, Cell Signaling, Danvers, MA, USA) in antibody diluent (Invitrogen, Carlsbad, CA, USA) at 4 °C. After thorough washes, slices were incubated overnight at 4°C with Alexa 488-labeled anti-rabbit IgG (1:500, Invitrogen, Carlsbad, CA, USA). After several PBS washes, slices were mounted on slides with Prolong antifade reagent with DAPI (Invitrogen, Carlsbad, CA, USA). Immunofluorescent images were obtained and analyzed using confocal microscopy (Olympus FV1000). In control experiments, primary antibodies were omitted and no fluorescence signals were detected.
Drugs
Insulin and insulin-like growth factor 1 (IGF-1) were purchased from Sigma. Wortmannin, triciribine, and SP600125 were from BIOMOL Research Laboratories Inc (Plymouth Meeting, PA, USA). PD98059 and U0126 were obtained from Cayman Chemical (Ann Arbor, Michigan, USA). Before each use, insulin was first solubilized in dH2O (pH 2.0, adjusted with HCl) at 0.4 mM, and then diluted in the testing solution to the final concentrations used. IGF-1 was dissolved in PBS containing 0.1% BSA. All other drugs were dissolved in dimethyl sulfoxide (DMSO, Sigma Chemical, St. Louis, MO, USA). The same amounts of solvents were added to the control slice cultures and were found to have no effects.
Statistical analysis
Data are expressed as mean ± SEM and analyzed for statistical significance by Student’s t-test and one-way ANOVA for within and between group comparisons respectively. Differences between mean values were considered significant when p < 0.05.
Results
Distinctive sensitivity of hippocampal sub-regions to OGD
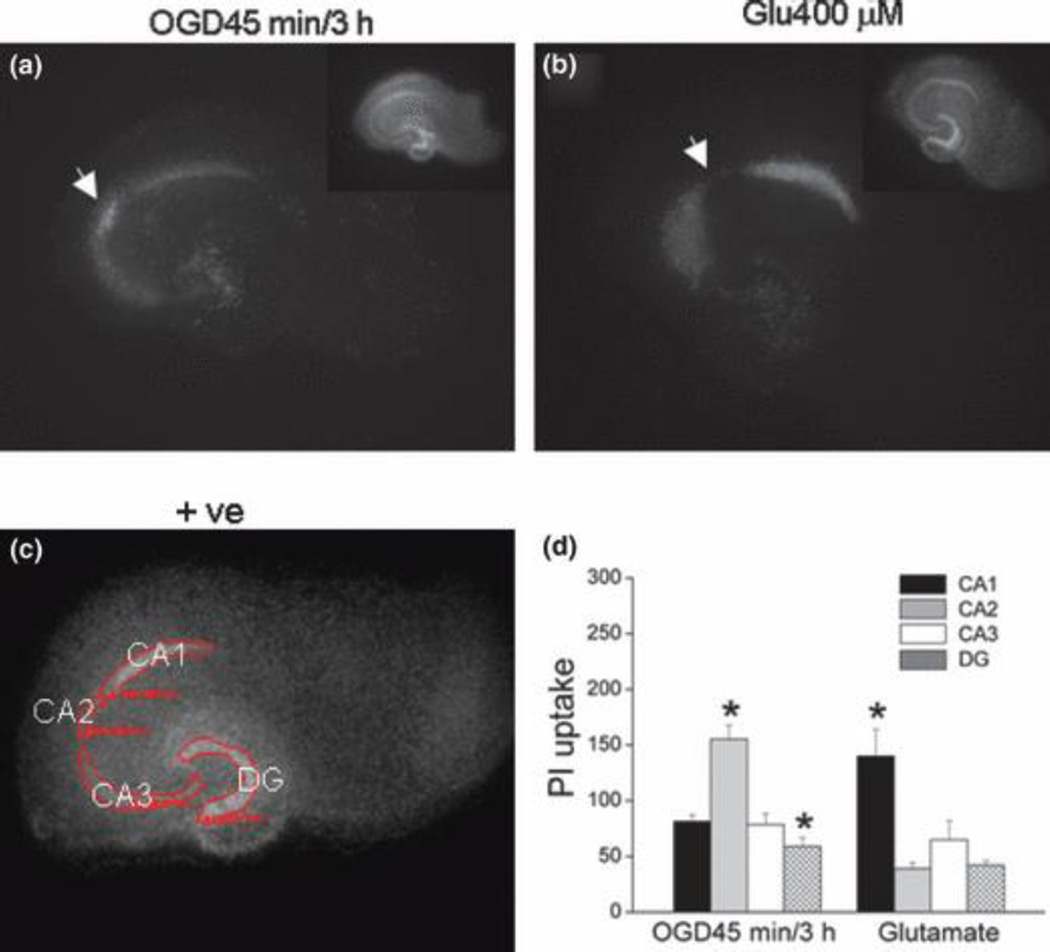
Among hippocampal sub-regions, the CA2 subfield has not been studied as much, especially in slice culture preparations, because CA2 is a rather small part and more difficult to identify than CA1 and CA3. In our experimental conditions, however, we identified a distinct group of cells in CA2 region that had a higher incorporation of PI 3h after exposure to OGD for 45 min or longer (Fig. 1a, d). This is distinctively different from the relative resistance that CA2 neurons exhibit to glutamate excitotoxicity (Fig. 1b, d). These and other properties we later characterized not only helped to identify the CA2 sub-region in our study (Fig. 1c), but also provided evidence that CA2 neurons are functionally distinctly different from their neighboring CA1 and CA3 neurons.
Fig. 1.
Distinctive hippocampal sub-regional sensitivity to OGD and glutamate. (a, b) Representative photomicrographs of slices stained with PI 3 h after a 45 min exposure to OGD (a) or 24 h after 2 h exposure to glutamate (400 µM, submerged, b). Insets: positive controls of the same slices after most of neurons were killed by submerging slices in ACSF/sucrose at 4°C for 24 h. (c) A representative photomicrograph of a positive control slice culture after most of cells was killed, showing four sub-regions measured for quantitative analysis of PI uptake. (d) Quantitative analysis of PI uptake in CA1 (black bars), CA2 (hatched bars), CA3 (open bars) and DG (crossed bars) after OGD or glutamate. OGD45min/3h group: 45 min OGD followed by 3 h reperfusion, n = 18 slices; Glutamate group: 2 h glutamate (400 µM) exposure (submerged) followed by 24 h reperfusion, n = 18. +ve: positive control. Bars represent the mean ± SEM. *, significantly different from other sub-regions with the same treatments, one-way ANOVA, p < 0.05.
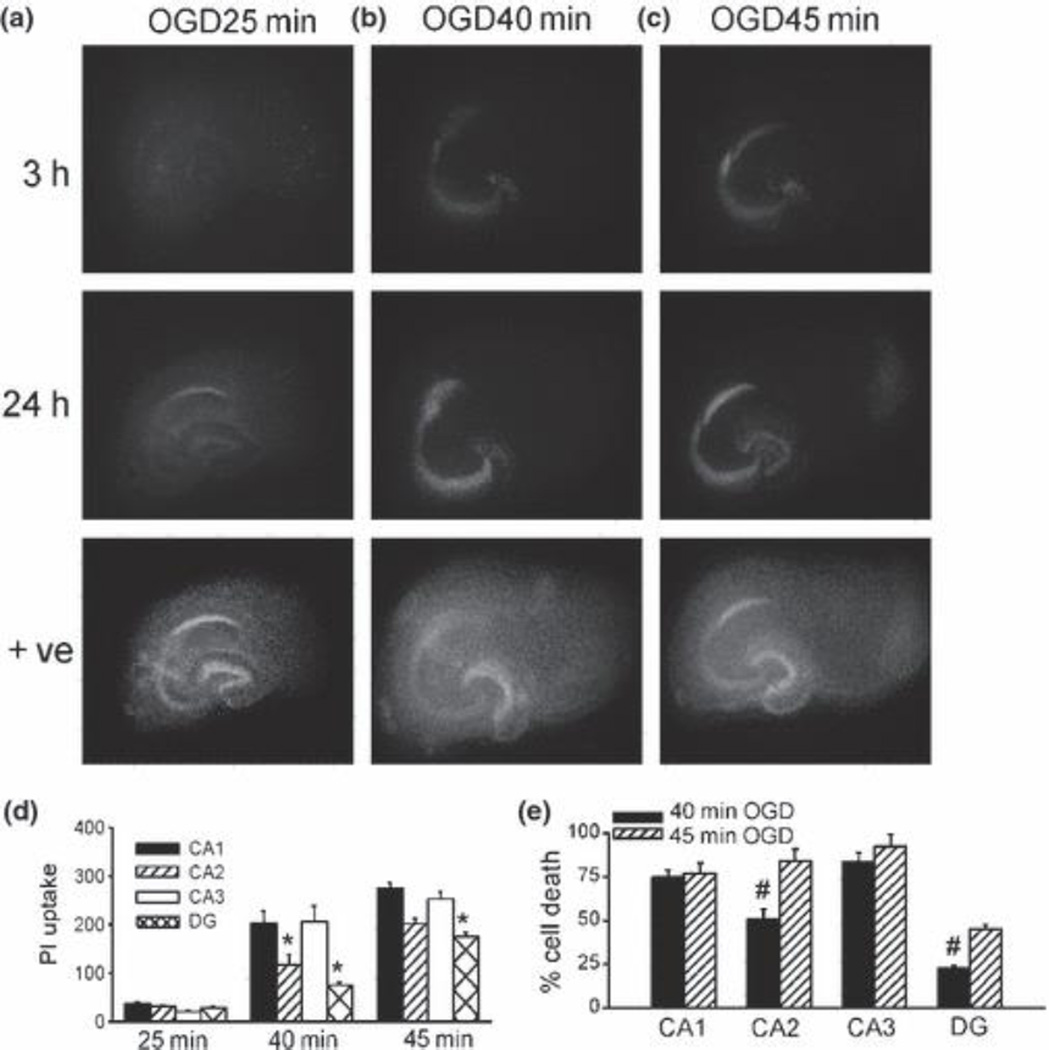
To further determine sub-region-, time- and duration-dependent sensitivity of hippocampal neurons to OGD, we subjected slice cultures to 25 min, 40 min and 45 min OGD and measured PI uptake in CA1, CA2, CA3 and dentate gyrus (DG) before, 3 h and 24 h after OGD exposure. Slice cultures exposed to OGD for 25 min exhibited little neuronal damage after 3 h reperfusion, and there was only very mild PI uptake in CA1 sub-region after 24 h (Fig. 2a, d). However, 40 min OGD induced marked PI fluorescence uptake in the CA1 and CA3 sub-regions (Fig. 2b, d, e), with less neuronal damage detected in DG granule neuronal layer and CA2 sub-regions. Significant injury was detected in all sub-regions including CA2 and DG after 24 h reperfusion following a further increase in duration of OGD exposure (Fig. 2c, d, e). There was marked PI uptake in all sub-regions after OGD for 45 min or longer (data not shown). These data suggest that CA1 and CA3 neurons are more vulnerable to OGD than CA2 neurons and DG granule neurons. Relative resistance of DG was exhibited in two ways: a), only more severe insults (longer duration of OGD) induced cell injury; b), a lower percentage of cell death was seen compared to other sub-regions subjected to the same milder insults. Since DG has higher cell density compared to CA sub-regions, a comparable PI uptake value represents a smaller percentage of cell death than CA-sub-regions (Fig. 2).
Fig. 2.
Hippocampal sub-regional duration-dependent sensitivity to OGD. (a-c) Representative photomicrographs of slices stained with PI 3 h and 24 h after exposure to OGD for 25 min (a), 40 min (b) or 45 min (c). +ve: positive control. (d) Quantitative analysis of PI uptake in CA1 (black bars), CA2 (hatched bars), CA3 (open bars) and DG (crossed bars), 24h after exposure to OGD for 25 min (n = 6), 40 min (n = 6) and 45 min (n = 59). *, significantly different from other sub-regions in the same OGD duration groups, one-way ANOVA, P < 0.05. (e) Percentage cell death (PI uptake normalized to maximum PI uptake from positive control) induced by OGD exposure for 40 min (black bars) or 45 min (hatched bars) followed by 24 h reperfusion. #, significantly different from 45min OGD group in the same sub-regions. Student’s unpaired t-test, p < 0.05. Bars represent the mean ± SEM.
Insulin/IGF protects against cell injury induced by OGD
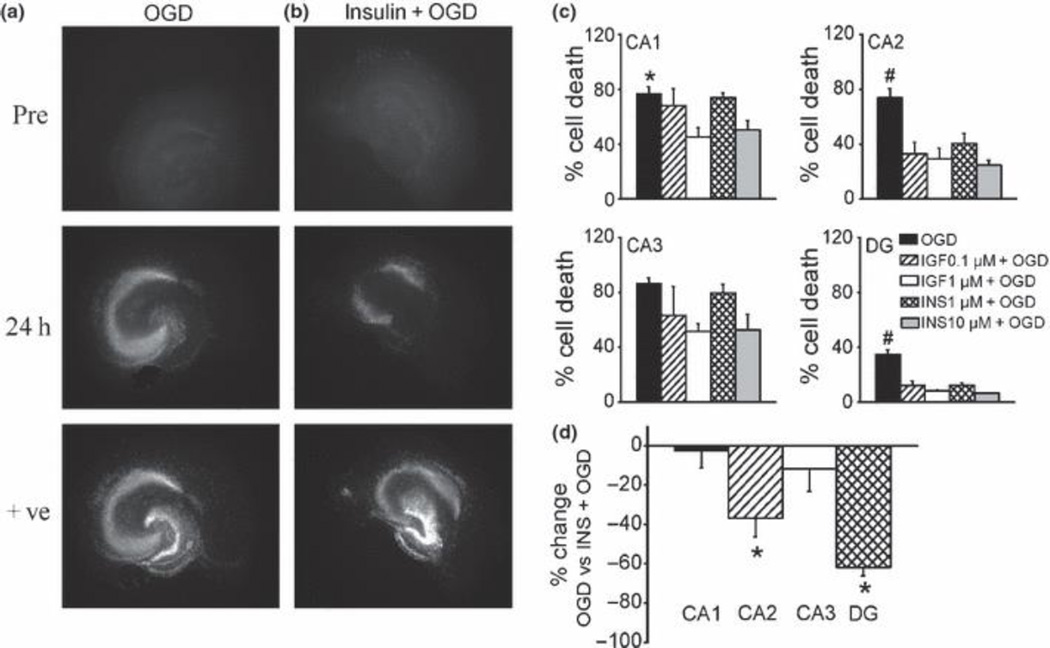
The hippocampus has a high number of insulin receptors (Steen et al. 2005). Insulin/IGF-1 has been shown to have neuroprotective effects against memory loss in humans (Benedict et al. 2007), and plays a role in promoting survival of newly formed neurons in DG in diabetic animal models (Lang et al. 2009). Thus we next examined the effects of insulin and IGF-1 on responses of hippocampal sub-regions to OGD. We chose a 45 min OGD for further study since there was marked PI uptake in DG after this exposure time (Fig. 2). Quantification of PI uptake showed that pretreatment for 24 h with insulin (1–10 µM) or IGF-1 (0.1–1 µM) dose-dependently reduced OGD-induced injury in all sub-regions with differential sub-regional sensitivity (Fig. 3 a–c). Both insulin and IGF treatments protected neurons with similar sub-regional selectivity. At lower concentrations, insulin/IGF markedly reduced injury in DG and CA2 sub-regions, but not CA1 and CA3 (Fig. 3). A reduction in PI uptake in CA1 and CA3 was observed at higher concentrations of insulin (10µM) and IGF (1µM), although only the latter reached statistical significance (Fig. 3c). The lack of effect of insulin in the CA1 sub-region is not due to a more severe injury in CA1 than the DG sub-region, because insulin did not protect CA1 even with less severe insults while there was little injury in DG (50 ± 5 % and 53 ± 5 % cell death induced by OGD in CA1 in the absence and presence of insulin respectively). Thus sensitivity of hippocampal sub-regions to insulin/IGF mediated neuroprotection is higher in DG and CA2 than CA1 and CA3, which is opposite to the order of their sensitivity to OGD insult (Fig. 2). Since 1µM insulin induced sub-maximal effects in the DG sub-region (Fig. 3d), we chose the dose of 1 µM insulin to further investigate a probable mechanism by which insulin exerts its sub-regional selective neuroprotective effects.
Fig. 3.
Protective effect of insulin/IGF against OGD-induced cell injury in organotypic hippocampal slice cultures. (a, b) Representative photomicrographs of slices stained with PI before and 24 h after 45 min exposure to OGD in a control slice (a) or a slice pretreated with insulin (1 µM) for 24 h (b). +ve: positive controls. (c) Quantitative analysis of hippocampal sub-regional cell injury (% cell death: PI uptake normalized by maximum PI uptake in positive controls) 24 h after exposure to 45 min OGD. Black bars, OGD alone, n = 59; hatched bars, OGD in IGF-1 (0.1 µM, 24 h) pretreated slices, n = 6; open bars, OGD in IGF-1 (1 µM) pretreated slice cultures, n = 6; crossed bars, OGD in insulin (1 µM) pretreated slices, n = 43; grey bars, OGD in insulin (10 µM) pretreated slices, n = 6; *, significantly different from 1µM IGF-1 treatment group in CA1; #, significantly different from all other treatments in the same sub-regions; One-way ANOVA, p < 0.05. (d) Percentage change in OGD-induced PI uptake (45 min OGD duration followed by 24 h reperfusion) in insulin (1µM) pretreated slices compared to those of untreated slices. For each experiment, data were averaged and a percentage change was calculated, n = 8 experiments. Bars represent the mean ± SEM. *, significantly different between untreated and insulin treated cultures, Student’s unpaired t-test, p < 0.05.
The neuroprotective effect of insulin involves PI3K/Akt pathway
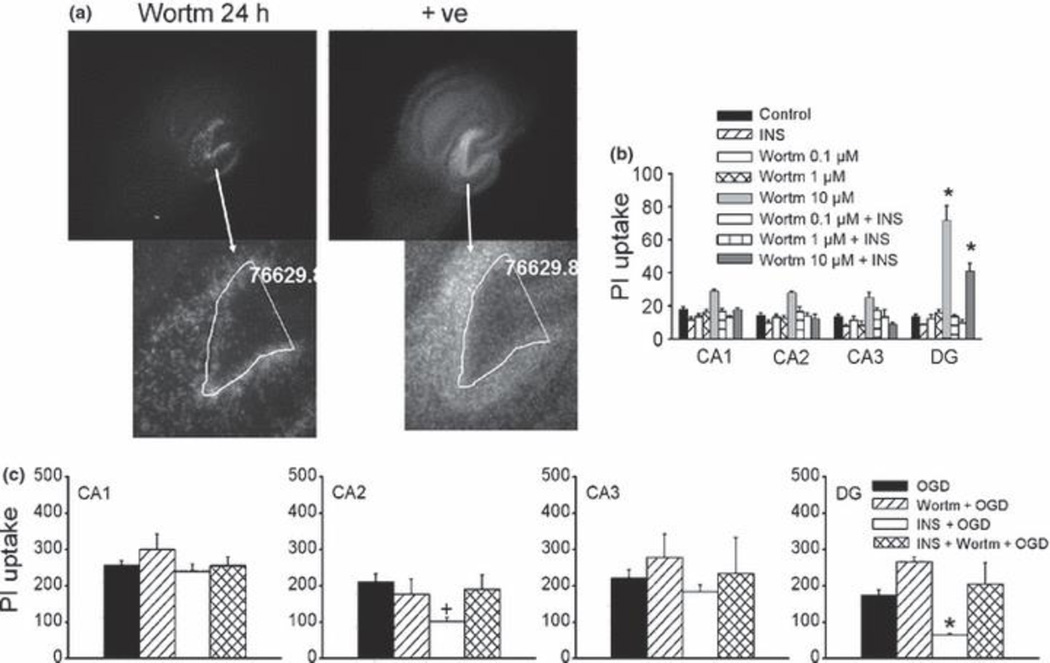
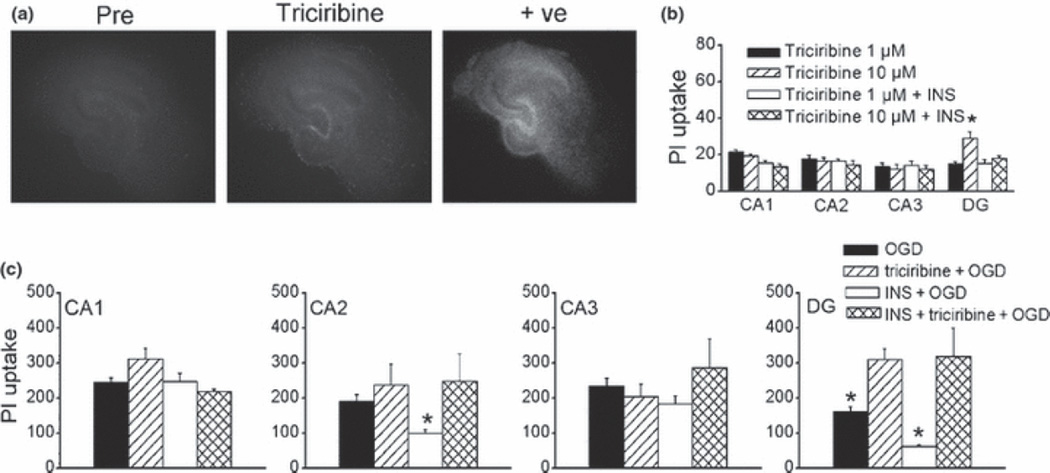
Insulin can activate several signaling pathways, including PI3K/Akt pathway. We next carried out experiments using inhibitors of PI3K signaling such as wortmannin (Wortm, a potent specific PI3K inhibitor), and triciribine (a specific inhibitor of Akt). Under basal conditions, wortmannin (0.1–1 µM, 24 h) did not have any effects on the PI uptake. However, at higher concentrations, Wortmannin (10 µM) selectively induced cell injury in the DG sub-region, which was attenuated in the presence of insulin (Fig. 4a-b). Inhibition of PI3K by wortmannin preferentially induced cell injury in the inner DG granule cell layer where newly formed neurons are found (Fig. 4a; Parent 2003). These data suggest that under normal conditions DG neuronal survival is more critically dependent on PI3K signaling than CA regions, possibly via neurogenesis-associated survival mechanisms. Although wortmannin alone did not appear to alter OGD-induced neuronal injury in CA and DG, the protective effects of insulin were prevented in the presence of Wortmannin, even at concentrations that had no effects on basal conditions (Fig. 4c). These data indicated that insulin protects neurons from OGD-induced injury through activating PI3K signaling. Similarly, triciribine selectively induced cell injury in the DG sub-region at a higher concentration (10 µM, 24 h) under basal conditions which was prevented by insulin (Fig. 5a, b). However, triciribine (1µM) prevented insulin-induced reduction in OGD-induced injury (Fig. 5c). CA1 and CA3 sub-regions were less sensitive to basal PI3K/Akt inhibition as well as protective effects of insulin against OGD. The CA2 sub-region is unique in that it was less sensitive to endogenous PI3K/Akt inhibition compared to DG, but more sensitive to insulin protection than CA1 and CA3 (Figs. 4, 5). Taken together, these data suggested that dentate neurons are more dependent on endogenous PI3K/AKt signaling pathways than CA neurons in the maintenance of neuronal survival/death homeostasis, and the action of insulin in protecting dentate neurons from OGD-induced injury is critically dependent on PI3K/Akt signaling pathway.
Fig. 4.
Effects of Wortmannin on sub-regional cell survival/death in organotypic hippocampal slice cultures. (a,) Representative photomicrographs of slices (upper) after 24 h wortmannin (10 µM) treatment (left) and post treatment to induce maximum death as positive control (right), and enlarged DG sub-region (lower). The white lines and numbers are reference lines and their enclosed areas indicating relative location of Wortmannin-induced injury in DG. (b) Quantitative analysis of hippocampal sub-regional PI uptake after wortmannin exposure in the presence and absence of insulin. Control, untreated slices; INS, insulin (1 µM, 24h) treated slices; Wortm0.1–10µM, slices were treated with 0.1–10 µM wortmannin for 24h; Wortm0.1–10µM+INS, slices were treated with 0.1–10 µM wortmannin in the presence of insulin (1 µM) for 24h. (c) Quantitative analysis of sub-regional PI uptake 24 h after 45 min exposure of OGD. OGD, OGD in untreated slices, n = 20; Wortm+OGD, wortmannin (1 µM) pretreated slices were exposed to OGD, n = 12; INS+OGD, OGD in insulin (1 µM) pretreated slices, n = 29; INS+Wortm+OGD : insulin (1 µM) and wortmannin (1 µM) pretreated slices were exposed to OGD, n = 12; Bars represent the mean ± SEM. *, significantly different from other treatment groups in the same sub-region; +, significantly different from OGD group in CA2. One-way ANOVA, p < 0.05.
Fig. 5.
Effects of triciribine on sub-regional cell survival/death in hippocampal slice cultures. (a,) Representative photomicrographs of slice cultures before (pre), after triciribine (10 µM) for 24 h (triciribine), and post treatment to induce maximum death as positive control (+ve). (b) Quantitative analysis of hippocampal sub-regional PI uptake after 24 h triciribine exposure (1–10 µM) in the presence and absence of insulin (1 µM). (c) Quantitative analysis of sub-regional PI uptake induced by 45 min OGD followed by 24 h reperfusion. OGD, OGD alone in untreated slices, n = 6; triciribine +OGD: triciribine (1 µM) pretreated slices were exposed to OGD, n = 6; INS+OGD, OGD in insulin (1 µM) pretreated slices, n = 12; INS+triciribine+OGD: insulin (1 µM) and triciribine (1 µM) pretreated slices were exposed to OGD, n = 12; Bars represent the mean ± SEM. *, significantly different from other treatment groups in the same sub-regions; One-way ANOVA, p < 0.05.
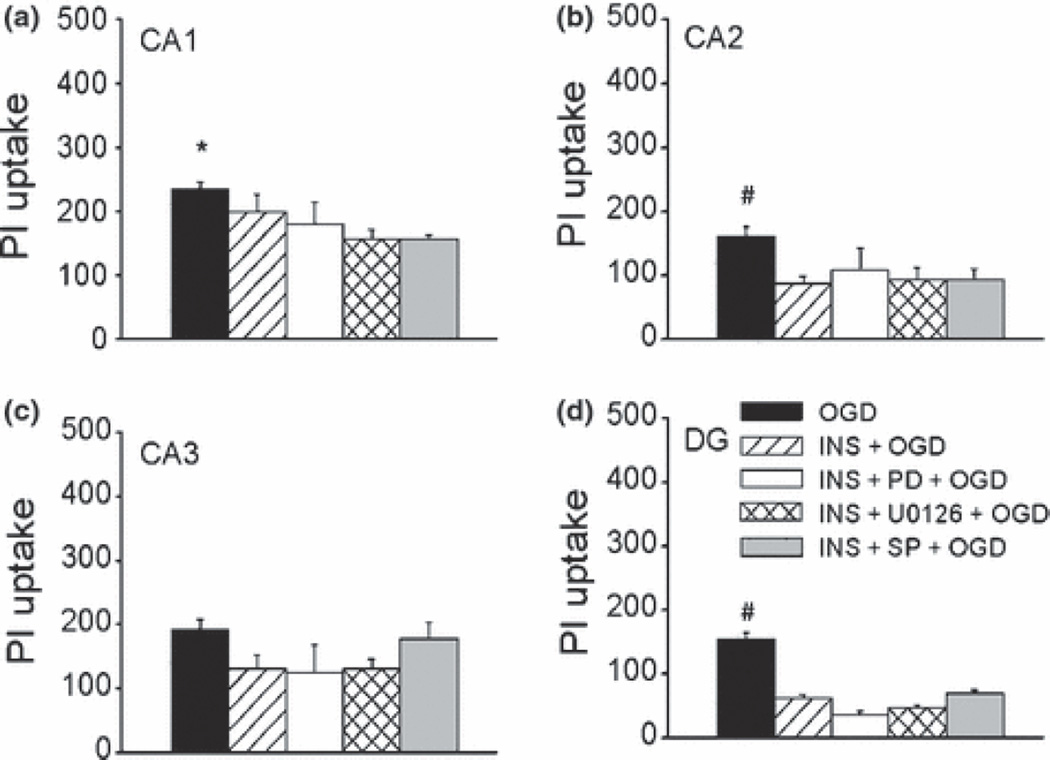
Insulin neuroprotection is not mediated by MAPK pathway
Since insulin was shown to activate both PI3K and MAPK pathways, we next studied the role of MAPK pathway in insulin action using PD98059 (10 µM), an inhibitor of MEK/ERK pathway, U0126 (1–10 µM), a potent and selective MEK inhibitor, and SP600125 (1 µM), a JNK inhibitor. We found that none of the three inhibitors had any effects on basal cell survival in CA and DG sub-regions at the concentrations used (data not shown), nor did they prevent insulin-induced reduction in PI uptake against OGD-induced cell injury in DG and CA2 (Fig. 6). These data suggested that the neuroprotective effects of insulin are not mediated by activating MAPK pathway. Furthermore, it appeared that blocking MAPK pathway further protected CA1 neurons in the presence of insulin (Fig. 6a), suggesting that activation of MAPK signaling by insulin may contribute to insensitivity to insulin/PI3K/Akt mediated protection. However, we did not see a consistent effect of MAPK inhibitors alone on OGD-induced neuronal injury, either pretreated for 24 h or 1 h prior to OGD (data not shown). Since PI3K and MAPK signaling has been shown to act antagonistically (van der Heide et al. 2003; Hui et al. 2005), we hypothesized that the effects of MAPK signaling may be enhanced by blocking PI3K. Indeed, compared to wortmannin (10 µM) alone, U0126 (10 µM) in the presence of wortmannin markedly reduced OGD-induced cell injury in CA sub-regions but to a lesser extent in DG (PI uptake was 310 ± 19 vs 187 ± 45 in CA1, 310 ± 22 before vs 260 ± 29 in DG, wortmannin alone vs wortmannin+U0126 respectively, n = 12). These data suggested that the insulin/MAPK pathway may act to antagonize insulin/PI3K/Akt and contribute to the relative resistance of CA1 and CA3 to insulin/IGF protection, as well as their relative vulnerability to OGD.
Fig. 6.
Effect of inhibitors of MAPK signaling on OGD-induced cell injury in insulin pretreated slices. (a-d) Histograms represent the quantitative analysis of PI uptake induced by 45 min OGD followed by 24 h reperfusion in CA1 (a), CA2 (b), CA3 (c), and DG (d). OGD, untreated slices were exposed to OGD, n = 29; INS+OGD, OGD in insulin (1 µM, 24 h) pretreated slices, n =12; INS+PD+OGD: insulin (1 µM) and PD98059 (10 µM) pretreated slices were exposed to OGD, n = 12; INS+U0126+OGD: insulin (1 µM) and U0126 (10 µM) pretreated slices were exposed to OGD, n = 18; INS+SP+OGD, insulin (1 µM) and SP600125 (10 µM) pretreated slices were exposed to OGD, n = 6; Bars represent the mean ± SEM. *, significantly different from INS+U0126+OGD and INS+SP+OGD groups in CA1; #, significantly different from other treatment groups within the same sub-regions; One-way ANOVA, p < 0.05.
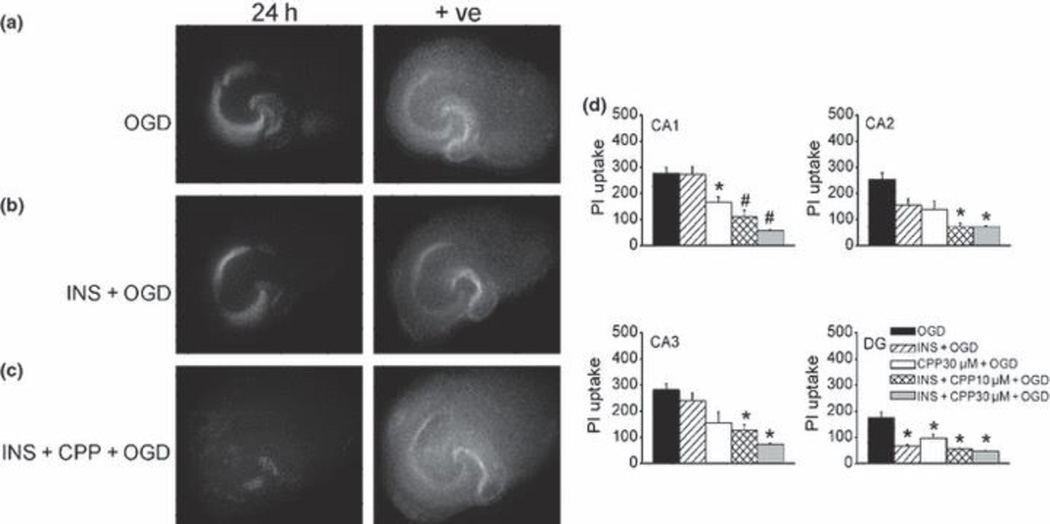
NMDA receptor-dependent OGD-induced cell injury
It was shown that insulin modulates CA1 neuronal activity in a NMDA receptor-dependent manner (van der Heide et al. 2005). Thus its action may involve direct (synaptic activity independent, insulin receptor mediated) or indirect (synaptic activity dependent, NMDA mediated) actions. Thus we next used NMDA receptor blocker CPP to examine the mechanisms of cell injury induced by OGD in insulin pretreated slices. We found that CPP (10 µM) in the presence of insulin (1µM) nearly completely blocked 45 min OGD-induced PI uptake in all sub-regions (Fig. 7a-c). We also tested protective effects of insulin and CPP against 50 min OGD. We found that 10 µM CPP alone did not significantly reduce PI uptake induced by OGD (PI uptake was 276 ± 22 vs 264 ± 47 in the absence vs presence of CPP, n = 11). At a higher concentration, CPP (30 µM) induced partial neuroprotection, as was seen with 1 µM insulin, which selectively protected DG and CA2 neurons (Fig. 7d). However, a combination of CPP (10–30 µM) and insulin (1 µM) nearly completely blocked the cell injury induced by OGD in all CA sub-regions (Fig. 7). These data suggested that with a more severe OGD insult, multiple mechanisms may need to be targeted to effectively prevent neuronal death.
Fig. 7.
Effect of NMDA receptor blockers and insulin on OGD-induced sub-regional injury. (a-c) Representative photomicrographs of slices stained with PI 24 h after 45 min OGD in a control slice (a), a slice pretreated with insulin (1 µM) for 24 h (b), and an insulin pretreated slice exposed to CPP (10 µM) for 1h before and during OGD (c); +ve: positive controls. (d) Quantitative analysis of hippocampal sub-regional PI uptake 24 h after 50 min OGD. OGD: untreated slices were exposed to OGD, n = 16; INS+OGD: insulin (1 µM) pretreated slices were exposed to OGD, n = 18; CPP30µM+OGD: slices were treated with 30 µM CPP 1 h before and during exposure to OGD, n = 5; INS+CPP10µM+OGD: insulin (1µM) pretreated slices were treated with CPP (10 µM, 1 h) before OGD exposure, n = 11; INS+CPP30µM+OGD: insulin (1µM) pretreated slices were treated with CPP(30 µM, 1 h) before OGD exposure, n = 5. Bars represent the mean ± SEM. *, significantly different from OGD groups in the same sub-regions; #, significantly different from OGD and INS+OGD groups in CA1, one-way ANOVA, p < 0.05.
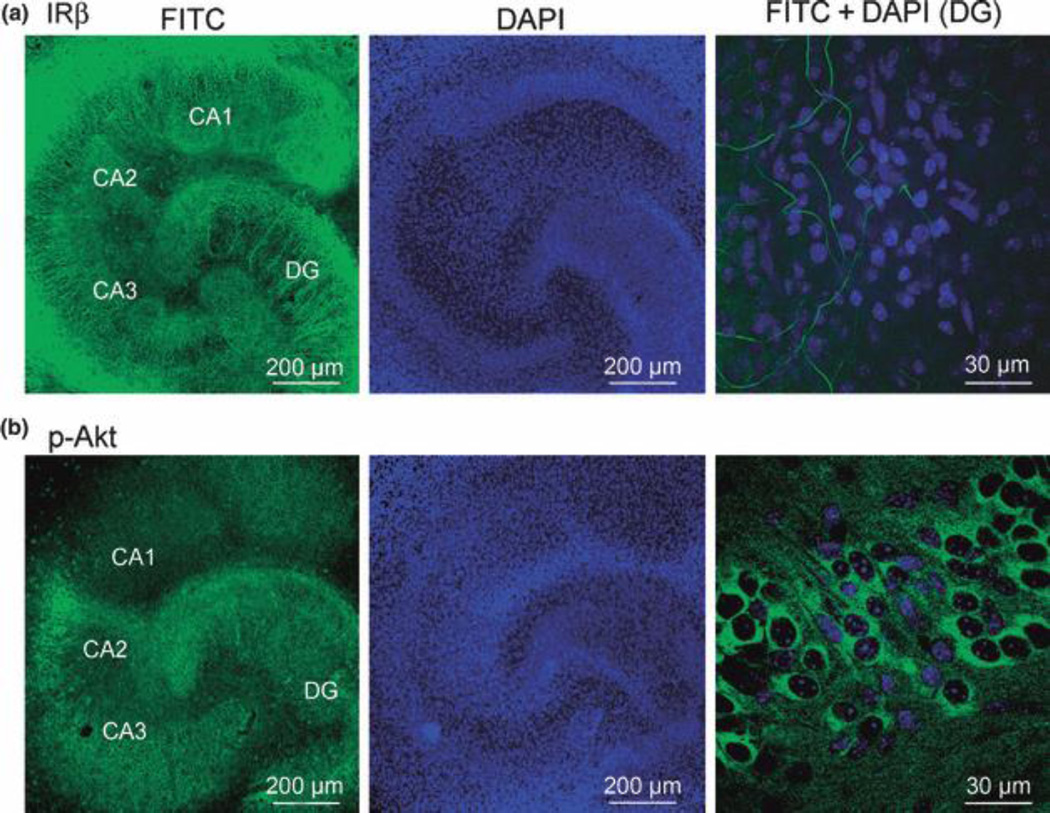
Insulin receptor β and basal phospho-Akt immunoreactivity in hippocampal slice cultures
Insulin receptor mRNA and protein have been found in brain regions including hippocampus from in vivo animal models (Marks et al. 1990; Zhao et al. 1999; Park et al. 2009). We next tested the distribution of insulin receptors and endogenous phospho-Akt (p-Akt) in our slice culture preparations, using antibodies against insulin receptor β subunit (IRβ, C-terminal) and p-Akt (Ser473) in untreated slice cultures. We found that IRβ immunoreaction was detected in all sub-regions of hippocampus, with higher expression in the neuropil than in cell body layers (Fig. 8a, n = 4). A strong signal was detected in the molecular and polymorphic layers of the DG, in the alveus and stratum lacunosum-moleculare, the mossy fibers and Schaffer collaterals in the CA regions (Fig. 8a). In contrast, higher levels of endogenous p-Akt were detected in the pyramidal cell layer of CA sub-regions and the molecular layer of the DG (Fig. 8b, n = 14), with lower expression in the polymorphic layer and the inner granule cell layer of DG, where neurons were most sensitive to blockade of PI3K/Akt (Figs 4 and 5; Fig. 8b). Our data confirmed that insulin signaling is detected in our slice cultures and its activation may protect neurons from OGD-induced injury.
Fig. 8.
Confocal images of insulin receptor and phospho-Akt immunofluorescence in hippocampal slice cultures. (a–b), images of two slice cultures with immunoreactivity staining (FITC in green) of insulin receptor β subunit (IRβ, a) or phospho-Akt (p-Akt, b), DAPI counterstained nucleus (blue), and higher magnification of insulin receptor β stained fibers (green, a) or p-Akt stained cell bodies (green. b), overlaid with DAPI stained nucleus (blue) in DG.
Discussion
In the present study, we found that there is a distinctive sub-regional sensitivity of hippocampus to OGD-induced injury and insulin neuroprotection. Insulin protects DG neurons via activation of PI3K/Akt pathway, whereas CA1 appears to be less sensitive to this signaling. Our data suggest that a severe ischemia insult, which results in injury in both vulnerable and resistant neurons, multiple mechanisms may need to be targeted for neuroprotection.
Insulin and insulin receptors are found in specific brain regions with poorly understood functions (Benedict et al. 2007). In our study, insulin receptors are detected predominantly in fiber pathways. Both insulin and IGF-1 protected hippocampal neurons with similar sub-regional selectivity. The concentrations we used for both insulin and IGF-1 would likely activate both receptors and the downstream signaling. Insulin receptor and IGF-1 receptor are structurally highly homologous to each other. Both receptors can bind insulin and IGF-1 and activate similar intracellular signaling pathways, yet differentially regulate expression of a subset of genes encoding adhesion, transcription, transport, and proliferation molecules. Insulin stimulates both the PI3K and the MAPK/ERK pathways (Halevy and Cantley 2004), which play an important regulatory role in cell survival and death. PI3K pathway is believed to be an important anti-apoptotic signaling pathway in neurons (Yuan and Yankner 2000), whereas MAPKs are activated by a variety of extracellular stimuli, and may contribute to either survival or death, possibly depending on the nature and severity of injury, or the specific cell type (Dash et al. 2002, Cavanaugh 2004; Hetman and Gozdz 2004; Slevin et al. 2000; Alessandrini et al. 1999; Runden et al. 1998; Chu et al. 2004). A substantial body of literature exists regarding the vulnerability of CA1 pyramidal neurons but not DG granule neurons to ischemia insults (Lipton 1999; Pulsinelli 1988). This differential sensitivity or vulnerability of the distinct neuronal populations allows for the examination of critical factors in cell death and survival. In the present study, we have shown that insulin/PI3K/Akt pathway plays different roles in different neuronal populations in their homeostasis of survival/death. DG neurons are sensitive to the regulation of insulin/PI3K/Akt signaling both in normal and ischemic conditions and this signaling may be critical in conferring relative resistance of these neurons to ischemia. MAPKs play a less dominant role in DG neuronal survival/death. On the other hand, as compared to DG neurons, CA1 neurons are less sensitive to disruptions of endogenous PI3K/Akt signaling, as well as the protective effects of insulin-induced activation of PI3K/Akt signaling against OGD-induced injury. The different sensitivity of CA1 and DG sub-regions to this insulin signaling pathway may be due to different expression levels of signaling molecules. As we have shown, in contrast to CA sub-regions which have high levels of basal p-Akt in the pyramidal cell layer, a relatively lower level of endogenous p-Akt is detected in the inner granule cell layer compared to the molecular layer of DG. This may contribute to its vulnerability to PI3K and Akt blockers under basal conditions, as well as a higher sensitivity to the activation of insulin signaling. In the presence of insulin, MEK inhibitors reduced injury induced by OGD in the CA1 sub-region. MAPK activation may contribute to the relative resistance of CA1 to insulin protection, as well as their relative vulnerability to ischemia, by antagonizing PI3K/Akt activation. Since OGD-induced injury in CA sub-regions was prevented by NMDA receptor antagonist in the presence of insulin, it is possible that CA sub-regions respond to insulin in a more complex manner involving direct activation of insulin receptor and indirect actions of insulin via synaptic activity-dependent stimulation of NMDA receptors (van der Heide et al. 2005; Moult and Harvey 2008), and activation of MAPK signaling (van der Heide et al. 2003; 2005; Hui et al. 2005). It is possible that PI3K and MAPK signaling play a role in the relative resistance/vulnerability of CA sub-regions to ischemia via modulation of synaptic plasticity (English and Sweatt 1997; van der Heide et al. 2005). Our data suggest that targeting multiple mechanisms involving NMDA receptor mediated excitotoxicity and disruption of basal survival/death homeostasis is critical for neuroprotection under ischema/reperfusion.
The hippocampus is critical for some forms of memory and spatial navigation, but previous research has mostly neglected the CA2, a unique region situated between CA3 and CA1. CA2 can be clearly delineated from its neighboring CA regions by expression patterns of cellular markers such as fibroblast growth factor 2 (Williams et al. 1996), Purkinje cell protein 4 (Zhao et al. 2001; Lein et al. 2005), vasopressin receptor (Young et al. 2006), and α-actinin 2 (Wyszynski et al. 1998). In addition, neurons in CA2 region are resistant to seizure generation (Sloviter, 1983), and hypoxia/ischemia (Sadowski et al. 1999), but vulnerable in schizophrenia (Benes et al. 1998). Thus, CA2 of hippocampus appears to be distinctively different from the other CA regions with respect to death and survival from injury. In the present study, we found that CA2 neurons respond to OGD and insulin differently from other CA regions. They are more resistant to glutamate, OGD, and appeared to be sensitive to the protective action of insulin after OGD insult. And yet, unlike DG neurons, they are insensitive to disruptions of PI3K/Akt signaling under basal conditions. There is a growing body of evidence to suggest that insulin can influence synaptic plasticity in the CNS. Insulin evokes long-term depression (LTD) of excitatory synaptic transmission via PI3K signaling in hippocampal slices (Man et al. 2000; van der Heide et al. 2005). It was shown that long-term potentiation (LTP) but not LTD is absent in CA2 neurons (Zhao et al. 2007). It is also possible that the sensitivity of CA2 neurons to insulin protection may be partly mediated by modulation of synaptic plasticity such as induction of LTD.
It is well established that there is a link between stroke and diabetes. In diabetic rat models infarct volumes are greater following middle cerebral artery occlusion as compared to non-diabetics (Rizk et al. 2005). Impaired insulin sensitivity is associated with stroke, independent of glucose level (Bravata et al. 2005). In rodent models of diabetes, compromised synaptic plasticity and learning are associated with decreased survival of neural progenitors and impaired neurogenesis (Zhang et al. 2008; Stranahan et al. 2008; Lang et al. 2009). On the other hand, diabetes and ischemic stroke can trigger endogenous neurotropic and protective activity, such as increasing the expression of IGF-1 and its receptors (Guan et al. 2001; Yan et al. 2006; Beilharz et al. 1998), and inducing neural progenitor proliferation in the dentate gyrus (Yan et al. 2006). IGF1 infusion significantly increases neural progenitor proliferation (Dempsey et al. 2003), and promotes survival of the newly formed cells (Lang et al. 2009). It is known that PI3K signaling promotes self-renewing properties of stem cells but inhibit differentiation, whereas MAPK signaling promotes differentiation (Qi et al. 2004; Hori et al. 2002). In our study, DG neurons are sensitive to alterations of PI3K/Akt signaling under normal and ischemic conditions. It is possible that the neurogenesis associated survival promoting signaling confers relative resistance of DG neurons to ischemia. Complex processes such as neuronal repair and remodeling after insult are mediated by the synergistic action of many factors. Selectively enhancing the survival promoting activity after the initial insult through the use of therapeutic agents may reduce brain injury and facilitate functional recovery after ischemic stroke.
Acknowledgements
This project was supported by NIH grant PO1HD032573 and the UCSD neuroscience microscopy shared facility grant P30NS047101. We wish to thank Orit Gavrialov, Shirley Reynolds and Jennifer Meerloo for their technical assistance.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz EJ, Russo VC, Butler G, Baker NL, Connor B, Sirimanne ES, Dragunow M, Werther GA, Gluckman PD, Williams CE, Scheepens A. Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic-ischemic injury. Brain Res. Mol. Brain Res. 1998;59:119–134. doi: 10.1016/s0169-328x(98)00122-3. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86:136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Wells CK, Kernan WN, Concato J, Brass LM, Gulanski BI. Association between impaired insulin sensitivity and stroke. Neuroepidemiology. 2005;25:69–74. doi: 10.1159/000086286. [DOI] [PubMed] [Google Scholar]
- Buchs PA, Stoppini L, Muller D. Structural modifications associated with synaptic development in area CA1 of rat hippocampal organotypic cultures. Developmetnal brain Res. 1993;71:81–91. doi: 10.1016/0165-3806(93)90108-m. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JE. Role of extracellular signal regulated kinase 5 in neuronal survival. Eur. J. Biochem. 2004;271:2056–2059. doi: 10.1111/j.1432-1033.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury-the dark side of ERK1/2. Eur. J. Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience. 2002;114:755–767. doi: 10.1016/s0306-4522(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J. Neurochem. 2003;87:586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase in hippocampal long-term potentiation. J. Biol. Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem. Biophys. Res. Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD. Insulin like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia-ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/s0306-4522(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Halevy O, Cantley LC. Differential regulation of the phosphoinositide 3-kinase and MAP kinase pathways by hepatocyte growth factor vs. insulin-like growth factor-I in myogenic cells. Exp. Cell Res. 2004;297:224–234. doi: 10.1016/j.yexcr.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur. J. Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Pei DS, Zhang QG, Guan QH, Zhang GY. The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res. 2005;1052:1–9. doi: 10.1016/j.brainres.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Vemuganti R, Dempsey RJ. Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells, role of Akt. Eur. J. Neurosci. 2007;25:1041–1048. doi: 10.1111/j.1460-9568.2007.05336.x. [DOI] [PubMed] [Google Scholar]
- Kirino T, Sano K. Changes in the contralateral dentate gyrus in Mongolian gerbils subjected to unilateral cerebral ischemia. Acta Neuropathol. 1980;50:121–129. doi: 10.1007/BF00692862. [DOI] [PubMed] [Google Scholar]
- Lang BT, Yan Y, Dempsey RJ, Vemuganti R. Impaired neurogenesis in adult type-2 diabetic rats. Brain Res. 2009;1258:25–33. doi: 10.1016/j.brainres.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee EO, Kang JL, Chong YH. Concomitant degradation of beta-catenin and GSK-3 beta potently contributes to glutamate-induced neurotoxicity in rat hippocampal slice cultures. J. Neurochem. 2008;106:1066–1077. doi: 10.1111/j.1471-4159.2008.05444.x. [DOI] [PubMed] [Google Scholar]
- Lein ES, Callaway EM, Albright TD, Gage FH. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J. Comp. Neurol. 2005;485:1–10. doi: 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- Martin DL. The role of glia in the inactivation of neurotransmitters. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford: Oxford University Press; 1995. pp. 732–745. [Google Scholar]
- Moult PR, Harvey J. Hormonal regulation of hippocampal dendritic morphology and synaptic plasticity. Cell Adh. Migr. 2008;2:269–275. doi: 10.4161/cam.2.4.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Park CW, Yoo KY, Hwang IK, Choi JH, Lee CH, Park OK, Cho JH, Lee YL, Shin HC, Won MH. Age-related changes in the insulin receptor beta in the Gerbil hippocampus. Neurochem Res. 2009 doi: 10.1007/s11064-009-0010-0. ahead of print. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog. Brain Res. 1988;63:29–37. doi: 10.1016/S0079-6123(08)61973-1. [DOI] [PubMed] [Google Scholar]
- Qi X, Li TG, Hao J, Hu J, Wang J, Simmons H, Miura S, Mishina Y, Zhao GQ. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. U S A. 2004;101:6027–6032. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res. 2005;1053:1–9. doi: 10.1016/j.brainres.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Runden E, Seglen PO, Haug FM, Ottersen OP, Wieloch T, Shamloo M, Laake JH. Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: evidence for a MAP kinase-dependent mechanism. J. Neurosci. 1998;18:7296–7305. doi: 10.1523/JNEUROSCI.18-18-07296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Wisniewski HM, Jakubowska-Sadowska K, Tarnawski M, Lazarewicz JW, Mossakowski MJ. Pattern of neuronal loss in the rat hippocampus following experimental cardiac arrest-induced ischemia. J. Neurol. Sci. 1999;168:13–20. doi: 10.1016/s0022-510x(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Sirén AL, Lewczuk P, Hasselblatt M, Dembowski C, Schilling L, Ehrenreich H. Endothelin B receptor deficiency augments neuronal damage upon exposure to hypoxia-ischemia in vivo. Brain Res. 2002;945:144–149. doi: 10.1016/s0006-8993(02)02911-6. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Slowik A, Rubio F, Szczudlik A, Gaffney J. Activation of MAP kinase (ERK-1/ERK-2), tyrosine kinase and VEGF in the human brain following acute ischaemic stroke. NeuroReport. 2000;11:2759–2764. doi: 10.1097/00001756-200008210-00030. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. “Epileptic” brain damage in rats induced by sustained electrical stimulation of the perforant path. I. Acute electrophysiological and light microscopic studies. Brain Res. Bull. 1983;10:675–697. doi: 10.1016/0361-9230(83)90037-0. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J. Alzheimer’s Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Müller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol. Cell. Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Hoekman MF, Biessels GJ, Gispen WH. Insulin inhibits extracellular regulated kinase 1/2 phosphorylation in a phosphatidylinositol 3-kinase (PI3) kinase-dependent manner in Neuro2a cells. J. Neurochem. 2003;86:86–91. doi: 10.1046/j.1471-4159.2003.01828.x. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Wang HD, Fukuda T, Suzuki T, Hashimoto K, Liou SY, Momoi T, Kosaka T, Yamamoto K, Nakanishi H. Differential effects of Bcl-2 overexpression on hippocampal CA1 neurons and dentate granule cells following hypoxic ischemia in adult mice. J. Neurosci. Res. 1999;57:1–12. doi: 10.1002/(SICI)1097-4547(19990701)57:1<1::AID-JNR1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Williams TE, Meshul CK, Cherry NJ, Tiffany NM, Eckenstein FP, Woodward WR. Characterization and distribution of basic fibroblast growth factor-containing cells in the rat hippocampus. J. Comp. Neurol. 1996;370:147–158. doi: 10.1002/(SICI)1096-9861(19960624)370:2<147::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kharazia V, Shanghvi R, Rao A, Beggs AH, Craig AM, Weinberg R, Sheng M. Differential regional expression and ultrastructural localization of alpha-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J. Neurosci. 1998;18:1383–1392. doi: 10.1523/JNEUROSCI.18-04-01383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur. J. Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Yao H, Sun X, Gu X, Wang J, Haddad GG. Cell death in an ischemic infarct rim model. J. Neurochem. 2007;103:1644–1653. doi: 10.1111/j.1471-4159.2007.04879.x. [DOI] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner A. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tan YF, Yue JT, Vranic M, Wojtowicz JM. Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol. Scand. 2008;117:205–210. doi: 10.1111/j.1600-0404.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Choi YS, Obrietan K, Dudek SM. Synaptic plasticity (and lack thereof) in hippocampal CA2 neurons. J. Neurosci. 2007;27:12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J. Biol. Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J. Comp. Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]