Abstract
Curcuma purpurascens BI. rhizome, a member of the Zingiberaceae family, is a popular spice in Indonesia that is traditionally used in assorted remedies. Dichloromethane extract of C. purpurascens BI. rhizome (DECPR) has previously been shown to have an apoptosis-inducing effect on colon cancer cells. In the present study, we examined the potential of DECPR to prevent colon cancer development in rats treated with azoxymethane (AOM) (15 mg/kg) by determining the percentage inhibition in incidence of aberrant crypt foci (ACF). Starting from the day immediately after AOM treatment, three groups of rats were orally administered once a day for 2 months either 10% Tween 20 (5 mL/kg, cancer control), DECPR (250 mg/kg, low dose), or DECPR (500 mg/kg, high dose). Meanwhile, the control group was intraperitoneally injected with 5-fluorouracil (35 mg/kg) for 5 consecutive days. After euthanizing the rats, the number of ACF was enumerated in colon tissues. Bax, Bcl-2, and proliferating cell nuclear antigen (PCNA) protein expressions were examined using immunohistochemical and Western blot analyses. Antioxidant enzymatic activity was measured in colon tissue homogenates and associated with malondialdehyde level. The percentage inhibition of ACF was 56.04% and 68.68% in the low- and high-dose DECPR-treated groups, respectively. The ACF inhibition in the treatment control group was 74.17%. Results revealed that DECPR exposure at both doses significantly decreased AOM-induced ACF formation, which was accompanied by reduced expression of PCNA. Upregulation of Bax and downregulation of Bcl-2 suggested the involvement of apoptosis in the chemopreventive effect of DECPR. In addition, the oxidative stress resulting from AOM treatment was significantly attenuated after administration of DECPR, which was shown by the elevated antioxidant enzymatic activity and reduced malondialdehyde level. Taken together, the present data clearly indicate that DECPR significantly inhibits ACF formation in AOM-treated rats and may offer protection against colon cancer development.
Keywords: colon cancer, PCNA, Zingiberaceae
Introduction
Diagnosis of more than 1 million new cases suffering from colorectal cancer worldwide each year has made colon cancer the fourth most prevalent cause of cancer mortality and third most common malignancy throughout the world.1,2 Environmental factors are established as the main cause of colorectal cancer incidence, and only 20% of cases have been linked to heritable genetic changes.3 The risk factors for the development of colorectal tumors include chronic intestinal inflammation, environmental and food-borne mutagens, and specific intestinal commensals and pathogens.4,5 Alcohol consumption, obesity, physical inactivity, smoking, diets high in fat and red meats, and inadequate intake of dietary fiber, fruits, and vegetables are other risk factors that contribute to colorectal cancer.6 Due to the widespread occurrence of the risk factors among populations throughout the world, extensive research is ongoing to develop new pharmaceutical agents with preventive and curative potential against colorectal cancer.7,8
The pathogenesis of colorectal cancer evolves from multistep deregulation of epithelial cells into a polyp, which may develop to a cancerous state.9 As prepolyp abnormalities, aberrant crypt foci (ACF) are recognizable precursors to colorectal cancer in both experimental models and humans. ACF were first identified as putative precancerous lesions in the colon of carcinogen-treated rodents.10 These lesions are microscopically characterized by crypts with elevated sizes above the normal mucosa composed of thickened luminal epithelia and easily discernible pericryptal zones.11 Due to similar genotypic and morphological characterizations of ACF between animal and human colons, ACF detection is employed as an easy biomarker to screen and diagnose early stages of pathogenesis of colon cancer in assorted studies.12 ACF monitoring is extensively used to investigate the effects of potential chemopreventive agents against different carcinogens.13–15 On the other hand, azoxymethane (AOM) (C2H6N2O), an oxide of azomethane, is a potent carcinogen widely used to induce ACF formation in experimental models.9
Nutraceuticals, including a wide range of products such as functional foods, spices, and dietary and herbal supplements, are a pivotal source of chemical diversity for pharmaceutical discovery.16 The genus Curcuma, in the Zingiberaceae family, has been a stable functional food source consumed by the Indonesian locals to control inflammation and cancer.17–19 Due to the popularity of Curcuma spices, various species in this genius are widely cultured in the Asia-Pacific region.20 One such plant that has been widely employed as a spice is Curcuma purpurascens BI.
C. purpurascens, commonly known as “temu tis” in Indonesia, has typical rhizomes similar to turmeric (Curcuma longa) and reaches a height of up to 1.75 m.21 In spite of different traditional uses of C. purpurascens rhizome against boils, cough, fever, itch, scabies, and wounds, detailed scientific investigations reported for this plant have been limited.22 The mixture of the rhizomes of C. purpurascens with other herbs is used to treat cough and skin infections and as a poultice after childbirth.21,22 In our previous study, we showed the selective cytotoxic effect of dichloromethane extract of C. purpurascens rhizome (DECPR) against different cancer cells and its apoptotic effect against HT-29 colon cancer cells. The aim of the present study was to examine the in vivo chemopreventive potential of DECPR on AOM-induced colon cancer in rats by analyzing the incidence of ACF.23
Materials and methods
Preparation of the rhizome extract
Dried rhizome of the C. purpurascens plant was collected from Yogyakarta, Indonesia, in September 2012. Botanical identification was carried out by Mr Teo Leong Eng, an ethnobotanist from the Faculty of Science, University of Malaya (Kuala Lumpur, Malaysia). A voucher specimen (KL 5793) has been deposited in the herbarium of the Department of Chemistry, Faculty of Science, University of Malaya. The air-dried and powdered rhizomes (2.5 kg) were macerated in dichloromethane at room temperature for 3 days. After maceration, the solution was filtered, concentrated to dryness under vacuum on reduced pressure at 40°C, and stored at −20°C until use. To provide 250 mg/kg and 500 mg/kg stocks for in vivo animal study, DECPR was dissolved in 10% Tween 20 (Sigma-Aldrich Co., St Louis, MO, USA). DECPR was dissolved in 10% Tween 20, in 25 mg/mL and 50 mg/mL for high- and low-dose treatment, respectively.
Animals and ethical approval
Thirty healthy adult male Sprague-Dawley rats (6 weeks old and 200–220 g weight) were provided by the Animal Experimental Unit of the University of Malaya. Rodents were housed in clean polyvinyl cages under 12/12-hour light/dark conditions of 50%–60% humidity and room temperature of 22°C–24°C. The rats were fed a normal pellet diet and water ad libitum. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the United States National Academy of Sciences and published by the National Institutes of Health.23 The Faculty of Medicine’s Institutional Animal Care and Use Committee of the University of Malaya approved all protocols involving the experiment (ethics number 2014-03-05/PHAR/R/ER).
Experimental protocols
The experiment was performed as previously described.24 Rats were randomly divided into five groups (n=6), namely, normal control, cancer control, low dose of DECPR (250 mg/kg), high dose of DECPR (500 mg/kg), and treatment control. The toxicity testing of the plant extract was previously investigated by in vitro studies using cell lines (HT-29 and CCD841) and in vivo exposure on experimental animals.25 The safe dose of the plant extract for the treatment was determined according to the acute toxicity results published by the present authors.25 No sign of toxicity in treated rats at a dose of 5 g/kg was reported by Rouhollahi et al.25 In addition, a range between 2 and 5 g/kg has been suggested for the dose of the plant extract in acute toxicity testing in previous studies.26,27
To induce ACF formation, all of the rats, except for rats in the normal control group, were subcutaneously injected with AOM (Sigma-Aldrich Co.) (CAS Number: 25843-45-2) once a week for 2 consecutive weeks (Table 1). The concentration of AOM used in this study was 15 mg/kg at a volume of 1 mL/kg. Normal control rats were subcutaneously injected with normal saline (15 mg/kg). Then, rats were orally administered with DECPR or Tween 20 (10%) once a day by oral gavage for 2 months according to the “Treatment” column in Table 1, except for the rats in the treatment control group. The volume of 1 mL/kg of 5-fluorouracil (5-FU) (Sigma-Aldrich Co.) at 35 mg/kg dose was intraperitoneally injected in the rats in the treatment control group for 5 consecutive days (Table 1). The dose of 35 mg/kg of 5-FU as chemotherapy regimen has been suggested for the investigation of colon cancer in animal studies.14,15,27
Table 1.
The experimental design and specifications
| Group | Description | Induction | Treatment |
|---|---|---|---|
| A | Normal control | Normal saline (15 mg/kg) | 10% Tween 20 (5 mL/kg) |
| B | Cancer control | AOM (15 mg/kg) | 10% Tween 20 (5 mL/kg) |
| C | Low dose | AOM (15 mg/kg) | DECPR (250 mg/kg) |
| D | High dose | AOM (15 mg/kg) | DECPR (500 mg/kg) |
| E | Treatment control | AOM (15 mg/kg) | 5-FU (35 mg/kg) |
Abbreviations: 5-FU, 5-fluorouracil; AOM, azoxymethane; DECPR, dichloromethane extract of Curcuma purpurascens rhizome.
Assessment of ACF
After 2 months of treatment, all the rats were sacrificed with a high dose of ketamine (30 mg/kg, 100 mg/mL) and xylazine (3 mg/kg, 100 mg/mL) anesthesia. The extracted colons of rats were opened longitudinally and rinsed with phosphate-buffered saline prior to fixation between two filter papers in 10% buffered formalin for 24 hours. Then, proximal and distal segments of samples with equal length were stained with methylene blue (0.2%; Sigma-Aldrich Co.), and enumeration of ACF was performed under a light microscope (Olympus Corporation, Tokyo, Japan).28
Immunohistochemistry
Immunohistochemical analyses of tissue sections were performed as previously described.29 In brief, after deparaffinizing and rehydrating tissue sections, sodium citrate buffer (10 mM) was employed to perform antigen retrieval for 10 minutes. Samples were then cooled in Tris-buffered saline prior to use of the commercial Dako ARK peroxidase kit (DAKO Denmark A/S, Glostrup, Denmark). Endogenous peroxidase was blocked by use of peroxidase blocking solution and mixed thoroughly for 5 minutes, followed by rinsing of the samples. The slides were incubated with biotinylated primary antibodies against Bax (1:100), Bcl-2 (1:100), and proliferating cell nuclear antigen (PCNA) (1:200) for 15 minutes and then supplemented with streptavidin–HRP for 30 minutes. Development of slides and counterstaining were performed using diaminobenzidine substrate chromogen and hematoxylin. For PCNA expression as a tumor marker, the proliferation index (PI) was determined using the following formula:
| (1) |
Enzymatic antioxidants
To prepare the colon tissue homogenates, samples were washed and homogenized in ice-cold phosphate-buffered saline (10%) using a Teflon homogenizer (Polytron; Heidolph Instruments GmbH & Co., KG, Schwabach, Germany). Then, samples were centrifuged at 4,500 rpm for 15 minutes at 4°C for disposal of cell debris. The supernatant was then employed to examine antioxidant activities using assay kits for catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) (Cayman Chemical Company, Ann Arbor, MI, USA), according to the manufacturer’s instructions.
Malondialdehyde
To determine the level of oxidative stress in colon tissue homogenates, a commercial kit for thiobarbituric acid reactive substance (TBARS; Cayman Chemical Company) was used according to the manufacturer instructions.30
Western blotting
Western blot analysis was performed as previously described in detail.15 In brief, the collected colon tissues from rats were subjected to protein extraction using protein extraction buffer (Pierce Biotechnology Inc., Rockford, IL, USA). After quantifying the extracted protein using the Bradford method, samples (30 µg) were run in a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel followed by transformation to polyvinylidene fluoride membranes (Pierce Biotechnology Inc.). Then, membranes were blocked using Blocker Casein (Pierce Biotechnology Inc.), and samples were incubated overnight with specific primary antibodies, PCNA, Bax, Bcl-2, and β-actin, which were obtained from Abcam Inc. (Cambridge, MA, USA). After washing of the samples with 0.1% Tris-buffered saline with Tween 20 for 5 minutes, they were probed with the appropriate peroxidase-coupled secondary antibodies for 2 hours. Bands were visualized using the Fusion FX7 system (Vilber Lourmat, Eberhardzell, Germany).
Statistical analysis
Results were analyzed by one-way analysis of variance followed by Tukey’s post hoc test and expressed as mean ± standard error of n animals per group. Statistical analyses were performed using the SAS 9.1 statistical program (SAS Institute Inc., Cary, NC, USA). All group comparisons were considered significant at P<0.05.
Results and discussion
ACF enumeration
To evaluate the chemopreventive effect of DECPR in animal models, ACF enumeration was applied as an easy indicator of the colon neoplasia. Methylene blue dye was employed to stain the proximal and distal parts of the separated colons, and quantitative results are reported in Table 2 and Figure 1. The rats that were treated with AOM experienced severe development of ACF, compared to the normal control group, which was evidenced by different numbers of crypts with increased sizes and by the reformed luminal epithelia (Figure 2). As shown in Table 2, the number of crypts was statistically significant in the cancer control group (182±6.47) compared to the treatment control (47±3.69) and low- and high-dose DECPR-treated groups (80±6.13 and 57±5.78, respectively). The groups treated with the DECPR showed no significant difference to the treatment control. Figure 1 presents the scattering of the ACF formation in the proximal and distal parts of the colons separated from the treated rats. The distal parts of the colons showed more aggregation of the ACF formation compared to the proximal parts in all experimental groups (Figure 1).
Table 2.
Effect of DECPR on the incidence of aberrant crypt categories (one, two, three, or four or more crypts) of the colons separated from the treated rats
| Group | Number of crypts per ACF
|
Total | Inhibition (%) | |||
|---|---|---|---|---|---|---|
| One crypt | Two crypts | Three crypts | Four or more crypts | |||
| A | 0 | 0 | 0 | 0 | 0 | N/A |
| B | 46±1.22 | 47±1.89 | 55±1.98 | 34±1.40 | 182±6.47 | N/A |
| C | 19±1.03* | 24±1.28* | 22±2.62* | 15±1.20* | 80±6.13* | 56.04 |
| D | 14±2.08* | 19±1.79* | 15±1.24* | 9±0.67* | 57±5.78* | 68.68 |
| E | 14±0.50* | 14±1.39* | 11±1.22* | 8±0.58* | 47±3.69* | 74.17 |
Notes: The five groups of rats were A: normal control; B: cancer control; C: low dose of DECPR; D: high dose of DECPR; and E: treatment control. Data are shown as mean ± standard error of the mean (n=6). Values are statistically significant at
P<0.05.
Abbreviations: ACF, aberrant crypt focus; DECPR, dichloromethane extract of Curcuma purpurascens rhizome; N/A, not applicable.
Figure 1.
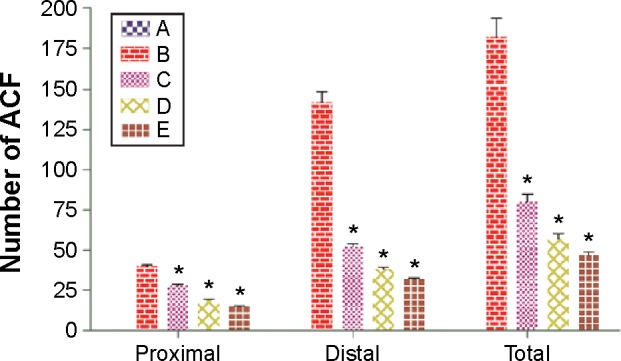
Effect of DECPR on ACF formation in proximal and distal parts of the colons separated from the treated rats.
Notes: The five groups of rats were A: normal control; B: cancer control; C: low dose of DECPR; D: high dose of DECPR; and E: 5-fluorouracil treatment control. Data are shown as mean ± standard error of the mean (n=6). Values are statistically significant at *P<0.05.
Abbreviations: ACF, aberrant crypt foci; DECPR, dichloromethane extract of Curcuma purpurascens rhizome.
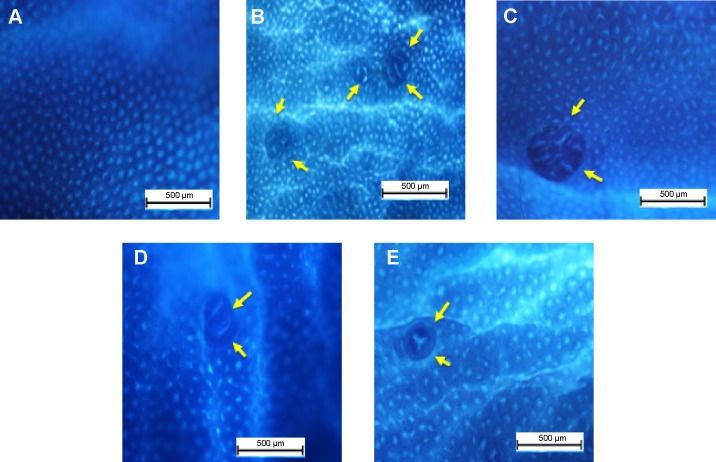
Figure 2.
Effect of DECPR on gross appearances of aberrant crypt foci (arrows) in the colon mucosa separated from the treated rats and stained with methylene blue dye.
Notes: The five groups of rats were (A) normal control; (B) cancer control; (C) low dose of DECPR; (D) high dose of DECPR; and (E) 5-fluorouracil treatment control. Scale bar: 500 µm.
Abbreviation: DECPR, dichloromethane extract of Curcuma purpurascens rhizome.
The result of this study appears to be in line with previous studies, which reported a higher number of ACF in distal parts of the colon compared to the proximal parts.31,32 The consequent ACF detriment was significantly attenuated by administration of DECPR at 250 mg/kg and 500 mg/kg concentrations with an inhibitory percentage of 56.04% and 68.68%, respectively. A number of published findings on other Curcuma spp. and their isolated phytochemicals demonstrated similar suppressive and protective effects against paraneoplastic lesions of colon carcinogenesis.33–36 This result demonstrates the promising chemopreventive potential of DECPR against chemical carcinogen-induced colon cancer in rats.
Immunohistochemical evaluation
DECPR induced downregulation of PCNA
Due to its critical role in several biological pathways such as chromatin remodeling, cell cycle, and DNA synthesis, repair, and methylation, PCNA is known to be a good index of cell proliferation.37,38 An association between PCNA activity and tumor evolution in clinical studies has indicated that this protein can be considered a prognostic marker for cancer.39,40 In addition, oncology investigations have found that excessive cell proliferation in epithelial colon tissues is inevitable in neoplasia development.41,42
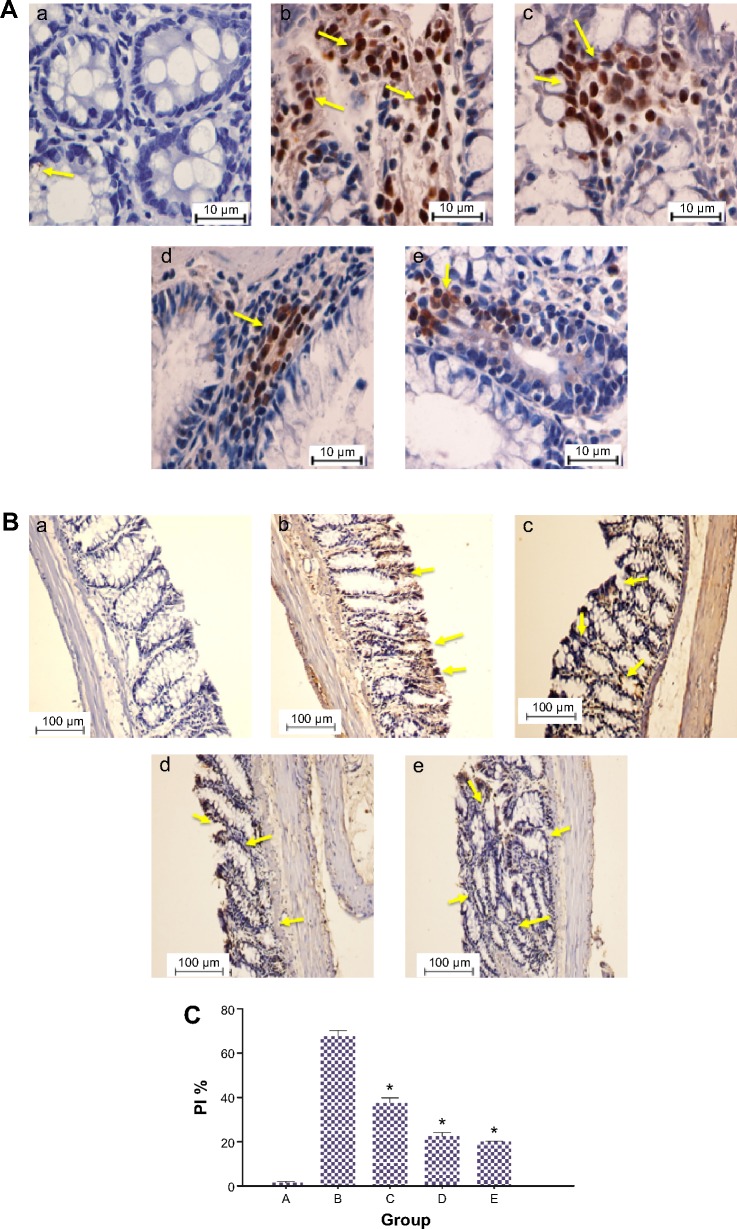
Since development of ACF requires aberrant cell proliferation in colon tissues, we performed immunohistochemical analysis on PCNA protein (Figure 3). In the AOM-induced colon cancer model, this chemical carcinogen caused significant elevation in PCNA expression (PI: 67.68%), compared to the normal control group (PI: 1.5%). This observation was previously shown with other chemical carcinogens.43,44 After administration of DECPR to rats, PI was significantly reduced to 37.45% and 22.46% for the low-dose and high-dose groups, respectively. The downregulation of PCNA protein expression was also detected in Western blot analysis. This result reveals that DECPR treatment causes a diminished proliferation zone in the colon tissues of rats and throws light on the involvement of proliferation pathways in the chemopreventive effect of DECPR.
Figure 3.
Immunohistochemical expression of PCNA in colon tissues of control and experimental groups of rats.
Notes: (A and B) (a) Group A. (b) Group B. (c) Group C. (d) Group D. (e) Group E. PCNA protein expression is illustrated as brown staining. (A) Scale bar: 10 µm. (B) Scale bar: 100 µm. (C) Quantitative data expressing the PCNA protein level illustrate that there was significant downregulation in groups C–E compared with the cancer control group. Data are shown as mean ± standard error of the mean (n=6). Values are statistically significant at *P<0.05. The five groups of rats were A: normal control; B: cancer control; C: low dose of DECPR; D: high dose of DECPR; and E: 5-fluorouracil treatment control. The yellow arrows show brown staining demonstrating a significant downregulation of PCNA in groups C–E compared with the cancer control group.
Abbreviations: DECPR, dichloromethane extract of Curcuma purpurascens rhizome; PI, proliferation index; PCNA, proliferating cell nuclear antigen.
DECPR induced upregulation of Bax and downregulation of Bcl-2
The Bcl-2 family of proteins, which has 25 members, has been established to consist of regulators and mediators of cellular life or death.45 This Bcl-2 family consists of two groups of proteins functioning as proapoptotic and antiapoptotic molecules. In addition, these proteins have a close relationship with mitochondria function. Therefore, changes in the expressions of these proteins can activate the intrinsic (mitochondrial) pathway of apoptosis.46 For example, proapoptotic protein of Bax dimerizes and translocates to the outer mitochondrial membrane and provides a channel for release of several proteins, including cytochrome c. However, this process can be suppressed by antiapoptotic mediators, including Bcl-2, Bcl-x, Bcl-w, and BAG.47
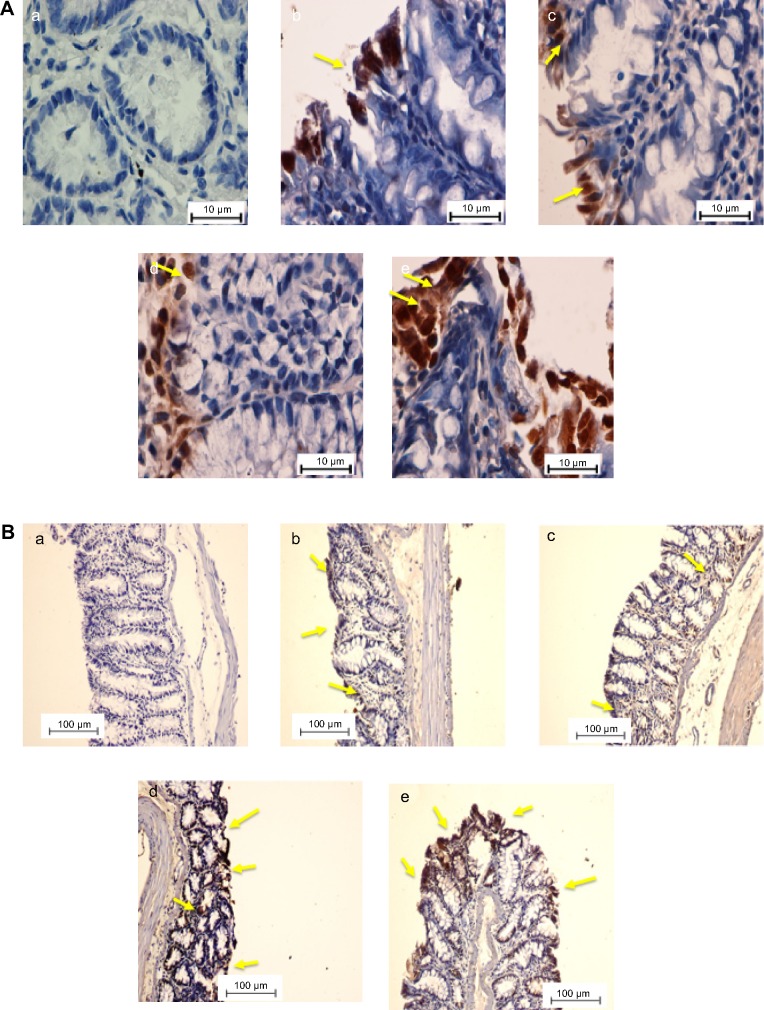
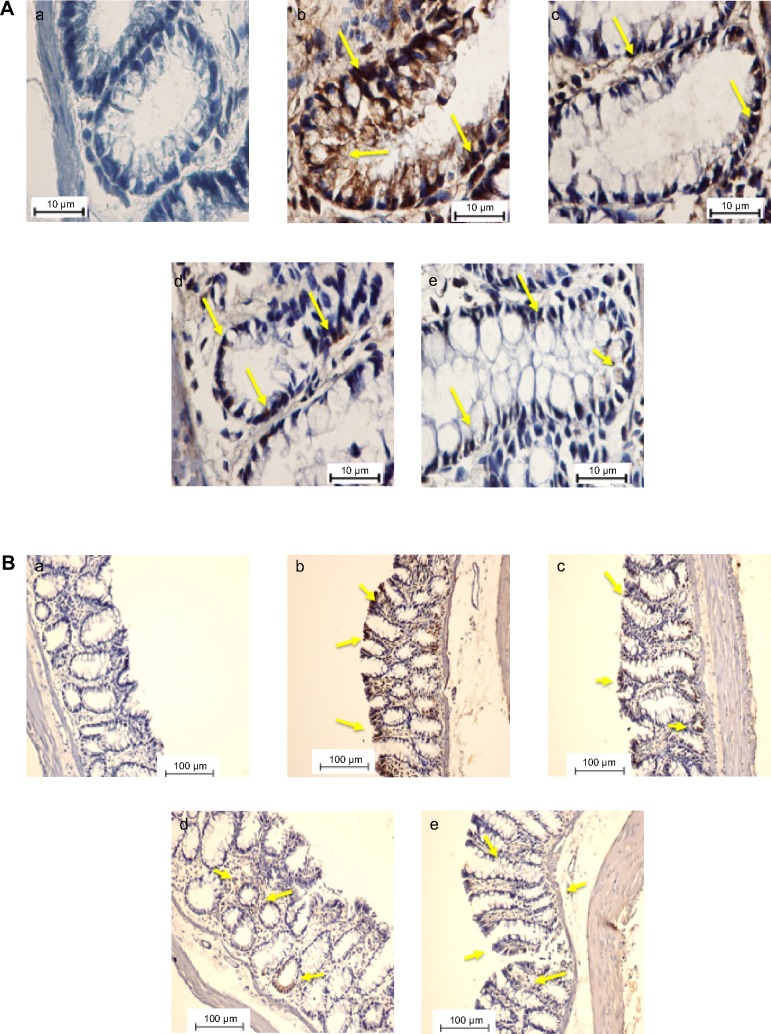
To determine the role of apoptosis in the chemopreventive potential of DECPR, we performed immunohistochemical analysis on Bax and Bcl-2 proteins. In the cancer control rats (group B), colon tissue sections revealed a small degree of immunostaining for Bax protein (Figure 4), while a marked degree of immunostaining was found for Bcl-2 protein (Figure 5). A conspicuous increase in the Bax protein expression and decrease in the Bcl-2 protein expression were detected after administration of DECPR at 250 mg/kg (group C) and 500 mg/kg (group D) doses. The 5-FU drug group (group E) showed a comparable result to groups treated with DECPR. Western blot analysis also confirmed the observed perturbations in the protein expression of Bax and Bcl-2 (Figure 6). It was previously reported that sufficient expression of Bax in cancer tissues may ameliorate the survival rate of cancer patients.48 On the other hand, defect in Bax function has a substantial effect on cancer progression.49 Our findings suggest that DECPR has a promising potential to activate mitochondrial-mediated apoptosis in cells exposed to a chemical carcinogen. This result is in agreement with our previous in vitro investigation, which showed induction of apoptosis in HT-29 cells through mitochondrial-mediated apoptosis.25
Figure 4.
Immunohistochemical expression of Bax in colon tissues of control and experimental groups of rats.
Notes: (A and B) The five groups of rats were (a) normal control; (b) cancer control; (c) low dose of DECPR; (d) high dose of DECPR; and (e) 5-fluorouracil treatment control. Bax protein expression is illustrated as brown staining. The yellow arrows depict the up regulation of Bax in groups C–E is shown as brown staining. (A) Scale bar: 10 µm. (B) Scale bar: 100 µm.
Abbreviation: DECPR, dichloromethane extract of Curcuma purpurascens rhizome.
Figure 5.
Immunohistochemical expression of Bcl-2 in colon tissues of control and experimental groups of rats.
Notes: (A and B) The five groups of rats were (a) normal control; (b) cancer control; (c) low dose of DECPR; (d) high dose of DECPR; and (e) 5-fluorouracil treatment control. Bcl-2 protein expression is illustrated as brown staining. The yellow arrows depict the down regulation of Bcl-2 in groups C–E is shown as brown staining. (A) Scale bar: 10 µm. (B) Scale bar: 100 µm.
Abbreviation: DECPR, dichloromethane extract of Curcuma purpurascens rhizome.
Figure 6.
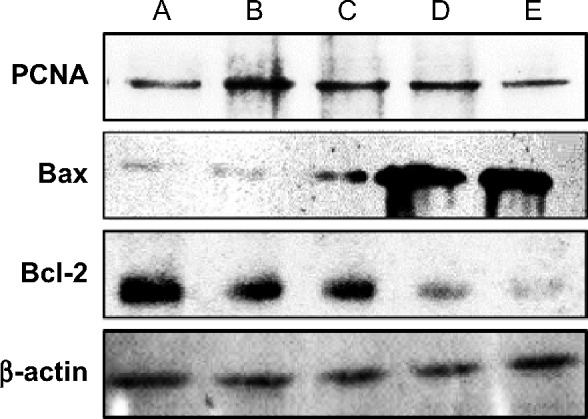
Western blot analysis of PCNA, Bax, and Bcl-2 proteins extracted from colon tissues of rats.
Notes: The five groups of rats were A: normal control; B: 10% Tween 20 (cancer control); C: low dose of DECPR; D: high dose of DECPR; and E: 5-fluorouracil treatment control. β-actin Western blotting was used as the control band.
Abbreviation: DECPR, dichloromethane extract of Curcuma purpurascens rhizome; PCNA, proliferating cell nuclear antigen.
DECPR augmented enzymatic antioxidant activities
A previous study showed that oxidative stress is involved in the pathogenesis of several diseases such as cancer and cardiovascular disorder.50 It has also been shown previously that concomitant use of antioxidant drugs with anticancer agents such as doxorubicin can potentiate their therapeutic effects and ameliorate the survival rate of patients.50–52 Hence, development of new chemotherapeutic drugs with inborn antioxidant activity is in the best interests of cancer researchers. Moreover, identifying different potent antioxidants derived from natural products has stimulated significant scientific attention in characterizing these phytochemicals to counteract the effects of oxidative stress.53,54
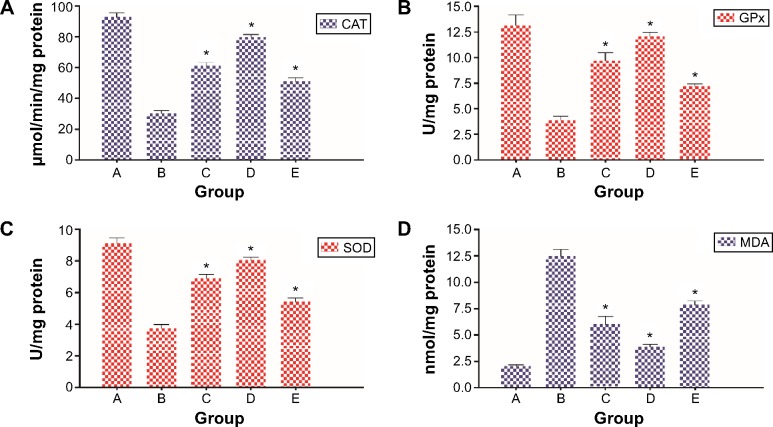
The activities of enzymatic antioxidants, including CAT, GPx, and SOD, in colon tissue homogenates from different groups of rats are depicted in Figure 7. The levels of CAT, GPx, and SOD were significantly decreased in cancer-bearing animals (group B), which shows similarity to previous studies.14,15
Figure 7.
Levels of enzymatic antioxidants and MDA in the colon tissues of control and experimental groups.
Notes: (A) CAT. (B) GPx. (C) SOD. (D) MDA. Data are shown as mean ± standard error of the mean (n=6). Values are statistically significant at *P<0.05. The five groups of rats were A: normal control; B: cancer control; C: low dose of DECPR; D: high dose of DECPR; and E: 5-fluorouracil treatment control.
Abbreviation: DECPR, dichloromethane extract of Curcuma purpurascens rhizome; CAT, catalase; GPx, glutathione peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde.
In rats treated with both doses of DECPR, these activities were significantly augmented compared to the cancer control group. As expected, DECPR elicited higher enzymatic activities compared to the 5-FU chemotherapeutic drug group (group E). This result is in line with a previous gastroprotective study on C. purpurascens rhizome, which showed potent antioxidative potential of this plant.55 Moreover, a number of investigations on other Curcuma spp. have established this genus as a source of phytochemicals with antioxidant and anti-inflammatory properties.56–58 For instance, turmerone and its derivatives, as the major phytochemicals of DECPR, have been shown to be potent antioxidants in previous studies.59–61 Our result demonstrates that DECPR protects the colons of rats from oxidative stress that was provoked by AOM treatment.
DECPR suppressed lipid peroxidation
Under cellular stress, neutrophils can cause the generation of superoxide radical anions, which leads to the formation of lipid peroxides as a result of reaction with cellular lipids.62 Several products, including lipid hydroperoxides, alkenes, and malondialdehyde (MDA), are degraded metabolites of this reaction, which eventually compromise the cell membrane integrity.63,64 Since MDA is a prime product of lipid peroxidation, it is defined as an effective marker of this process.14,65 As shown in Figure 7, AOM administration to rats provoked significant MDA production due to lipid peroxidation. This result is in agreement with previous reports showing augmented MDA generation in tissue and plasma of patients suffering from colorectal cancer.66,67 Administration of rats with DECPR at 250 and 500 mg/kg doses attenuated MDA production in tissue homogenates. This protective effect against lipid peroxidation was more effective than that produced by 5-FU. Our data verified the antioxidative potential of DECPR against the oxidative stress induced by a chemical carcinogen, which was reflected by a decreased level of MDA.
Conclusion
Data from the present study demonstrate, for the first time, experimental evidence that DECPR exerts a noteworthy chemopreventive activity against chemical-induced colon cancer. In a previous study conducted by the present authors, it was reported that γ-elemene, a-elemenone, ar-turmerone, turmerone, and curlone were detected in the Gas chromatography-mass spectrometry analysis of DECPR, with turmerone being the major compound.26 This prominent chemopreventive effect of DECPR at both doses is reflected in its ability to induce apoptosis by reducing PCNA and Bcl-2 and enhancing Bax protein expression. However, it is pivotal to investigate in future studies if DECPR anticancer activity can be verified in other colon cancer models as well as in genetically modified animal models. In addition, further studies should focus on investigating the in vivo chemopreventive effect of turmerone, the major compound of DECPR.
Acknowledgments
This work was supported by the University Malaya Research Grant (UMRG: RG539-13HTM) and High Impact Research (HIR) MOHE Grant (UM HIR MOHE E000049-20001).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hajiaghaalipour F, Kanthimathi MS, Sanusi J, Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015;169:401–410. doi: 10.1016/j.foodchem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Tenesa A, Dunlop MG. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet. 2009;10(6):353–358. doi: 10.1038/nrg2574. [DOI] [PubMed] [Google Scholar]
- 3.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21(20):2525–2538. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Pei Z. Bacteria, inflammation, and colon cancer. World J Gastroenterol. 2006;12(42):6741–6746. doi: 10.3748/wjg.v12.i42.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9(4):405–410. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Martínez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 7.Rajamanickam S, Agarwal R. Natural products and colon cancer: current status and future prospects. Drug Dev Res. 2008;69(7):460–471. doi: 10.1002/ddr.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randhawa MA, Alghamdi MS. Anticancer activity of Nigella sativa (black seed) – a review. Am J Chin Med. 2011;39(6):1075–1091. doi: 10.1142/S0192415X1100941X. [DOI] [PubMed] [Google Scholar]
- 9.Raju J. Azoxymethane-induced rat aberrant crypt foci: relevance in studying chemoprevention of colon cancer. World J Gastroenterol. 2008;14(43):6632–6635. doi: 10.3748/wjg.14.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alrawi SJ, Schiff M, Carroll RE, et al. Aberrant crypt foci. Anticancer Res. 2006;26(1A):107–119. [PubMed] [Google Scholar]
- 11.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93(1):55–71. doi: 10.1016/0304-3835(95)03788-X. [DOI] [PubMed] [Google Scholar]
- 12.Fenoglio-Preiser CM, Noffsinger A. Aberrant crypt foci: a review. Toxicol Pathol. 1999;27(6):632–642. doi: 10.1177/019262339902700604. [DOI] [PubMed] [Google Scholar]
- 13.Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339(18):1277–1284. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 14.Zorofchian Moghadamtousi S, Rouhollahi E, Karimian H, et al. The chemopotential effect of Annona muricata leaves against azoxymethane-induced colonic aberrant crypt foci in rats and the apoptotic effect of Acetogenin Annomuricin E in HT-29 cells: a bioassay-guided approach. PLoS One. 2015;10(4):e0122288. doi: 10.1371/journal.pone.0122288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajrezaie M, Hassandarvish P, Moghadamtousi SZ, et al. Chemopreventive evaluation of a Schiff base derived copper (II) complex against azoxymethane-induced colorectal cancer in rats. PLoS One. 2014;9(3):e91246. doi: 10.1371/journal.pone.0091246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moghadamtousi SZ, Kamarudin MNA, Chan CK, Goh BH, Kadir HA. Phytochemistry and biology of Loranthus parasiticus Merr, a commonly used herbal medicine. Am J Chin Med. 2014;42(1):23–35. doi: 10.1142/S0192415X14500025. [DOI] [PubMed] [Google Scholar]
- 17.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29(2):197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 19.Schaffer M, Schaffer PM, Zidan J, Bar Sela G. Curcuma as a functional food in the control of cancer and inflammation. Curr Opin Clin Nutr Metab Care. 2011;14(6):588–597. doi: 10.1097/MCO.0b013e32834bfe94. [DOI] [PubMed] [Google Scholar]
- 20.Nahar L, Sarker SD. Phytochemistry of the genus Curcuma. In: Ravindran PN, Babu KN, Sivaraman K, editors. Turmeric: The Genus Curcuma. Boca Raton: CRC Press; 2007. pp. 71–106. [Google Scholar]
- 21.Hong SL, Lee GS, Syed Abdul Rahman SN, et al. Essential oil content of the rhizome of Curcuma purpurascens Bl. (Temu Tis) and Its antiproliferative effect on selected human carcinoma cell lines. Scientific World Journal. 2014;2014:397430. doi: 10.1155/2014/397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller E. Javanese Medical Plants Used in Rural Communities. Vienna: University of Vienna; 2009. [Google Scholar]
- 23.Garber JC, Barbee RW, Bielitzki JT, et al. Guide for the care and use of laboratory animals. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 24.Shwter AN, Abdullah NA, Alshawsh MA, et al. Chemoprevention of colonic aberrant crypt foci by Gynura procumbens in rats. J Ethnopharmacology. 2014;151(3):1194–1201. doi: 10.1016/j.jep.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 25.Rouhollahi E, Zorofchian Moghadamtousi S, Paydar M, et al. Inhibitory effect of Curcuma purpurascens BI. rhizome on HT-29 colon cancer cells through mitochondrial-dependent apoptosis pathway. BMC Complement Altern Med. 2015;15:15. doi: 10.1186/s12906-015-0534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghadamtousi SZ, Rouhollahi E, Karimian H, Fadaeinasab M, Abdulla MA, Kadir HA. Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther. 2014;8:2099–2110. doi: 10.2147/DDDT.S70096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almagrami AA, Alshawsh MA, Saif-Ali R, Shwter A, Salem SD, Abdulla MA. Evaluation of chemopreventive effects of Acanthus ilicifolius against azoxymethane-induced aberrant crypt foci in the rat colon. PLoS One. 2014;9(5):e96004. doi: 10.1371/journal.pone.0096004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37(2):147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 29.Abdulaziz Bardi A, Halabi MF, Hassandarvish P, et al. Andrographis paniculata leaf extract prevents thioacetamide-induced liver cirrhosis in rats. PLoS One. 2014;9(10):e109424. doi: 10.1371/journal.pone.0109424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med. 1988;4(3):155–161. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 31.Gourineni V, Verghese M, Boateng J, Shackelford L, Bhat K. Chemopreventive potential of synergy1 and soybean in reducing azoxymethane-induced aberrant crypt foci in fisher 344 male rats. J Nutr Metab. 2011;2011:983038. doi: 10.1155/2011/983038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo YW, Chen YH, Chiu WC, Liao H, Lin SH. Soy saponins meditate the progression of colon cancer in rats by inhibiting the activity of β-glucuronidase and the number of aberrant crypt foci but not cyclooxygenase-2 activity. ISRN Oncol. 2013;2013:645817. doi: 10.1155/2013/645817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993;14(11):2219–2225. doi: 10.1093/carcin/14.11.2219. [DOI] [PubMed] [Google Scholar]
- 34.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55(2):259–266. [PubMed] [Google Scholar]
- 35.Kwon Y, Malik M, Magnuson BA. Inhibition of colonic aberrant crypt foci by curcumin in rats is affected by age. Nutr Cancer. 2004;48(1):37–43. doi: 10.1207/s15327914nc4801_6. [DOI] [PubMed] [Google Scholar]
- 36.Bounaama A, Djerdjouri B, Laroche-Clary A, Le Morvan V, Robert J. Short curcumin treatment modulates oxidative stress, arginase activity, aberrant crypt foci, and TGF-β1 and HES-1 transcripts in 1,2-dimethylhydrazine-colon carcinogenesis in mice. Toxicology. 2012;302(2–3):308–317. doi: 10.1016/j.tox.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71(8):2454–2460. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116(Pt 15):3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 39.Isozaki H, Okajima K, Ichinona T, et al. The significance of proliferating cell nuclear antigen (PCNA) expression in cancer of the ampulla of vater in terms of prognosis. Surg Today. 1994;24(6):494–499. doi: 10.1007/BF01884567. [DOI] [PubMed] [Google Scholar]
- 40.Naryzhny SN, Lee H. Characterization of proliferating cell nuclear antigen (PCNA) isoforms in normal and cancer cells: there is no cancer-associated form of PCNA. FEBS Lett. 2007;581(25):4917–4920. doi: 10.1016/j.febslet.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Lipkin M, Blattner WE, Fraumeni JF, Jr, Lynch HT, Deschner E, Winawer S. Tritiated thymidine (phi p, phi h) labeling distribution as a marker for hereditary predisposition to colon cancer. Cancer Res. 1983;43(4):1899–1904. [PubMed] [Google Scholar]
- 42.Ponz de Leon M, Roncucci L, Di Donato P, et al. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res. 1988;48(14):4121–4126. [PubMed] [Google Scholar]
- 43.Deschner EE, Long FC, Hakissian M, Herrmann SL. Differential susceptibility of AKR, C57BL/6J, and CF1 mice to 1,2-dimethylhydrazine-induced colonic tumor formation predicted by proliferative characteristics of colonic epithelial cells. J Natl Cancer Inst. 1983;70(2):279–282. [PubMed] [Google Scholar]
- 44.Bishayee A, Mandal A. Trianthema portulacastrum Linn. exerts chemoprevention of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats. Mutat Res. 2014;768:107–118. doi: 10.1016/j.mrfmmm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 46.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 47.Ocker M, Höpfner M. Apoptosis-modulating drugs for improved cancer therapy. Eur Surg Res. 2012;48(3):111–120. doi: 10.1159/000336875. [DOI] [PubMed] [Google Scholar]
- 48.Sturm I, Petrowsky H, Volz R, et al. Analysis of p53/BAX/p16(ink4a/CDKN2) in esophageal squamous cell carcinoma: high BAX and p16(ink4a/CDKN2) identifies patients with good prognosis. J Clin Oncol. 2001;19(8):2272–2281. doi: 10.1200/JCO.2001.19.8.2272. [DOI] [PubMed] [Google Scholar]
- 49.Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci U S A. 2000;97(20):10872–10877. doi: 10.1073/pnas.190210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granados-Principal S, El-Azem N, Pamplona R, et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem Pharmacol. 2014;90(1):25–33. doi: 10.1016/j.bcp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Lissoni P, Barni S, Mandalà M, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 1999;35(12):1688–1692. doi: 10.1016/s0959-8049(99)00159-8. [DOI] [PubMed] [Google Scholar]
- 52.Cerea G, Vaghi M, Ardizzoia A, et al. Biomodulation of cancer chemotherapy for metastatic colorectal cancer: a randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticancer Res. 2003;23(2C):1951–1954. [PubMed] [Google Scholar]
- 53.Liao JC, Deng JS, Chiu CS, et al. Chemical compositions, anti-inflammatory, antiproliferative and radical-scavenging activities of Actinidia callosa var. ephippioides. Am J Chin Med. 2012;40(5):1047–1062. doi: 10.1142/S0192415X12500772. [DOI] [PubMed] [Google Scholar]
- 54.Zhang XQ, Kim JH, Lee GS, et al. In vitro antioxidant and in vivo anti-inflammatory activities of Ophioglossum thermale. Am J Chin Med. 2012;40(2):279–293. doi: 10.1142/S0192415X1250022X. [DOI] [PubMed] [Google Scholar]
- 55.Rouhollahi E, Moghadamtousi SZ, Hamdi OA, et al. Evaluation of acute toxicity and gastroprotective activity of curcuma purpurascens BI. rhizome against ethanol-induced gastric mucosal injury in rats. BMC Complement Altern Med. 2014;14:378. doi: 10.1186/1472-6882-14-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramsewak RS, DeWitt DL, Nair MG. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine. 2000;7(4):303–308. doi: 10.1016/S0944-7113(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 57.Mau J-L, Lai EYC, Wang N-P, Chen C-C, Chang C-H, Chyau C-C. Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem. 2003;82(4):583–591. [Google Scholar]
- 58.Srinivas L, Shalini VK, Shylaja M. Turmerin: a water soluble antioxidant peptide from turmeric [Curcuma longa] Arch Biochem Biophys. 1992;292(2):617–623. doi: 10.1016/0003-9861(92)90040-4. [DOI] [PubMed] [Google Scholar]
- 59.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch C. 2002;57(9–10):828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 60.Sacchetti G, Maietti S, Muzzoli M, et al. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91(4):621–632. [Google Scholar]
- 61.Tsai S-Y, Huang S-J, Chyau C-C, Tsai C-H, Weng C-C, Mau J-L. Composition and antioxidant properties of essential oils from Curcuma rhizome. Asian Journal of Arts and Sciences. 2011;2(1):57–66. [Google Scholar]
- 62.Kobayashi T, Ohta Y, Yoshino J, Nakazawa S. Teprenone promotes the healing of acetic acid-induced chronic gastric ulcers in rats by inhibiting neutrophil infiltration and lipid peroxidation in ulcerated gastric tissues. Pharmacol Res. 2001;43(1):23–30. doi: 10.1006/phrs.2000.0748. [DOI] [PubMed] [Google Scholar]
- 63.Vaca CE, Wilhelm J, Harms-Ringdahl M. Interaction of lipid per-oxidation products with DNA. A review. Mutat Res. 1988;195(2):137–149. doi: 10.1016/0165-1110(88)90022-x. [DOI] [PubMed] [Google Scholar]
- 64.Pandurangan AK, Dharmalingam P, Ananda Sadagopan SK, Ganapasam S. Effect of luteolin on the levels of glycoproteins during azoxymethane-induced colon carcinogenesis in mice. Asian Pac J Cancer Prev. 2012;13(4):1569–1573. doi: 10.7314/apjcp.2012.13.4.1569. [DOI] [PubMed] [Google Scholar]
- 65.Demircan B, Çelik G, Süleyman H, Akçay F. Effects of indomethacin, celecoxib and meloxicam on glutathione, malondialdehyde and myeloperoxidase in rat gastric tissue. The Pain Clinic. 2005;17(4):383–388. [Google Scholar]
- 66.Hendrickse C, Kelly RW, Radley S, Donovan IA, Keighley MR, Neoptolemos JP. Lipid peroxidation and prostaglandins in colorectal cancer. Br J Surg. 1994;81(8):1219–1223. doi: 10.1002/bjs.1800810849. [DOI] [PubMed] [Google Scholar]
- 67.Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11(3):403–406. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]