Abstract
Rationale
Mechanisms of angiogenesis in skeletal muscle remain poorly understood. Efforts to induce physiological angiogenesis hold promise for the treatment of diabetic microvascular disease and Peripheral Artery Disease (PAD), but are hindered by the complexity of physiological angiogenesis and by the poor angiogenic response of aged and diabetic patients. To date, the best therapy for diabetic vascular disease remains exercise, often a challenging option for patients with leg pain. PGC-1α, a powerful regulator of metabolism, mediates exercise-induced angiogenesis in skeletal muscle.
Objective
To test if, and how, PGC-1α can induce functional angiogenesis in adult skeletal muscle.
Methods and Results
We show here that muscle PGC-1α robustly induces functional angiogenesis in adult, aged, and diabetic mice. The process involves the orchestration of numerous cell types, and leads to patent, non-leaky, properly organized, and functional nascent vessels. These findings contrast sharply with the disorganized vasculature elicited by induction of VEGF alone. Bioinformatic analyses revealed that PGC-1α induces the secretion of secreted phosphoprotein 1 (SPP1), and the recruitment of macrophages. SPP1 stimulates macrophages to secrete monocyte chemoattractant protein-1 (MCP-1), which then activates adjacent endothelial cells, pericytes, and smooth muscle cells. In contrast, induction of PGC-1α in SPP1 −/− mice leads to immature capillarization and blunted arteriolarization. Finally, adenoviral delivery of PGC-1α into skeletal muscle of either young or old and diabetic mice improved the recovery of blood flow in the murine hind-limb ischemia model of PAD.
Conclusions
PGC-1α drives functional angiogenesis in skeletal muscle and likely recapitulates the complex physiological angiogenesis elicited by exercise.
Keywords: Angiogenesis, secreted phosphoprotein 1, PGC-1, macrophages, skeletal muscle, peripheral artery disease, gene regulation, gene therapy, growth factors and cytokines
INTRODUCTION
Exercise is a potent stimulus for angiogenesis in adult skeletal muscle, and is one of the few instances of non-pathological angiogenesis that occurs in mammals post development1, 2. However, how exercise orchestrates the complex process of physiological angiogenesis in skeletal muscle remains poorly understood. One prevailing notion has been that the metabolic needs of exercising muscle lead to local hypoxia, activation of the transcription factor Hypoxia Inducible Factor-1α (HIF-1α), and induction of VEGF and angiogenesis1–4. However, hypoxia has been difficult to show in muscles undergoing endurance exercise, a potent stimulus for angiogenesis. Moreover, deletion of HIF-1α increases, rather than decreases, capillary density in skeletal muscle5.
The transcriptional coactivator peroxisome Proliferation Activator Receptor-γ (PPAR-γ) coactivator -1α (PGC-1α) is a powerful regulator of mitochondria and metabolism in multiple tissues6, 7. In skeletal muscle, PGC-1α orchestrates the induction of hundreds of genes involved in mitochondrial biology, including components of the electron transport chain and beta oxidation. Transgenic animals over-expressing PGC-1α in skeletal myocytes have markedly more mitochondria, and as a result have improved endurance running capacity8, 9. We recently showed that PGC-1α also regulates VEGF and other angiogenic factors. PGC-1α induces VEGF in a HIF-independent fashion by coactivating ERR-α on a novel enhancer in the first intron of the VEGF gene10. We also recently showed that PGC-1α is required for exercise-induced angiogenesis11. Together, these observations suggest that PGC-1α likely orchestrates physiological angiogenesis in skeletal muscle. Indeed, transgenic induction of PGC-1α in myocytes beginning pre-natally (via the MCK promoter) leads to dramatic induction of capillary density10. It is unclear, however, if this can be recapitulated outside the plastic environment of in utero development, and especially in older and diabetic contexts where endothelial dysfunction is prominent. The cellular and molecular mechanisms by which PGC-1α orchestrates angiogenesis are also not known.
We show here, using an inducible transgenic model, that PGC-1α robustly induces angiogenesis in adult, aged, and diabetic mice. The vessels are abundant and functional, likely recapitulating physiological angiogenesis. Mechanistically, we uncover a novel role for macrophages, and the secreted factors secreted phosphoprotein 1 (SPP1) (also known as osteopontin) and monocyte chemoattractant protein-1 (MCP-1), not previously known to be involved in physiological angiogenesis. Finally, we show that adenoviral delivery of PGC-1α to skeletal muscle accelerates recovery from limb ischemia in mice.
METHODS
Animals
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee. MCK-TTA and TRE-PGC-1α-inducible mice12 were obtained from Dr. Daniel Kelly. sVEGFR1 mice were kindly provided by Dr. Eli Keshet, Jerusalem, Israel13. SPP1 −/− mice were purchased from Jackson Labs. TRE-VEGFA mice were generated by homologous recombination at the HPRT locus. All transgenic animals were maintained hemizygous on a mixed C57Bl/6 and 129 strain unless otherwise stated. Full details are provided in the Online Supplement.
Cells and reagents
Human umbilical cord endothelial cells (HUVECs), 10T1/2, THP-1 and C2C12 cells were maintained using standard growth media conditions. Primary skeletal myocytes, pericytes and smooth muscle cells were isolated, cultured and differentiated from hindlimbs of as described previously14. Full details are provided in the Online Supplement on culture conditions, conditioned media preparation, transwell migration assays viral infections and reagent procurement including antibodies and ELISAs.
Real-time PCR and microarrays
Total RNA was isolated from mouse tissue and cultured cells using the TRIZOL (Invitrogen) and Turbocapture (Qiagen) method, respectively and subjected to reverse transcription and relative expression levels determined. For microarrays, RNA was probed with Affymetrix mouse 1.0 gene arrays data obtained was analyzed using the Gene Set Enrichment Analysis (Broad Institute of MIT and Harvard). Please see Online Supplement for full details.
Measurement of intravascular volume
Intravascular volume was measured by injecting 125I-BSA intravenously into wild type and PGC-1α transgenic mice after 4 weeks of transgene induction. The tracer was allowed to circulate for 5 minutes and then the amount of radioactivity in the muscle was measured in a gamma counter15.
Animal surgeries
Unless otherwise specified animals were anesthetized with ketamine-xylazine prior to all surgical procedures. Vascular leak was determined by measurement of Evans’s blue leak as previously described16. Hind limb ischemia surgeries were performed, measured and scored as previously described17. Refer to Online Supplement for specific details on all surgical procedures.
Histological analysis
Quantification of capillaries was performed computationally. Please refer to Online Supplement for detailed protocol.
Statistical analysis
The data are presented as means ± SE. Statistical analysis was performed with Student's t-test for all in vitro and in vivo experiments. P-values of <0.05 were considered statistically significant.
RESULTS
PGC-1α induces robust angiogenesis in adult skeletal muscle
We have shown before that constitutive expression of PGC-1α, beginning in utero, increases vascular density in skeletal muscle10. To test if PGC-1α could induce angiogenesis in adult skeletal muscle, we used a previously described double transgenic mouse model12. TRE-PGC-1α “transponder” mice, in which PGC-1α expression can be induced by the tetracycline-sensitive transactivator (tTA), were crossed with “inducer” mice that express the “tet-off” tTA under the control of the muscle-specific MCK (muscle creatine kinase) promoter. The resulting double transgenic animals express PGC-1α in a muscle specific manner, and only in the absence of doxycycline in the chow (Figure 1A). Transgenic expression of PGC1α in these mice begins approximately 1 week after removal of doxycycline (doxy), shortly after which the mice display increased mitochondrial biogenesis and fatty acid oxidation in skeletal muscle12.
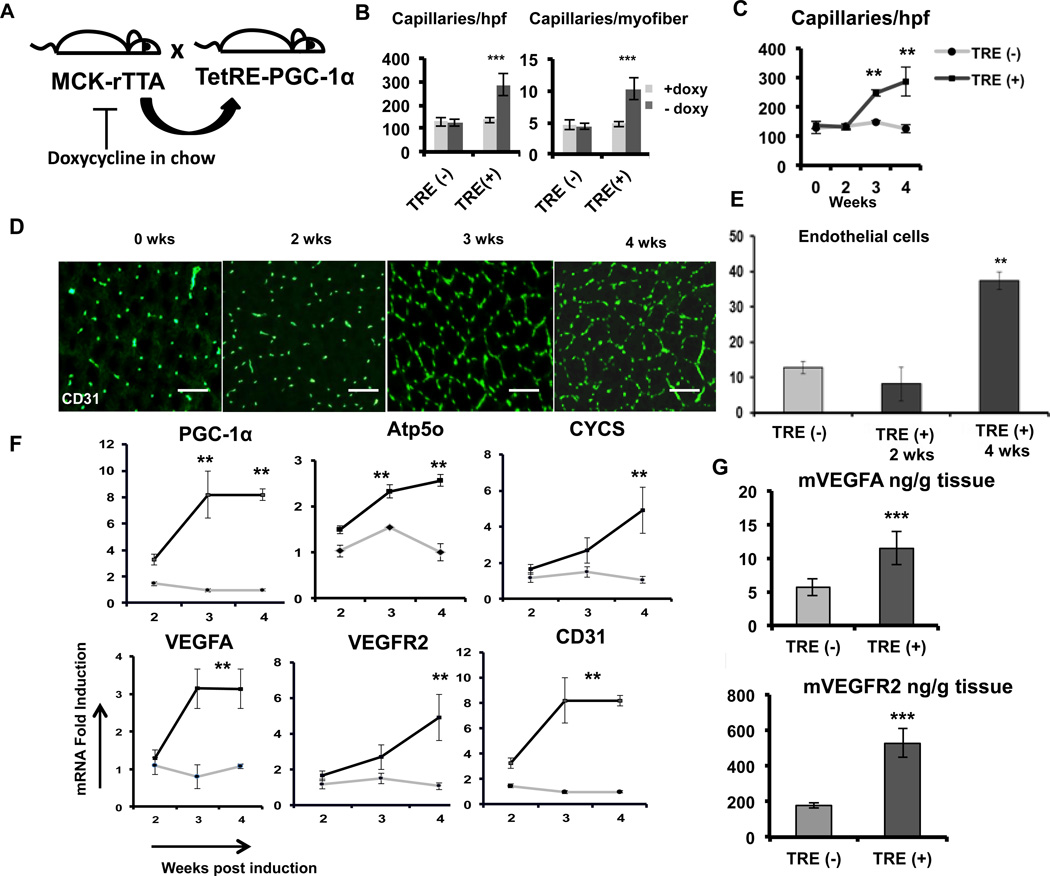
Figure 1. PGC-1α induces robust angiogenesis in adult skeletal muscle.
A, Generation of the PGC-1α-expressing double transgenic TRE (+) mice: Mice expressing the TTA transactivator in muscle (MCK-rTTA) were crossed with the TRE-PGC-1α ‘transponder’ mice to generate double transgenic TRE (+) mice. MCK-rTTA single transgenic mice (TRE (−) mice) were used as controls. B, Quantification of capillary density per high power field (hpf) and per myofiber in transverse sections of the single transgenic TRE (−) and double transgenic TRE (+) mice with (Grey) or 4 weeks without doxy (black) chow. C, Capillary density in the quadriceps muscle of TRE (−) and TRE (+) mice at 1week intervals after doxy removal. D, Representative CD31-stained transverse sections of TRE (+) mice after 0, 2, 3 and 4 weeks of doxy removal. E, Flow cytometry quantification of CD31 positive endothelial cells in the quadriceps muscle from TRE (−) and TRE (+) mice. F, Expression of mitochondrial and angiogenic genes in quadriceps muscle at the indicated times after induction of PGC-1α. G–H, VEGF (G) and VEGFR2 (H) ELISA of quadriceps muscle lysate after 3 weeks of PGC-1α induction. N>4 for each group, *P<0.05, **P<0.01, ***P<0.001. scale bar = 100µm. Error Bars indicate SE.
Doxy-chow was replaced with normal chow at 10–12 weeks of age. At 1-week intervals thereafter, groups of mice were sacrificed, quadriceps muscles were harvested, and transverse frozen sections were cut and evaluated by immuno-histochemical staining for CD31 (platelet-endothelial cell adhesion molecule -1 i.e. PECAM-1), an endothelial cell enriched marker. As shown in Figure 1B, marked increases in angiogenesis were noted in the double transgenic mice (TRE (+)), but not in single transgenic mice (TRE (−)) or double transgenic mice maintained on doxy chow. Rebound from potential doxycycline-mediated inhibition of angiogenesis18 is not likely, because the single transgenic mice, which were switched to normal chow, did not induce angiogenesis. Capillary density in the induced animals more than doubled from 100 capillaries per high-power field (caps/hpf) to 250 caps/hpf (Figure 1B). The number of capillaries surrounding each fiber increased from 5 to 10 (Figure 1B), consistent with the absence of significant change in fiber size after transgene induction12. Similar increases in capillary density were seen in tibialis anterior and gastrocnemius, and the increase in capillary density was also detectable by staining with CD144 (VE-Cadherin) (Supplemental Figure I A–D).
We next tested the time course of angiogenesis induction at 0, 2, 3 and 4 weeks after doxy removal. As shown in Figure 1C and D, capillary density was induced as early as 3 week after doxy removal. Flow cytometry analysis of cells enzymatically removed from quadriceps revealed a near quadrupling of CD31+ endothelial cells at 4 weeks (Figure 1E), indicating that the marked increase in CD31 staining seen in stained transverse sections reflects increases in endothelial cell number, rather than size or projections. Real time qPCR revealed 2–3 fold increases in the expression of mitochondrial genes (ATP5o and CYCS) after 3 weeks of induction, as shown previously12. qPCR analysis of angiogenic genes revealed 3-fold increase in VEGFA and VEGFR2 expression at 3 and 4 weeks, and a 8-fold increase in CD31 mRNA expression (Figure 1F). Consistent with these findings, levels of VEGFA and VEGFR2 protein were induced 2.5-fold at 3 weeks, as measured by ELISAs with protein extracts from whole muscle (quadriceps) (Figure 1G and H). Together, these results demonstrate that PGC-1α can robustly induce angiogenesis in adult skeletal muscle, even after the increased plasticity present during the developmental period.
PGC-1α-mediated angiogenesis in skeletal muscle is VEGFA dependent
Endothelial migration is a hallmark of angiogenesis. We therefore tested if PGC-1α expression in myocytes could stimulate the migration of adjacent endothelial cells. C2C12 cells were made to differentiate into myotubes in the bottom wells of modified Boyden chambers (Transwell system) and human umbilical vein endothelial cells (HUVECs) were seeded into the top chambers. As shown in Supplemental Figure II A, infection of C2C12s with adeno-PGC-1α markedly stimulated the migration of the endothelial cells. VEGFA is crucial for many, but not all, forms of angiogenesis19, 20, and we have shown previously that PGC-1 regulates VEGFA expression10. To test if the migration of endothelial cells was dependent on VEGF secretion, the conditioned medium in the above co-culture experiment was supplemented with soluble VEGF receptor 1 (sFlt1-soluble fms-like tyrosine kinase 1), which binds to and inhibits VEGF family members. As shown in Supplemental Figure II B, blocking of VEGF led to complete inhibition of HUVEC migration.
To test if VEGF is required for PGC-1α induced angiogenesis in intact animals, we next used transgenic mice that express human sFlt1 fused to an IgG1 Fc fragment13, under control of a tTA-responsive promoter (a kind gift of Eli Keshet). These mice were crossed with the PGC-1α-inducible transgenic mice, yielding triple transgenic mice in which removal of doxy leads to the simultaneous induction of PGC-1α and sFlt1 in skeletal muscle (Supplemental Figure II C). Groups of 12-week old triple transgenic mice, and controls, were switched from doxy chow to normal chow, and the capillary density was evaluated in quadriceps 4 weeks after induction. As shown in Supplemental Figure II D–F, sFlt1 expression completely inhibited the increase in capillary density mediated by PGC-1α. The induction of angiogenesis by PGC-1α in skeletal muscle thus requires VEGFA in intact animals.
PGC-1α can induce angiogenesis in aged and diabetic animals
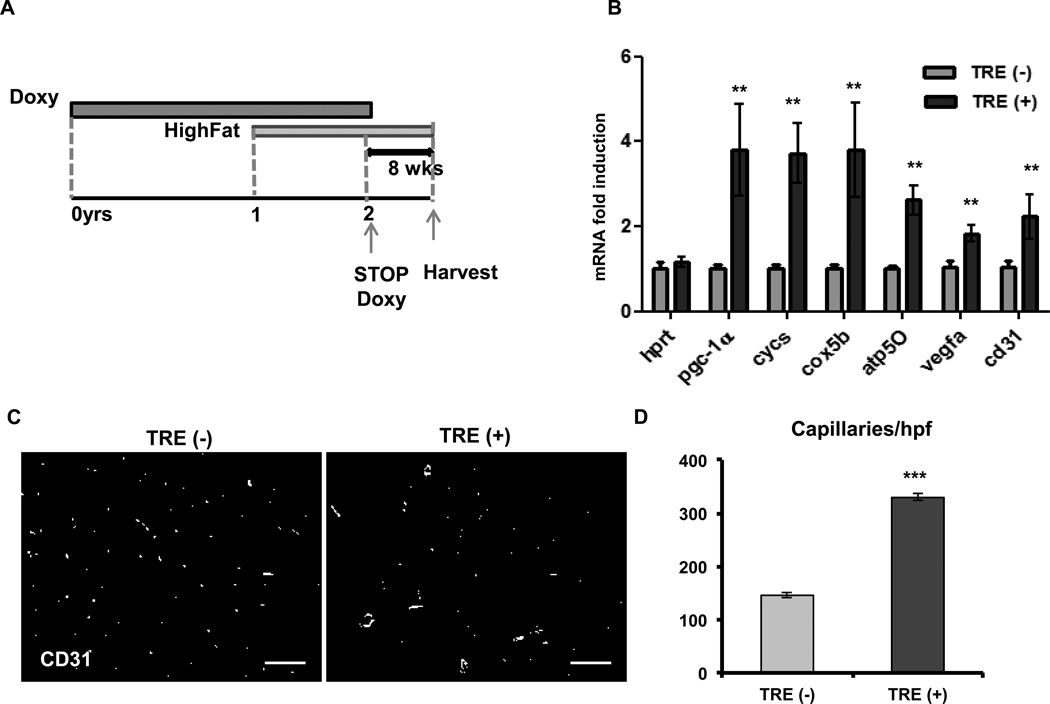
To test whether PGC-1α can induce angiogenesis in old and diabetic mice, we maintained PGC-1α double transgenic mice on a 45% high fat diet (containing doxycycline to maintain the transgene inhibited) starting at the age of 12 months. 12 months later, at 2 years of age, doxycycline was removed from the diet for 8 weeks to allow induction of PGC-1α (Figure 2A). Glucose tolerance tests (GTT) confirmed that the mice had become diabetic (Supplemental Figure III A and B). There was no difference in the body weights of the wild type and transgenic mice. After 8 weeks of PGC-1α induction, there was a 4-fold increase in PGC-1α expression (Figure 2B) and a 2-fold increase in VEGFA and CD31 in muscle (Figure 2B). Transverse sections of the gastrocnemius stained for CD31 revealed a 2-fold increase in capillary density in the PGC-1α-induced old diabetic animals, from 120 caps/hpf in the TRE (−) animals to 325 caps/hpf in the TRE (+) double transgenics (Figure 2C and D). Similar results were obtained from 2-year old transgenic non-diabetic animals maintained on a regular chow diet (Supplemental Figure III C–E). These data thus demonstrate that aged and diabetic muscle beds remain exquisitely responsive to angiogenic stimulation by PGC-1α.
Figure 2. PGC-1α can induce angiogenesis in old diabetic animals.
A, Experimental model to test PGC-1α induced angiogenesis in old diabetic mice. B, Expression of mitochondrial and angiogenic genes in the quadriceps muscle after 8 weeks of doxy removal. C, Capillary density in the gastrocnemius muscle. D, Quantification of capillary number per high power field. Error Bars are SE. N=4/group, * significance with P<0.05, **P<0.01, ***P<0.001. scale bar = 100µm.
PGC-1α induces the formation of patent, functional, non-leaky blood vessels
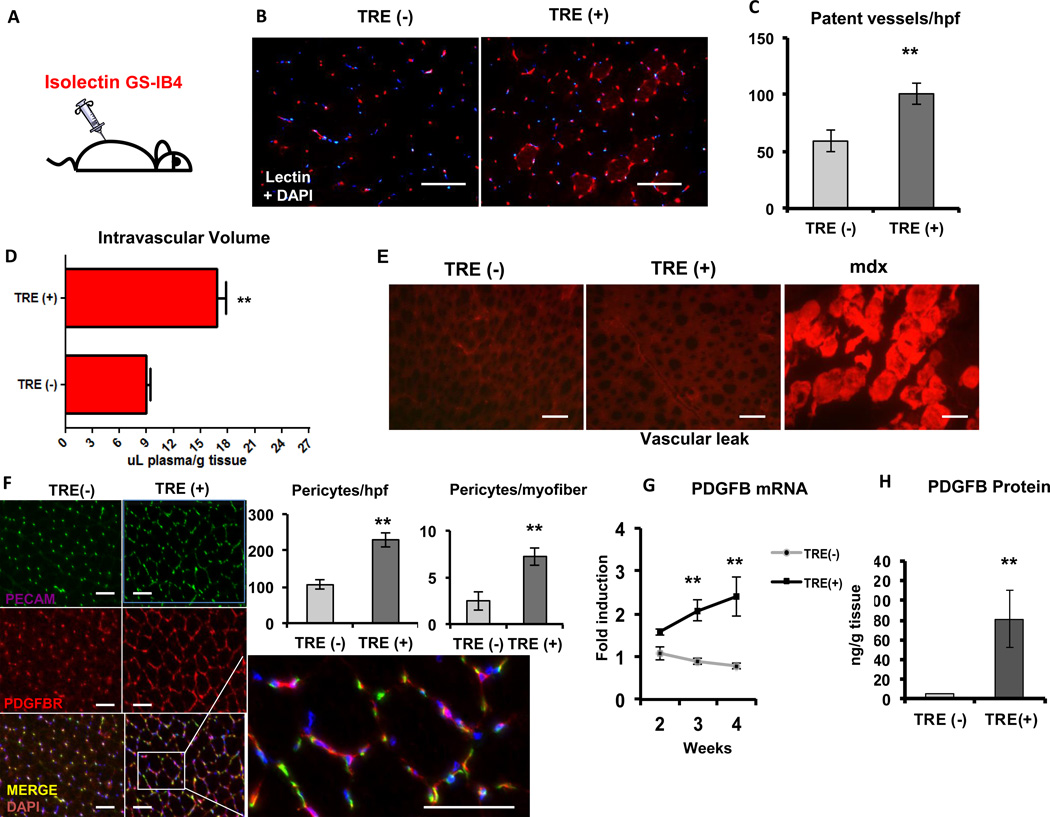
We next sought to test the functional integrity of the vessels induced by PGC-1α. A critical parameter of functional vessels is patency. To gauge the blood vessel patency, intravascular injections of isolectin GS-IB4 were used. GS-IB4 lectin avidly binds N-glucosamine oligomers on the luminal side of the endothelium, but does not escape the vessel lumen, thus staining only patent vessels21. Mice were injected intravenously and euthanized 15 min later and the muscles were harvested and sectioned. Fluorescent imaging showed a 2-fold increase in the number of patent vessels per high power field in the induced TRE (+) mice when compared to the TRE (−) mice (Figure 3A–C).
Figure 3. PGC-1α-induced blood vessels are functional.
A, Isolectin GS-IB4 was injected intravenously into wild type and PGC-1α 12–16 week-old transgenic mice after 4 weeks of doxy removal. B, Visualization of intravascular isolectinin the gastrocnemius muscle after 4 weeks of doxy removal. C, Quantification of lectin-stained patent capillaries per hpf. D, Measurement of intravascular volume in the quadriceps muscle of wild type and PGC-1α transgenics 5 min after injection of 125I-BSA. E, Vascular leak in the quadriceps muscle of TRE (−), TRE (+) and mdx mice. F, Capillary and pericyte density in the gastrocnemius muscle after 3 weeks of PGC-1α expression. Graphs show quantification of pericytes/hpf and /myofiber. G, PDGFB gene expression after 0, 2, 3 and 4 weeks of doxy removal. H, PDGFB protein expression after 3 weeks of PGC-1α expression. Error bars are SE. N=3–4/group, * significance with P<0.05, **P<0.01, ***P<0.001. scale bar = 100µm.
Another measure of vessel patency is the amount of intravascular volume contained in a muscle bed. To measure this we used intravenous injections of 125I-labelled albumin, which remains intravascular and can be quantified. Mice placed on a doxycycline-free diet for 4 weeks were injected with 125I labelled albumin, and 5 minutes later quadriceps and tibialis anterior muscles were removed, and the radioactive content was measured by gamma counting. As shown in Figure 3D, the PGC-1α-expressing double transgenic mice (TRE (+)) revealed a 2-fold increase in intravascular volume in muscle beds when compared to the single transgenic TRE (−) mice. This strong increase in intravascular volume is consistent with the increase in capillary content seen by immunostaining (Figure1B–D and Supplemental Figure I D) and isolectin staining (Figure 3B–C). The blood vessels induced by PGC-1α are thus patent and capable of sustaining blood flow.
One critical parameter of vessel integrity is the impermeability to macromolecules. To test this, we used injections of Evans Blue (EB) dye, a macromolecule that normally remains intravascular but easily extravasates in the presence of aberrantly permeable vessels22. A mouse model of muscle dystrophy (mdx mouse23), in which EB is well known to extravasate, was used as a positive control. Double transgenic mice that had been induced for 4 weeks, and controls, were injected with EB intraperitoneally, and muscles were harvested after 16 hrs. Fluorescent microscopy showed that blood vessels induced by PGC-1α did not leak EB, despite the dramatic increase in vessel density. In contrast, as a positive control, mdx mice had strong EB staining (Figure 3E). PGC-1α-induced blood vessels are thus not leaky, and thus differ from those in most tumor vessels, or vessels induced by VEGFA injection alone into muscle24–26.
In sharp contrast to the above findings, induction of VEGFA alone, achieved by similar skeletal muscle specific double-transgenic expression, led to induction of dramatically disorganized neovascularization, with the appearance of large sinusoid formations (Supplemental Figure IV A), and a dramatic increase in capillary leak (Supplemental Figure IV B–C). Induction of VEGFA is thus necessary (Supplemental Figure II) but not sufficient for complete PGC-1α-mediated angiogenesis.
Vessel maturation, including the establishment of patency and impermeability, also involves the recruitment of perivascular cells such as pericytes, and the formation of new arterioles via recruitment of smooth muscle cells. Immuno-staining of transverse sections of quadriceps muscle with antibodies against PDGFRB (platelet derived growth factor receptor-β), a pericyte-enriched marker, showed a >2-fold increase in the number of pericytes per high power field in the TRE+ mice when compared to the TRE (−) mice after three weeks of induction of PGC-1α (Figure 3F). Higher order vessels (arterioles) were also increased in number in TRE (+) mice, as measured by SMA staining (Supplemental Figure V A–B). The formation of larger arteries, however, did not appear to be induced, as evaluated by microCT in either the inducible model (Supplemental Figure V C–D) or the constitutive model of PGC-1α muscle expression (Supplemental Figure V E–F). Pericytes are known to migrate to angiogenic areas in response to the ligand PDGFBB27, 28. Real time qPCR analysis of the RNA from quadriceps muscle at 0, 2, 3 and 4 weeks of PGC-1α expression showed that the TRE (+) mice had up to a 2-fold increase in PDGFB gene expression when compared to the TRE (−) mice (Figure 3G). Adenoviral delivery of PGC-1α to primary myotubes also induced PDGFB dramatically, and this required the presence of the transcription factor ERRα (Supplemental Figure V G). PDGFBB protein levels were elevated 30-fold in TRE (+) mice when compared to the TRE (−) mice (Figure 3H). Together, these data demonstrate that PGC-1α orchestrates the formation of patent, non-leaky, and functional, pericyte-covered blood vessels in adult skeletal muscle. Interestingly, the large increase in capillaries and arterioles had no impact on systemic blood pressure, systemic arterial resistance, cardiac output, or Frank Starling relationships, as evaluated by invasive hemodynamic measurements (Supplemental Figure VI A–B).
Induction of PGC-1α recruits macrophages to skeletal muscle
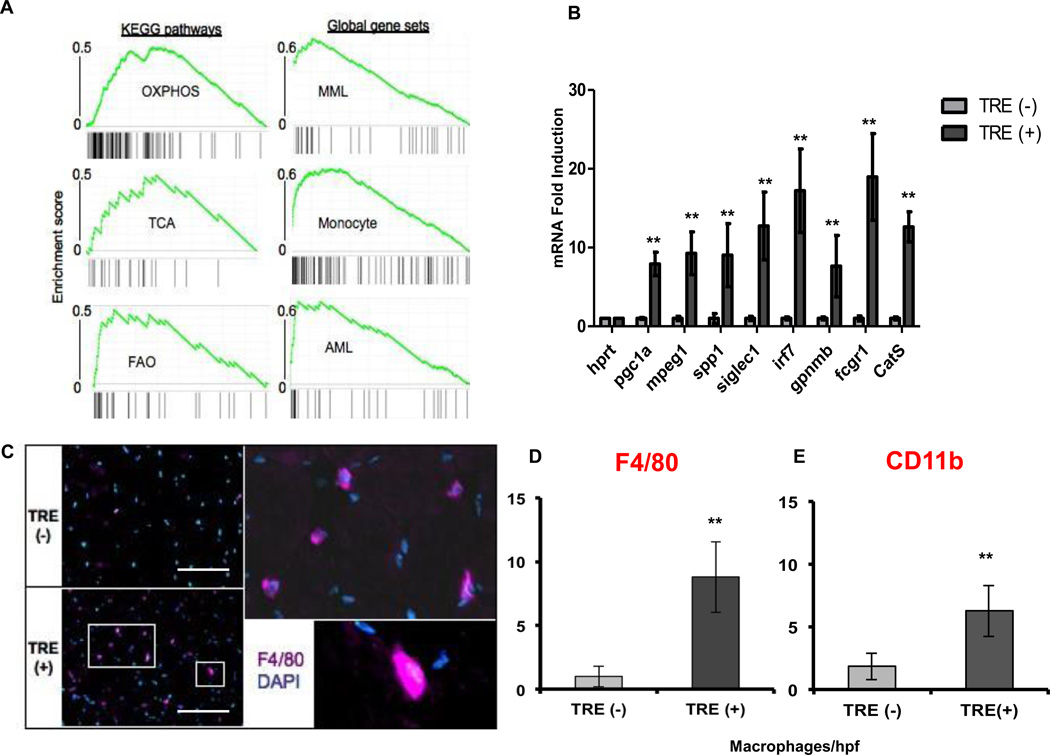
To gain understanding of the genes and pathways activated by PGC-1α during the angiogenesis process, we conducted an expression microarray analysis using RNA from muscles of the PGC-1α transgenic and control mice after 4 weeks of induction. Gene set enrichment analysis (GSEA) revealed a strong activation of oxidative phosphorylation, TCA cycle and fatty acid oxidation programs in the PGC-1α-expressing TRE (+) mice (Figure 4A), as expected12. Surprisingly, the gene sets most strongly induced by PGC-1α were those of myeloid cells (Figure 4A). Indeed, of the 30 most-induced genes on the microarray, 14 were genes known to be expressed strongly in macrophages (Supplemental Table I). Real time qPCR analysis of RNA from the quadriceps muscle confirmed these findings (Figure 4B). These observations suggested that induction of PGC-1α in skeletal muscle leads to infiltration of macrophages. To test this, sections from quadriceps of transgenic animals were immunostained with antibodies to the monocyte-specific marker CD11b and the macrophage-specific marker F4/80. This revealed a dramatic 4-fold increase in the number of monocytes/macrophages, from 2 to 8 per high power field, in the double transgenic TRE (+) mice when compared to the TRE (−) single transgenics (Figure 4C–E). Thus, surprisingly, the induction of PGC-1α in skeletal muscle cells leads to a strong recruitment of macrophages, despite the absence of any overt damage, or of any biomechanical or ischemic stress.
Figure 4. PGC-1α recruits macrophages to adult skeletal muscle.
A, Gene Set Enrichment Analysis of microarray data from gastrocnemius muscle of TRE (−) and TRE (+) mice after 4 weeks of doxy removal. B, Real Time qPCR validation of macrophage specific genes induced in the microarray. C, F4/80 positive macrophage density in gastrocnemius muscle of TRE (−) and TRE (+) mice. D, Quantification of F4/80 positive macrophages. E, Quantification of CD11b positive cells. Error bars are SE. N>4/group, *P<0.05, **P<0.01, ***P<0.001. scale bar = 100µm.
Grunewald and colleagues have reported that VEGF-activated endothelial cells can recruit monocytes and macrophages during the process of angiogenesis29. To test if endothelial activation and angiogenesis was required for the PGC-1α-induced recruitment of macrophages, we used the transgenic mice that express human sFlt1 fused to an IgG1 Fc fragment described above, crossed with the PGC-1α-inducible transgenic mice. As shown in Supplemental Figure VII A–B, sFlt1 expression had no impact on the recruitment of macrophages, even though it completely inhibited the increase in capillary density seen in the inducible PGC-1α transgenics (Supplemental Figure II). The recruitment of macrophages by PGC-1α thus occurs independently of angiogenesis or signaling by VEGF family members.
Myocytes expressing PGC-1 secrete SPP1 to regulate macrophage activation
The findings above suggested that PGC-1α causes the secretion from myocytes themselves of factors that can affect macrophage function. To test this idea, conditioned medium (CM) was harvested from differentiated C2C12 myotubes over-expressing PGC-1α and then placed on peripheral blood monocytes/macrophages. Within 24 hours, striking changes were seen in the morphology of the macrophages treated with PGC-1α CM when compared to control CM (Figure 5A). The morphological changes, including the appearance of pseudopodia, suggested that macrophages were activated by the PGC-1α CM. Consistent with this notion, macrophages treated with PGC-1α CM showed a marked elevation in the expression of macrophage activation genes (Figure 5B).
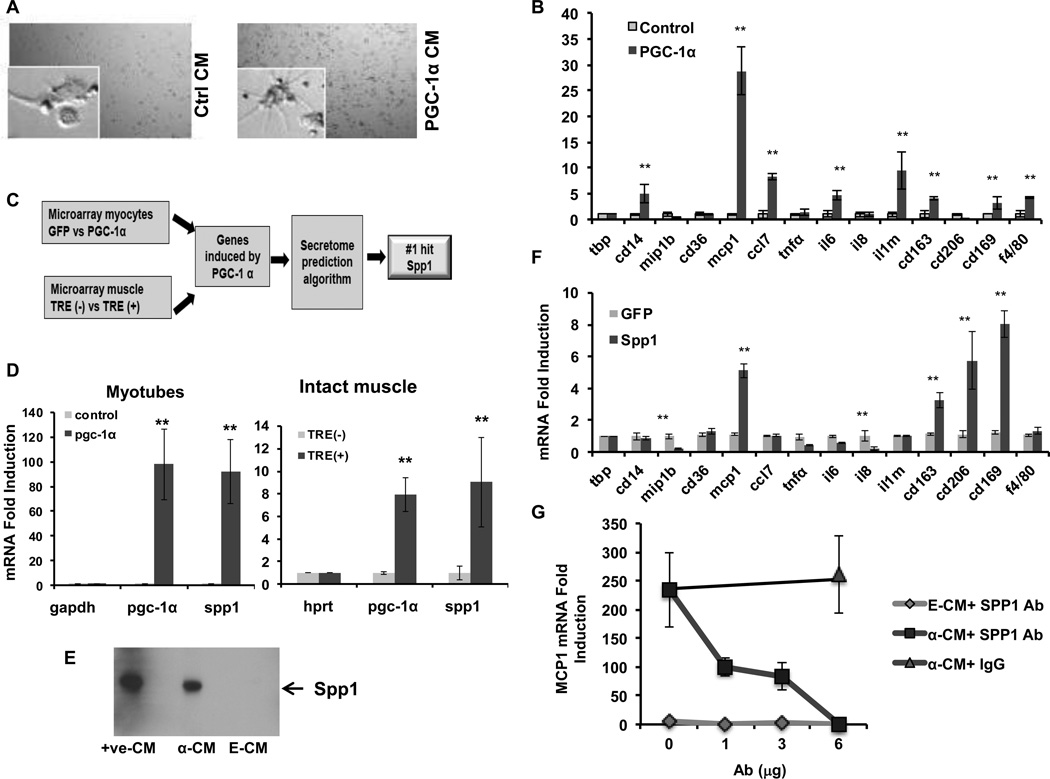
Figure 5. PGC-1α induces the secretion of SPP1 to regulate macrophage activation.
A, Conditioned medium from myotubes overexpressing PGC-1α (PGC-1α CM) dramatically changes the morphology of macrophages. B, Expression of macrophage activation genes in THP1 cells treated with control and PGC-1α CM. C, Bioinformatic analyses of PGC-1α-expressing myotyubes and muscle yield SPP1 as a lead candidate secreted factor that could impact macrophages. D, SPP1 expression in myotubes infected with control or PGC-1α retrovirus (left) and in gastrocnemius muscle from TRE (−) and TRE (+) mice. E, Western blot of SPP1protein present in control and PGC-1α CM. +ve-CM: CM from 293 cells overexpressing SPP1. F, Expression of macrophage activation genes in THP1 cells treated with control and SPP1 CM. G, MCP-1 gene expression after addition of SPP1 neutralizing antibody to control or PGC-1α CM. Error bars are SE. N=3/group., *P<0.05, **P<0.01, ***P<0.001.
To begin to identify key factors secreted by muscle cells expressing PGC-1α, we used bioinformatic approaches to generate a list of candidate proteins induced and secreted by PGC-1α. RNA from myotubes and from intact muscles overexpressing PGC-1α were probed with Affymetrix arrays, and genes induced by PGC-1α in both conditions were subjected to publicly available algorithms to predict the subset of genes that are likely secreted (SignalP 3.0) (Figure 5C). The most dramatically induced gene on this list was SPP1, a protein known to modulate monocyte/macrophage biology30, 31. Increased expression of SPP1 gene was noted in both myotubes (80-fold) and intact muscle (9-fold) overexpressing PGC-1α (Figure 5D). SPP1 protein was abundantly detectable in PGC-1α CM, compared to control CM (Figure 5E). PGC-1α thus strongly induces the secretion of SPP1 from myocytes.
Treatment of macrophages with SPP1 led to the induction of a subset of the same genes that were induced by CM from myotubes expressing PGC-1α (Figure 5F, compared to 5B), including MCP-1 (6-fold), CD163 (2-fold) and CD169 (4-fold). These data suggested that SPP1 mediates some of the effects of the PGC-1α-CM on macrophages. To test this directly, we treated the PGC-1α CM with SPP1 neutralizing antibody before adding the CM to the THP-1 cells, revealing a dose dependent decrease in MCP-1 gene expression, with a complete block upon addition of 6 ug of SPP1 antibody, but not control IgG (Figure 5G). Together, these results show that PGC-1α induces SPP1 secretion from myotubes, which in turn activates macrophages to express MCP-1 and other markers of activation.
Conditioned macrophages secrete MCP-1 to recruit vascular cells
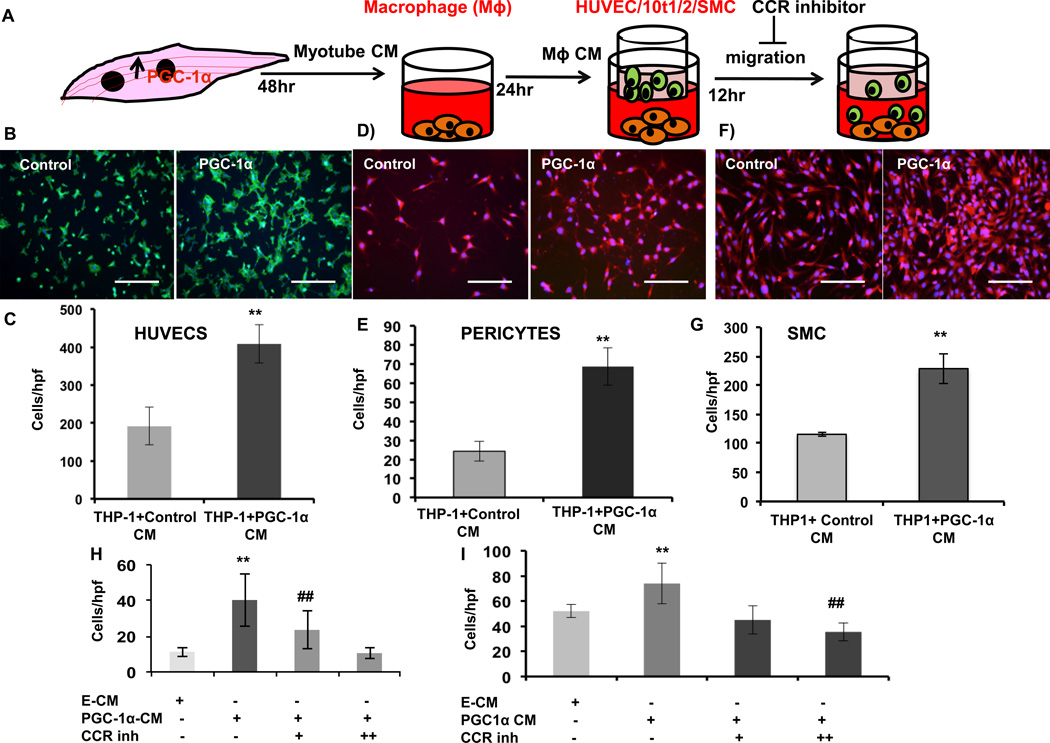
The pronounced activation of macrophages by PGC-1α-expressing myocytes suggested that the macrophages may assist the orchestration of PGC-1α induced angiogenesis. We first investigated this notion by testing if macrophages that had been “educated” by PGC-1α expressing myocytes would, in turn, affect the function of vascular cells. THP-1 monocytes were first plated into bottom chambers of Transwells and treated with phorbol myristate acetate (PMA) to induce differentiation into macrophages. The cells were then treated with conditioned medium from myotubes overexpressing PGC-1α, versus control myotubes. 24 hours later, the medium was removed and replaced with fresh medium, thus removing the myotube CM and allowing the THP-1 macrophages to condition the fresh medium (Figure 6A). HUVECs were then seeded into the top chamber, and migrated cells were counted 12 hours later. As shown in Figure 6B–C, macrophages that had been “educated” by CM from PGC-α-expressing myotubes dramatically increased their ability to stimulate adjacent endothelial cells to migrate. Similar experiments with the differentiated 10 T1/2 model of pericytes32, 33 (Figure 6D–E) and smooth muscle cells (Figure 6F–G) yielded similarly strong 2- to 3-fold increases in cell migration. These results thus indicate that PGC-1α expression in myotubes has a strong, indirect, effect on numerous vascular cells, via activation of macrophages.
Figure 6. Conditioned macrophages secrete MCP-1 to recruit vascular cells.
A, Experimental design for migration assay with HUVECs and 10 t1/2 (pericytes). B, Migration of Human umbilical vein endothelial cells (HUVECS). C, Quantification of migrated cells. D, Migration of differentiated 10 t1/2 cells. E, Quantification of migrated cells. F, Migration of A0184 cells. G, Quantification of migrated cells. H, Quantification of migrated HUVECs with or without CCR inhibitors. I, Quantification of migrated 10 t1/2 cells with or without CCR inhibitors. Error bars are SE. N=4/group. *P<0.05, **P<0.01, ***P<0.001. scale bar = 100µm.
We showed above that CM from PGC-α expressing myotubes strongly induced the expression of MCP-1, in a manner dependent on the secretion of SPP1 (Figure 5G). MCP-1 secreted from macrophages can recruit endothelial cells, pericytes, and smooth muscle cells.34–37 This suggested that the activity present in THP-1-conditioned medium in the above experiments maybe be, at least in part, MCP-1. To test this, we used inhibitors to the MCP-1 receptors. Endothelial cells express two receptors for MCP-1, CCR1 and 2. Treatment of THP-1 CM with a cocktail of CCR-1 and 2 antagonists completely abrogated the ability of the CM to induce endothelial cell migration (Figure 6H). Induction of pericyte migration was similarly abrogated by addition of the inhibitor cocktail (Figure 6I). Together, these results indicate that PGC-1α in myotubes induces the secretion of SPP1, which in turn activates macrophages to secrete MCP-1, ultimately contributing to the stimulation of endothelial cell and pericyte migration.
PGC-1α induces aberrant vessel formation in vivo in the absence of SPP1
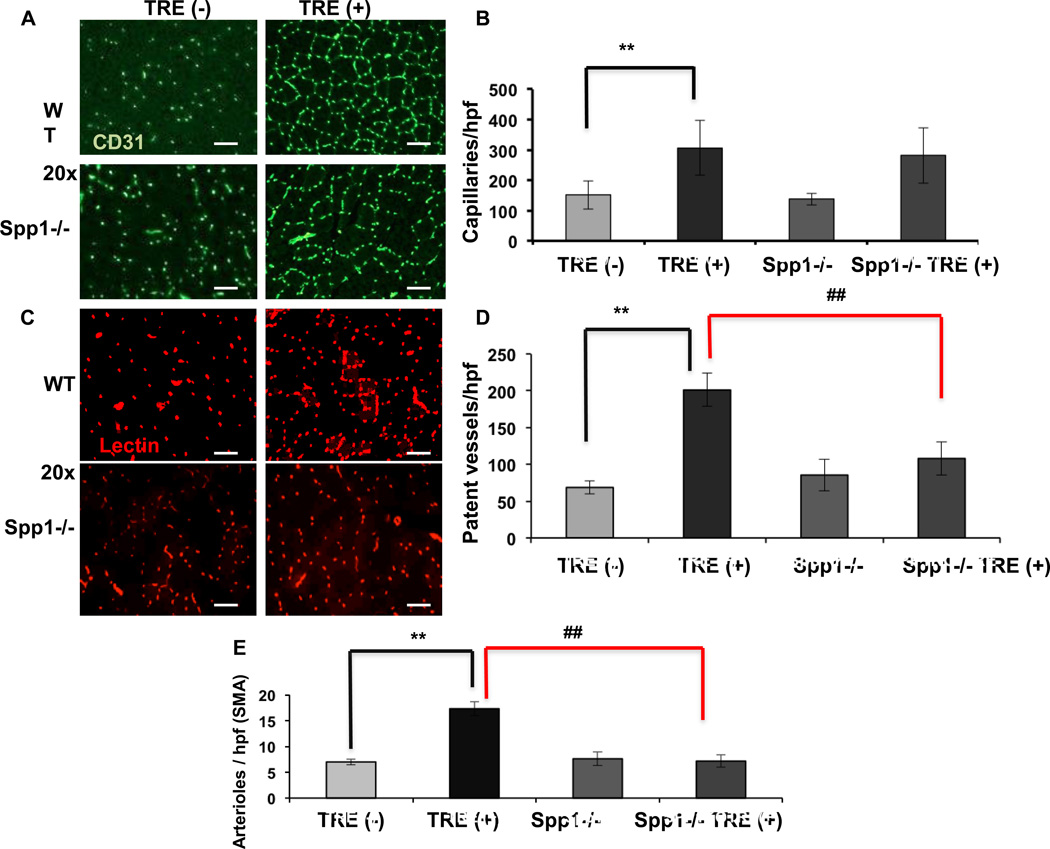
To directly test in vivo the role of SPP1 in vessel formation in response to PGC-1α, SPP1 −/− mice were crossed with the double transgenic TRE (+) mice to produce SPP1−/− TRE (+) mice. Expression of PGC-1α was induced for 4 weeks, after which the mice were injected with lectin (as described above), and muscles were harvested and stained for CD31 and SMA, as described above. As shown in Figure 7A–B, the absence of SPP1 had no impact on the marked increase in vascular density (CD31 staining) seen after induction of PGC-1α. In sharp contrast, the absence of SPP1 almost completely abrogated the 3-fold increase in isolectin staining seen after induction of PGC-1α (Figure 7C–D). These data indicate that SPP1, though dispensable for capillary formation per se, is essential for the development of vessel patency in vivo. Vessel patency allows for blood flow, and blood flow is thought to be crucial for the formation of new arterioles19, 38–40. As shown in Figure 7E, the absence of SPP1 also completely abrogated the increase in arterioles (SMA staining) seen after induction of PGC-1α. Together, these data demonstrate that SPP1 is essential for proper maturation of vessels induced by PGC-1α in intact animals.
Figure 7. SPP1 is required for vessel patency and arteriolar formation.
A, Capillary density in the quadriceps after 4 weeks of doxy removal. B, Quantification of capillary endothelial cells/high power field (20×). C, Patent capillaries in quadriceps muscle after 4 weeks of doxy removal. D, Quantification of patent vessels per high power field (20×). E, Quantification of arterioles (SMA)/lpf (5×). Error bars are SE. N=4/group. *, # significance with P<0.05, **P<0.01, ***P<0.001. scale bar = 100µm.
Adenoviral injection of PGC-1α improves blood flow recovery in young and old diabetic mice
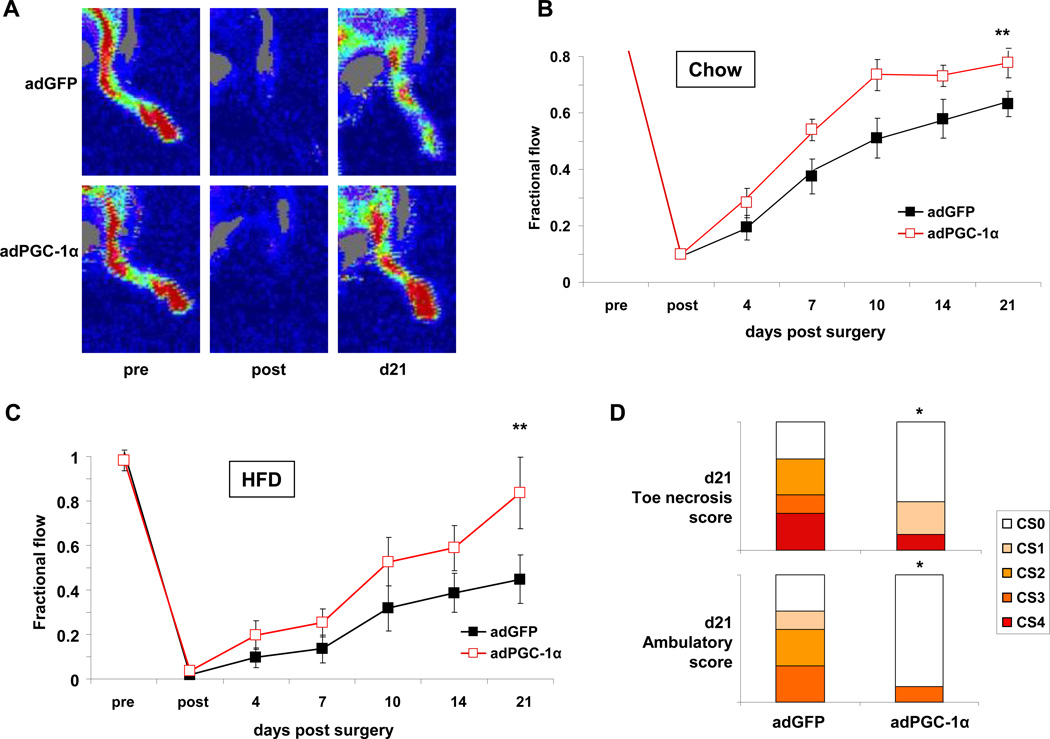
To begin to address the translational potential of PGC-1α induction in skeletal muscle, we used adenovirus expressing PGC-1α, versus control virus expressing GFP, in the murine hindlimb ischemia model17. We first used young 16-week old C57/Bl6 mice on normal chow. Adenovirus encoding either PGC-1α or GFP was injected into the hindlimb at the time of hindlimb ischemia surgery, as described17. Blood flow recovery was then monitored over the ensuing 21 days, using non-invasive laser Doppler imaging. As shown in Figure 8A–B, approximately 60% recovery of blood flow was seen in control-injected mice after 21 days, as observed by others10, 17, 30. In contrast, animals injected with PGC-1α virus had significantly accelerated recovery of blood flow, reaching 60% recovery within 10 days (Figure 8A–B). PGC-1α thus improves recovery from hindlimb ischemia in young animals. We next tested the PGC-1α virus in one year-old, high-fat fed, diabetic animals. As shown in in Figure 8C, blood flow recovery in the control-injected animals was markedly reduced compared to the young control animals (Figure 8B), reaching only 40% recovery after 21 days. Consistent with this, most animals developed severe ambulation defects and/or limb necrosis (Figure 8D), outcomes that are rarely seen in younger mice (not shown). In contrast, animals injected with PGC-1α virus had significantly accelerated recovery of blood flow, reaching >70% recovery after 21 days (Figure 8C), and the clinical outcomes were markedly improved in PGC-1α-injected animals (Figure 8D).
Figure 8. Adenoviral delivery of PGC-1α improves blood flow recovery after hindlimb ischemia.
A, Sample laser Doppler images of blood flow recovery pre, immediately post, and 21 days post hindlimb ischemia surgery with concurrent injection of GFP vs. PGC-1α adenovirus at the time of ligation. B, Quantification of blood flow of animals in A. Data presented as fractional flow compared to non-ischemic leg. N=7 per group. C, Quantification of blood flow in the ischemic limbs of high fat-fed diabetic mice after injection of GFP or PGC-1α adenovirus. N=7 per group. D, Measurement of toe necrosis and ambulatory scores 21days after hind limb ischemia surgery and adenoviral injection. CS0–4 = clinical scores 0–4. *P<0.1 by Mann-Whitney U test, **P<0.01 by 2-way ANOVA with bonferroni correction
DISCUSSION
Therapeutic angiogenesis has been pursued extensively as a potential treatment for ischemic disorders like peripheral artery disease.41–43 Thus far, however, clinical trials have largely failed.44–55 A number of clinical factors likely underlie these failures, including trial designs, difficulties with patient recruitment, and heterogeneous patient populations. In addition, important biological hurdles have also become evident.41, 45 Angiogenesis is a complex process, not easily recapitulated by simple delivery of one or two angiogenic factors.44, 50 Agents capable of coordinating this complex process have remained elusive. One approach has been to target so-called “master regulators”. This has been tried primarily with HIF-1α, with promising early results56, but a recent phase III trial failed to show benefit57. However, activation of HIF-1α typically inhibits metabolism, in particular oxidative metabolism5, which likely worsens oxidative tasks like walking57. A second, equally important biological hurdle to therapeutic angiogenesis is that most patients receiving such therapy have significant endothelial dysfunction that renders them less responsive to many angiogenic stimuli, but most pre-clinical trials have involved young, healthy animals. Here we show that PGC-1α can produce sustainable functional angiogenesis in adult skeletal muscle. PGC-1α orchestrates the complex recruitment of multiple cells types (Supplemental Figure VIII), resulting in new blood vessels that are patent, non-leaky, and functional. PGC-1α adenoviral injections improved blood flow in a murine model of peripheral arterial disease. Moreover, PGC-1α robustly induced angiogenesis, and improved blood flow after hindlimb ischemia, in old and diabetic animals. These data highlight PGC-1α as a potential therapeutic agent in the treatment of PAD.
Recovery of blood flow after hindlimb ischemia in the mouse, and improvements in blood flow and function in humans with PAD, is a complex process that likely includes the formation of new collaterals, capillaries, and arterioles. The induction of PGC-1α in skeletal muscle does not appear to induce the formation of new higher order arteries beyond arterioles. We propose here that PGC-1α likely predominantly affects local effects at the level of capillaries and arterioles. These effects likely allow optimal redistribution of local blood flow to match metabolic demand. In addition, increased cutaneous capillary flow could contribute to reduced tissue necrosis, via improved wound healing, independently of flow to the muscle component of the hindlimb. Tissue flow is know to be auto-regulated to match oxygen supply to demand, but because the transgenic mice are not more active than their control littermates, and demand is not altered, supply is therefore also not altered. Thus we do not see evidence that resistance at baseline is decreased, or that flow is increased. Interestingly, this suggests that, in these mice, the formation of new arterioles is not occurring in response to increase in mass baseline flow, although local fluctuations cannot be excluded. Exercise, on the other hand, not only robustly induces PGC-1α expression in skeletal muscle in rodents and humans, but also has numerous other effects, including increases in blood flow, cardiac output, etc. The genetic model used here thus separates the effects of PGC-1α from other exercise-induced effects. The data thus allow us to conclude that PGC-1α is sufficient to induce functional angiogenesis, but it is likely that other factors such as hemodynamic effects of exercise (or ischemia) contribute to subsequent formation of higher order vessels.
The induction of PGC-1α likely recapitulates normal angiogenesis in muscle. Post-natal physiological (i.e. non-pathological) angiogenesis is relatively rare, limited primarily to uterine changes during the estrous cycle, and to exercise-induced angiogenesis in skeletal muscle. Mechanisms of physiological angiogenesis are poorly understood, but likely differ significantly from those of pathological angiogenesis, e.g. in cancer or retinopathy. HIF-1α, for example, plays a prominent role in pathological angiogenesis58–61 while deletion of HIF-1α in skeletal muscle leads to more blood vessels, rather than fewer5. By contrast, PGC-1α expression is strongly induced by exercise, and deletion of PGC-1α in skeletal muscle does block exercise-induced angiogenesis, indicating PGC-1α likely mediates this process11. PGC-1α-induced angiogenesis thus likely closely recapitulates exercise-induced angiogenesis. In addition, PGC-1α likely contributes to the increase in capillary:fiber ratio consistently seen in oxidative fibers, where PGC-1α expression is high. This coordination allows the delivery of oxygen/fuel via the vasculature to match the consumption of oxygen/fuel in mitochondria-rich oxidative fibers.
The mechanisms of physiological angiogenesis in muscle remain poorly understood. Several studies have revealed a robust infiltration of macrophages in pathological angiogenesis such as found in ischemia and cancer62–64. In these conditions the macrophage infiltrate is felt to be part of the underlying inflammatory response. Exposure of macrophages to hypoxia results in their secretion of angiogenic molecules such as VEGF, PIGF, FGF2 and PDGF65–67. However, these processes are unlikely to be at play in our inducible PGC-1α model because 1) there is no hypoxia, 2) there is no trauma, and 3) there is no generalized inflammation, but instead a specific recruitment of macrophages. The data thus reveal an alternative mechanism for macrophage recruitment during angiogenesis: via PGC-1α-induced secretion of SPP1. SPP1 is a secreted non-collagenous sialic acid-rich protein that plays an important role in modulating numerous cell behaviors68–70. The protein is a powerful regulator of leukocyte migration and an inducer of angiogenic cytokines71–74.
Our data indicate that the recruited macrophages act, at least in part, to help orchestrate multicellular angiogenesis. SPP1 stimulates the macrophages to induce MCP-1, which in turn activates endothelial cells, pericytes, and smooth muscle cells. Deletion of SPP1 in intact animals leads to aberrant PGC-1α-induced vasculature, most notably the diminution of patent blood vessels, and blunted arteriolarization. An SPP1-macrophage-MCP-1 axis thus appears to be important for vessel maturation during physiological angiogenesis. Interestingly, previous work has shown that SPP1 expression is induced by hindlimb ischemia, and that SPP1 knockout mice have decreased recruitment of macrophages and significantly impaired blood flow recovery after hind-limb ischemia30, 75. In these contexts, it is also interesting to note that macrophages have recently been implicated in the process of tip cell to tip cell anastomosing in neuronal angiogenesis76, an important maturation step in angiogenesis. Physiological angiogenesis in skeletal muscle likely does not occur by sprouting angiogenesis (i.e. tip cell formation), however, but rather via intussusception, a process that remains poorly understood2, 77. Our data thus suggest that macrophages may be involved in both forms of vessel maturation.
In summary, the current study demonstrates that PGC-1α is a powerful orchestrator of functional angiogenesis in skeletal muscle. Angiogenesis can be robustly activated in either young or old and diabetic mice. And adenoviral delivery of PGC-1α improves the response to hindlimb ischemia in both of those contexts. The study thus provides novel mechanistic insights into physiological angiogenesis in skeletal muscle.
Supplementary Material
Novelty and Significance.
What Is Known?
Peripheral Artery Disease (PAD) and microvascular disease are major complications of diabetes.
Exercise is currently the best-known therapeutic intervention in the treatment of PAD.
PGC-1alpha is induced by exercise in skeletal muscle, and is required for exercise-induced angiogenesis.
What New Information Does this Manuscript Contribute?
Induction of PGC-1alpha in the adult murine muscle induces the formation of new functional blood vessels.
PGC-1alpha activates a cascade of events including the production of SPP1, leading to the recruitment of macrophages required for vessel maturation.
Viral delivery of PGC-1alpha in significantly improves blood flow recovery in the murine hindlimb ischemia model of PAD.
PAD and microvascular disease are major complication of diabetes and are major contributors to limb amputations. Therapeutic attempts at increasing the formation of new blood vessels have thus far been unsuccessful, likely in part due to poorly formed or leaky blood vessels. Exercise is currently the best-known treatment for PAD, although patient participation is often difficult. We use here a known mimetic of the exercise response, the transcriptional regulator PGC-1alpha, to induce new capillary blood vessels in the adult mouse. The blood vessels formed are patent, not leaky, and fully functional. PGC-1alpha activates a complex cascade of events, including the secretion of SPP1, which recruits macrophages involved in vessel maturation. Adenoviral delivery of PGC-1alpha to a limb ischemic model improved blood flow recovery, highlighting the therapeutic potential of targeting the PGC-1alpha pathway. Taken together the results show that PGC-1alpha coordinates the formation of new blood vessels analogous to those achieved with exercise, and potentially offers a new therapeutic target for the treatment of PAD.
Acknowledgments
SOURCES OF FUNDING
GCR is supported by NIH grant KO1 grant AR062128. SR is supported by funding from a NHLBI NRSA Fellowship. HD is supported by NIH grant PO1 CA92644. WA is funded by NIH grant HL076540. ZA is funded by NIH grant RO1 HL094499.
Nonstandard Abbreviations and Acronyms
- PAD
Peripheral Arterial Disease
- VEGF
Vascular Endothelial Growth Factor
- PGC-1α
Peroxisome Proliferation Activator Receptor-γ (PPAR-γ) coactivator-1α
- SPP1
Secreted Phosphoprotein 1 (also known as osteopontin)
- MCP-1
Monocyte Chemoattractant Protein-1
- HIF-1α
Hypoxia Inducible Factor-1α
- ERR-α
Estrogen Related Receptor-α
- MCK
Muscle Creatine Kinase
- TTA
Tetracycline Transactivator
- TRE
Tetracycline Response Element
- CD31
Cluster of Differentiation 31 also known as Platelet-Endothelial cell Adhesion Molecule-1 (PECAM-1)
- PDGFRβ
Platelet Derived Growth Factor Receptor beta
- SMA
Smooth Muscle Actin
- CCR-1 and 2
C-C chemokine receptor 1 and 2
- qPC
Quantitative Polymerase Chain Reaction
- BSA
Bovine Serum Albumin
- GS-IB4
Griffonia simplicifolia
- GFP
Green Fluorescent Protein
- PBS
Phosphate Buffered Saline
- HEK293T
Human Embryonic Kidney 293 containing SV40 T-antigen
- ATP5o
ATP synthase Subunit O
- CYCS
Cytochrome C oxidase
- VEGFR2
Vascular Endothelial Growth Factor Receptor 2
- FGF2
Fibroblast Growth Factor 2 (bFGF)
- ELISA
Enzyme Linked Immuno-sorbent Assay
- Sflt1
Soluble fms-like tyrosine kinase-1 (also known as sVEGFR1: soluble VEGF Receptor 1)
- PIGF
Placental Induced Growth Factor
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: Perspectives of various models. Physiol Rev. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- 2.Egginton S. Invited review: Activity-induced angiogenesis. Pflugers Arch. 2009;457:963–977. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 3.Bloor CM. Angiogenesis during exercise and training. Angiogenesis. 2005;8:263–271. doi: 10.1007/s10456-005-9013-x. [DOI] [PubMed] [Google Scholar]
- 4.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 5.Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, Johnson RS. Hif-1alpha in endurance training: Suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–R2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- 6.Arany Z. Pgc-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18:426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finck BN, Kelly DP. Pgc-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of ppargamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator pgc-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 10.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. Hif-independent regulation of vegf and angiogenesis by the transcriptional coactivator pgc-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 11.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator pgc-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator pgc-1alpha in muscle refueling. J Biol Chem. 2007;282:36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- 13.May D, Gilon D, Djonov V, Itin A, Lazarus A, Gordon O, Rosenberger C, Keshet E. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc Natl Acad Sci U S A. 2008;105:282–287. doi: 10.1073/pnas.0707778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe GC, Patten IS, Zsengeller ZK, El-Khoury R, Okutsu M, Bampoh S, Koulisis N, Farrell C, Hirshman MF, Yan Z, Goodyear LJ, Rustin P, Arany Z. Disconnecting mitochondrial content from respiratory chain capacity in pgc-1-deficient skeletal muscle. Cell Rep. 2013;3:1449–1456. doi: 10.1016/j.celrep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. Pgc-1alpha regulates the neuromuscular junction program and ameliorates duchenne muscular dystrophy. Genes Dev. 2007;21:770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 18.Cox CA, Amaral J, Salloum R, Guedez L, Reid TW, Jaworski C, John-Aryankalayil M, Freedman KA, Campos MM, Martinez A, Becerra SP, Carper DA. Doxycycline's effect on ocular angiogenesis: An in vivo analysis. Ophthalmology. 2010;117:1782–1791. doi: 10.1016/j.ophtha.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry. Biokhimiia. 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 21.Merrilees MJ, Beaumont B, Scott LJ. Comparison of deposits of versican, biglycan and decorin in saphenous vein and internal thoracic, radial and coronary arteries: Correlation to patency. Coronary artery disease. 2001;12:7–16. doi: 10.1097/00019501-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Plante GE, Chakir M, Ettaouil K, Lehoux S, Sirois P. Consequences of alteration in capillary permeability. Canadian journal of physiology and pharmacology. 1996;74:824–833. doi: 10.1139/cjpp-74-7-824. [DOI] [PubMed] [Google Scholar]
- 23.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tafuro S, Ayuso E, Zacchigna S, Zentilin L, Moimas S, Dore F, Giacca M. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovascular research. 2009;83:663–671. doi: 10.1093/cvr/cvp152. [DOI] [PubMed] [Google Scholar]
- 25.Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, Della Siega A, Bilodeau L, Burton JR, Proulx G, Radhakrishnan S. Vegf gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: Results of the northern trial. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su H, Takagawa J, Huang Y, Arakawa-Hoyt J, Pons J, Grossman W, Kan YW. Additive effect of aav-mediated angiopoietin-1 and vegf expression on the therapy of infarcted heart. International journal of cardiology. 2009;133:191–197. doi: 10.1016/j.ijcard.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutcher ME, Herman IM. The pericyte: Cellular regulator of microvascular blood flow. Microvascular research. 2009;77:235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. American journal of hematology. 2010;85:593–598. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 29.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. Vegf-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arterioscler Thromb Vasc Biol. 2008;28:290–295. doi: 10.1161/ATVBAHA.107.158485. [DOI] [PubMed] [Google Scholar]
- 31.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. The Journal of clinical investigation. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated vegf is differentiation-dependent and is associated with endothelial survival. Developmental biology. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Ding R, Darland DC, Parmacek MS, D'Amore PA. Endothelial-mesenchymal interactions in vitro reveal molecular mechanisms of smooth muscle/pericyte differentiation. Stem cells and development. 2004;13:509–520. doi: 10.1089/scd.2004.13.509. [DOI] [PubMed] [Google Scholar]
- 34.Arefieva TI, Kukhtina NB, Antonova OA, Krasnikova TL. Mcp-1-stimulated chemotaxis of monocytic and endothelial cells is dependent on activation of different signaling cascades. Cytokine. 2005;31:439–446. doi: 10.1016/j.cyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express ccr2 and respond to mcp-1: Direct role of mcp-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 36.Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of ccr2 by endothelial cells : Implications for mcp-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2085–2093. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Wang Q, Fei T, Han JD, Chen YG. Mcp-1 mediates tgf-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007;109:987–994. doi: 10.1182/blood-2006-07-036400. [DOI] [PubMed] [Google Scholar]
- 38.Troidl K, Schaper W. Arteriogenesis versus angiogenesis in peripheral artery disease. Diabetes/metabolism research and reviews. 2012;28(Suppl 1):27–29. doi: 10.1002/dmrr.2232. [DOI] [PubMed] [Google Scholar]
- 39.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacological reviews. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 40.Reginato S, Gianni-Barrera R, Banfi A. Taming of the wild vessel: Promoting vessel stabilization for safe therapeutic angiogenesis. Biochemical Society transactions. 2011;39:1654–1658. doi: 10.1042/BST20110652. [DOI] [PubMed] [Google Scholar]
- 41.Giacca M, Zacchigna S. Vegf gene therapy: Therapeutic angiogenesis in the clinic and beyond. Gene therapy. 2012;19:622–629. doi: 10.1038/gt.2012.17. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 43.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karvinen H, Pasanen E, Rissanen TT, Korpisalo P, Vahakangas E, Jazwa A, Giacca M, Yla-Herttuala S. Long-term vegf-a expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene therapy. 2011;18:1166–1172. doi: 10.1038/gt.2011.66. [DOI] [PubMed] [Google Scholar]
- 45.Germani A, Di Campli C, Pompilio G, Biglioli P, Capogrossi MC. Regenerative therapy in peripheral artery disease. Cardiovascular therapeutics. 2009;27:289–304. doi: 10.1111/j.1755-5922.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 46.Mikroulis D, Papanas N, Maltezos E, Bougioukas G. Angiogenic growth factors in the treatment of peripheral arterial disease. Current vascular pharmacology. 2007;5:195–209. doi: 10.2174/157016107781024136. [DOI] [PubMed] [Google Scholar]
- 47.Schalch P, Rahman GF, Patejunas G, Goldschmidt RA, Carbray J, Retuerto MA, Kim D, Esser K, Crystal RG, Rosengart TK. Adenoviral-mediated transfer of vascular endothelial growth factor 121 cdna enhances myocardial perfusion and exercise performance in the nonischemic state. The Journal of thoracic and cardiovascular surgery. 2004;127:535–540. doi: 10.1016/j.jtcvs.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Rajagopalan S, Mohler E, 3rd, Lederman RJ, Saucedo J, Mendelsohn FO, Olin J, Blebea J, Goldman C, Trachtenberg JD, Pressler M, Rasmussen H, Annex BH, Hirsch AT. Regional angiogenesis with vascular endothelial growth factor (vegf) in peripheral arterial disease: Design of the rave trial. American heart journal. 2003;145:1114–1118. doi: 10.1016/S0002-8703(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 49.Semenza GL. Therapeutic angiogenesis: Another passing phase? Circ Res. 2006;98:1115–1116. doi: 10.1161/01.RES.0000223485.43020.9e. [DOI] [PubMed] [Google Scholar]
- 50.Carmeliet P. Vegf gene therapy: Stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6:1102–1103. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 51.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The viva trial: Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 52.Rajagopalan S, Mohler ER, 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: A phase ii randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 53.Makinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, Yla-Herttuala S. Increased vascularity detected by digital subtraction angiography after vegf gene transfer to human lower limb artery: A randomized, placebo-controlled, double-blinded phase ii study. Molecular therapy : the journal of the American Society of Gene Therapy. 2002;6:127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 54.Muona K, Makinen K, Hedman M, Manninen H, Yla-Herttuala S. 10-year safety follow-up in patients with local vegf gene transfer to ischemic lower limb. Gene therapy. 2012;19:392–395. doi: 10.1038/gt.2011.109. [DOI] [PubMed] [Google Scholar]
- 55.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH, Investigators T. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the traffic study): A randomised trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 56.Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer of hif-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18769–18774. doi: 10.1073/pnas.0910561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Creager MA, Olin JW, Belch JJ, Moneta GL, Henry TD, Rajagopalan S, Annex BH, Hiatt WR. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765–1773. doi: 10.1161/CIRCULATIONAHA.110.009407. [DOI] [PubMed] [Google Scholar]
- 58.Rezvani HR, Ali N, Nissen LJ, Harfouche G, de Verneuil H, Taieb A, Mazurier F. Hif-1alpha in epidermis: Oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. The Journal of investigative dermatology. 2011;131:1793–1805. doi: 10.1038/jid.2011.141. [DOI] [PubMed] [Google Scholar]
- 59.Coulon C, Georgiadou M, Roncal C, De Bock K, Langenberg T, Carmeliet P. From vessel sprouting to normalization: Role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2331–2336. doi: 10.1161/ATVBAHA.110.214106. [DOI] [PubMed] [Google Scholar]
- 60.Semenza GL. Regulation of vascularization by hypoxia-inducible factor 1. Annals of the New York Academy of Sciences. 2009;1177:2–8. doi: 10.1111/j.1749-6632.2009.05032.x. [DOI] [PubMed] [Google Scholar]
- 61.Semenza GL. Hif-1 inhibitors for cancer therapy: From gene expression to drug discovery. Current pharmaceutical design. 2009;15:3839–3843. doi: 10.2174/138161209789649402. [DOI] [PubMed] [Google Scholar]
- 62.Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Experimental oncology. 2010;32:153–158. [PubMed] [Google Scholar]
- 63.Sica A. Macrophages give gas(6) to cancer. Blood. 2010;115:2122–2123. doi: 10.1182/blood-2009-12-255869. [DOI] [PubMed] [Google Scholar]
- 64.Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 65.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 66.Lewis C, Murdoch C. Macrophage responses to hypoxia: Implications for tumor progression and anti-cancer therapies. The American journal of pathology. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. International journal of cancer. Journal international du cancer. 2005;117:701–708. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- 68.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. The American journal of pathology. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 69.Chae S, Jun HO, Lee EG, Yang SJ, Lee DC, Jung JK, Park KC, Yeom YI, Kim KW. Osteopontin splice variants differentially modulate the migratory activity of hepatocellular carcinoma cell lines. International journal of oncology. 2009;35:1409–1416. doi: 10.3892/ijo_00000458. [DOI] [PubMed] [Google Scholar]
- 70.Senger DR, Perruzzi CA. Cell migration promoted by a potent grgds-containing thrombin-cleavage fragment of osteopontin. Biochimica et biophysica acta. 1996;1314:13–24. doi: 10.1016/s0167-4889(96)00067-5. [DOI] [PubMed] [Google Scholar]
- 71.Wang KX, Denhardt DT. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1087–1097. doi: 10.1158/1055-9965.EPI-06-1008. [DOI] [PubMed] [Google Scholar]
- 73.Weber GF. The cancer biomarker osteopontin: Combination with other markers. Cancer genomics & proteomics. 2011;8:263–288. [PubMed] [Google Scholar]
- 74.Rittling SR. Osteopontin in macrophage function. Expert reviews in molecular medicine. 2011;13:e15. doi: 10.1017/S1462399411001839. [DOI] [PubMed] [Google Scholar]
- 75.Lyle AN, Joseph G, Fan AE, Weiss D, Landazuri N, Taylor WR. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1383–1391. doi: 10.1161/ATVBAHA.112.248922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of vegf-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gustafsson T. Vascular remodelling in human skeletal muscle. Biochemical Society transactions. 2011;39:1628–1632. doi: 10.1042/BST20110720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.