Abstract
Pulmonary edema clearance is necessary for patients with lung injury to recover and survive. The mechanisms regulating edema clearance from the lungs are distinct from the factors contributing edema formation during injury. Edema clearance is effected via vectorial transport of Na+ out of the airspaces which generates an osmotic gradient causing water to follow the gradient out of the cells. This Na+ transport across the alveolar epithelium is mostly effected via apical Na+ and chloride channels and basolateral Na,K-ATPase. The Na,K-ATPase pumps Na+ out of the cell and K+ into the cell against their respective gradients in an ATP-consuming reaction. Two mechanisms contribute to the regulation of the Na,K-ATPase activity:recruitment of its subunits from intracellular compartments into the basolateral membrane, and transcriptional/translational regulation. Na,K-ATPase activity and edema clearance are increased by catecholamines, aldosterone, vasopressin, overexpression of the pump genes, and others. During lung injury, mechanisms regulating edema clearance are inhibited by yet unclear pathways. Better understanding of the mechanisms that regulate pulmonary edema clearance may lead to therapeutic interventions that counterbalance the inhibition of edema clearance during lung injury and improve the lungs’ ability to clear fluid, which is crucial for patient survival.
Keywords: Acute lung injury; alveolar epithelium; alveolar fluid clearance; Na,K-ATPase; pulmonary edema
INTRODUCTION
Pulmonary edema is a life-threatening condition of fluid excess in the lungs that causes impaired gas exchange with consequent symptoms that range from mild shortness of breath to acute respiratory failure. Approximately 56% of intensive care unit patients suffer from acute respiratory failure (ARF), with one-third of those subsequently dying.1 The pathogenesis of ARF can be classified to cardiogenic and non-cardiogenic pulmonary edema. Acute heart failure is the most common cause of increased hydrostatic pressure and is very prevalent, with almost 658,000 emergency department visits in the United States per year. The mortality rate from acute cardiogenic pulmonary edema ranges from 12% to 15%.2 In the USA, overall costs of heart failure in 2010 have been estimated at $39.2 billion, with hospitalization representing approximately 80% of direct treatment costs for heart failure.3
Acute lung injury (ALI) that is due to increased permeability pulmonary edema is also common, with an incidence of 86 per 100,000 person-years, and equates to over 190,000 cases and 74,500 fatalities annually in the United States. Although mortality has declined, recent studies still report an approximate 25% death rate.2
For many years, it was believed that fluid accumulation in the lung depends only on the abrogation of balanced Starling forces—the hydrostatic pressure and oncotic pressures.4,5 However, more recently it has been demonstrated that the alveolar epithelium has an active role in clearing edema out of the alveoli, a process called alveolar fluid clearance (AFC).
LUNG STRUCTURE
The lung is responsible for gas exchange: enriching the circulation with oxygen (O2) and extruding carbon dioxide (CO2). The structure of the lungs is designed to facilitate gas exchange by enabling the transit of gases through the respiratory airways, and, as the gases reach alveolar sacs and alveolus clusters, gas exchange actually occurs. The alveoli are tightly wrapped with blood vessels allowing the diffusion of oxygen from the alveoli to the blood-stream of the alveolar blood vessels. Then, oxygenated blood is perfused throughout the body where gas exchange occurs in the capillary beds.6
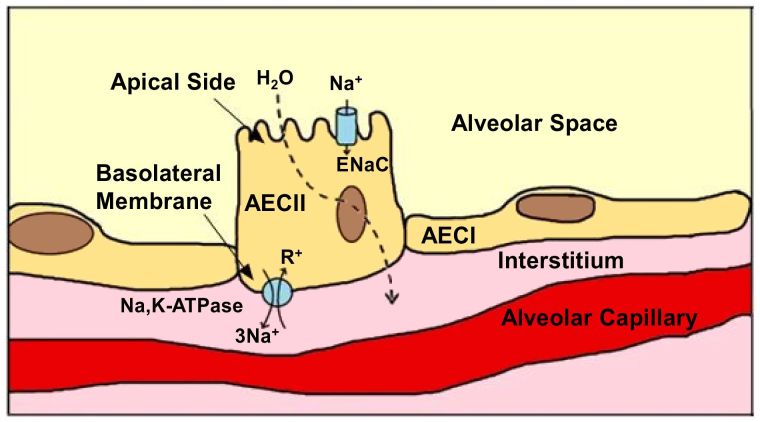
Since gas exchange relies on diffusion, it is crucial that the layer separating the alveolar space from the interstitium is thin and permeable.6 To ensure this environment, alveoli are built of a monolayer epithelium that contains two alveolar epithelial cells (Figure 1), types I and II (AECI and AECII, respectively), and macrophages.7 Moreover, the alveolar space must be free of fluids and open. In utero, the fetal lung is filled with fluid that is removed shortly after birth, mainly because active reabsorption of sodium ions (Na+) across the alveolar epithelium creates an osmotic force favoring reabsorption of alveolar fluid.8
Figure 1. Schematic Representation of Alveolar Epithelial Cells with the Components that Contribute to the Alveolar Fluid Clearance Process.
AECI, alveolar epithelial cell type I; AECII, alveolar epithelial cell type II; ENaC, epithelial Na+ channel; Na,K-ATPase, sodium-potassium pump.
Alveolar epithelial cells type I are squamous with a diameter of about 50–100 μm; however, they are very thin, thus minimizing the diffusion distance between the alveolar airspace and the pulmonary capillaries, which facilitates gas exchange. Although they constitute 5%–10% of all lung cells,9 AECI cover more than 90% of the alveolar surface as they are very large and have thin cytoplasmic extensions. Recently, it was reported that AECI play an active role in water permeability and the regulation of alveolar fluid homeostasis.10,11
The alveolar epithelial cells type II are smaller and cuboidal, with a diameter of 21 μm in rats and 50 μm in humans. They occupy only ~5% of the surface area, yet AECII constitute ~15% of all lung cells and 60% of alveolar epithelial cells. They produce, secrete, and recycle lung surfactant; they transport ions, participate in lung immune responses, and can also be converted to AECI to repair damaged epithelium or during fetal lung development. The AECII has a distinct morphology with characteristic lamellar bodies and a bipolar plasma membrane, consisting of an apical side that has short microvilli and a basolateral domain. These cells contain a wide range of transport proteins, including epithelial Na+ channels (ENaCs), Na,K-ATPase, Na-H exchanger (NHE), and aquaporin-3 (AQP3).12–16
HISTORIC PERSPECTIVE
Normand et al. demonstrated that fetal lamb lungs can absorb fluid from the airspaces at birth,17 and by using 131I tracer Walters and Olver calculated the rate of lung liquid secretion in fetal lambs.18 Matthay et al. reported that mature sheep lungs have the ability to clear edema.19 Since then, alveolar fluid clearance has been extensively investigated.4,20–25
ALVEOLAR ACTIVE SODIUM TRANSPORT MECHANISM
Alveolar fluid clearance (AFC) is an active process carried out mostly by the apical epithelial Na+ channels, and the basolateral Na,K-ATPase is involved in AFC.13,24
Briefly, Na+ enters the alveolar epithelial cells through the apical amiloride-sensitive Na+ channels (ENaC), is transported through alveolar epithelial cells, and by a process that consumes energy is pumped out of the cell by the Na,K-ATPase located in the basolateral membrane in exchange for potassium entry in a ratio of 3:2 Na+–K+ against their chemical gradient. This active vectorial Na+ flux produces a transepithelial osmotic gradient that causes water to move from the airspaces following the gradient.8,26–28
Alveolar fluid reabsorption can be modulated by pharmacologic agents, gene therapy, and other interventions. Catecholamines, growth factors, vasopressin, aldosterone, overexpression of Na,K-ATPase subunits and chronic heart failure model increase alveolar fluid reabsorption (Figure 2A).16,29–40
Figure 2. The Effect of Various Pharmacologic and Pathophysiologic Conditions on Alveolar Fluid Clearance.
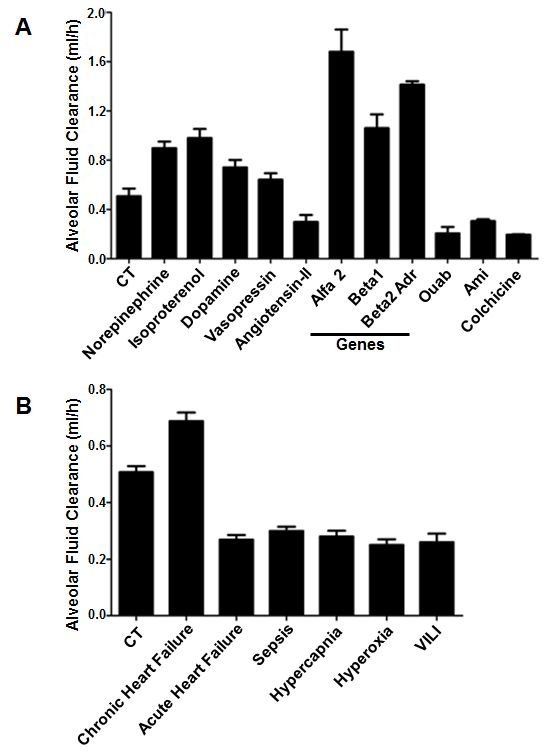
A: The rate of alveolar fluid clearance (AFC) was modulated following therapeutic interventions: catecholamines, vasopressin, and gene therapy upregulated AFC; however, the administration of endothelin, angiotensin, amiloride, ouabain, or colchicine inhibited active sodium transport and thus AFC. The data were adapted from references 13, 27, 28, 31, 33, 36, 37.
B: Alveolar fluid clearance (AFC) was decreased in the various states of acute lung injury, such as sepsis, hyperoxia, hypercapnia, and ventilation-induced lung injury. Moreover, in rats exposed to acutely increased left atrial pressure (e.g. acute left heart failure) AFC was inhibited; whereas AFC was significantly upregulated in chronic heart failure rats. The data were adapted from references 34, 40–43.
The bars represent mean ± SEM. Alfa2, α2-subunit of Na,K-ATPase; Ami, amiloride; beta1, β1-subunit of Na,K-ATPase; beta2 adr, β2 adrenergic receptor; CT, control; Ouab, ouabain; VILI, ventilation-induced lung injury.
Active Na+ transport and edema clearance are inhibited by interventions that can be divided into several categories:
General AFC inhibitors such as the sodium channel blocker, amiloride,41 and the Na,K-ATPase inhibitor, ouabain.5
Consequences of acute lung injury (ALI), hypoxia,42 and hypercapnia that impair the alveolar epithelial function by increasing intracellular calcium levels.43,44
Mechanisms of ALI including sepsis,45 hyperoxia,46 high tidal volume ventilation and ventilation-induced lung injury,47 acute left atrial hypertension,48 andendothelin (Figure 2).49
Notably, aerosolized or intravenous β2-agonist therapy did not improve clinical outcomes in patients with lung injury; therefore, the use of β2-agonist therapy was not recommended in mechanically ventilated patients with lung injury.50,51 A more recent study appears to shed light onto the disparate effect of β2-agonist therapy in vitro in animals, and in patients with lung injury. Apparently, the β2-adrenergic receptor (β2AR) on alveolar macrophages can augment the release of IL-6, thus linking the sympathetic nervous system, by β2AR signaling, with lung inflammation and enhanced susceptibility to thrombotic cardiovascular events, which could have negative effects on the outcome of patients with acute lung injury.52
Alveolar fluid clearance reflects active sodium transport, and several mediators participate in this process, including Na,K-ATPase, Na+ channels, aquaporins, and others. The sodium–potassium pump, an energy-consuming enzyme (Na,K-ATPase), is a heterodimeric integral membrane protein that is synthesized in polysomes related to the rough endoplasmic reticulum. It is composed of two subunits: an α-subunit (a catalytic 110-kDa unit) and a β-subunit (a regulatory 55-kDa unit). The α-subunit contains binding sites for ATP hydrolysis, Na+, K+, and cardiac glycosides. There are at least three isoforms of α (α1, α2, and α3); they differ by their affinity to sodium, ouabain, and tissue distribution. The β-subunit is thought to be responsible for incorporating the α-subunit into the plasma membrane; there are three known isoforms of β-subunit.
A critical role is played by Na,K-ATPase in the homeostasis of Na+ and K+ during altered salt intake and pH regulation, besides its other important functions in several organs; therefore it is strictly regulated.8,53 The Na,K-ATPase can be regulated at the level of expression, internalization, and recruitment of the pump proteins to the basolateral membrane (Figure 3). For example, catecholamines enhance the ability of the lungs to clear edema by recruiting Na,K-ATPase from the alveolar epithelial cell (AEC) cytosol to the basolateral membranes within minutes and thus increasing the activity of the pump. This process is mediated via the cAMP/PKA pathway.30,54,55 In contrast, the adverse effects on AFC of hypoxia, hypercapnia, sepsis, and endothelin are due to the AMPK/PKC pathway, in which Na,K-ATPase is phosphorylated, leading to its endocytosis.42,45,49,56
Figure 3. Schematic Representation of Active Sodium Transport in the Alveolar Epithelial Cell Depicting Apical Na+ Channels, Basolaterally Located Na,K-ATPase, Aquaporins, and Co-transporters.
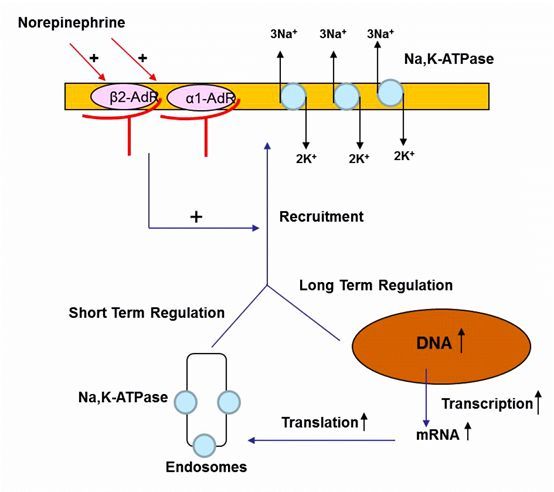
Sodium enters through the apical membrane via Na+ channels and is extruded by the Na,K-ATPase, with water following iso-osmotically. Proposed mechanisms by which norepinephrine upregulates active sodium transport and alveolar clearance.
An important contributor to alveolar sodium transport is the sodium channel (ENaC). The ENaC is a heterotrimeric protein that can be composed by different combinations of three known subunits: αENaC, βENaC, and γENaC. Alveolar epithelial cells contain three types of channels with different selectivity properties: (1) a Ca2+-activated non-selective Na+ channel (NSC) composed of α-subunits alone, (2) a Na+-selective (moderately selective) channel composed of a combination of αENaC and βENaC or γENaC, and (3) the highly NSC composed of the three different subunits. The ENaC is located on the apical portion of AECs and plays a crucial role in sodium transport and AFC.28,30,57 Moreover, it has been shown that knocking out αENaC leads to defective AFC and premature death in newborn mice.
Water channels or aquaporins (AQPs) are expressed in the lungs. AQP1 is located in both the apical and basolateral aspects of endothelial cells and fibroblasts, and AQP3, AQP4, and AQP5 are expressed in the respiratory epithelium. In the human respiratory tract, AQP5 is expressed in the apical surface of AECI and AQP3 in the apical and basal membrane of AECII. Targeted deletions of AQPs in transgenic mice suggest that AQPs are not essential for alveolar fluid clearance; however, other compensatory mechanisms could have taken place instead of the deletion of AQPs.58
While the contribution of AECI to alveolar fluid clearance has been suggested, further studies to evaluate the role of distal epithelial cells are necessary. We also need to explore the role of chloride channels and their regulation, particularly in the pathways of activation as well as functional contributions of AQPs and cystic fibrosis transmembrane conductance regulator (CFTR). Additionally, signal transduction pathways as well as translation and post-translational pathways need to be studied as they may inform the field and help with novel therapies to modulate these pathways to enhance pulmonary edema clearance in patients with lung injury.
CONCLUSIONS
The mechanisms of lung edema clearance contrast with the regulation of pulmonary edema formation. Clearance of edema fluid is an active process that requires active transport of Na+ out of alveolar airspaces, with water following the osmotic gradient. The regulation of vectorial Na+ transport across the alveolo-capillary barrier is mediated mostly by apical Na+ channels and basolaterally expressed Na,K-ATPases. In patients with acute respiratory distress syndrome and lung injury the mechanisms regulating alveolar fluid reabsorption are impaired, and the restoration of the alveolar epithelial function to keep the lungs dry is important for normal gas exchange to occur and patients to survive. More knowledge about the mechanisms regulating lung edema clearance is needed in order to develop novel therapeutic strategies to accelerate fluid clearance and improve the alveolar epithelial function.
Abbreviations
- AEC
alveolar epithelial cell
- AFC
alveolar fluid clearance
- AQP
aquaporin
- β2AR
β2-adrenergic receptor
- CFTR
cystic fibrosis transmembrane conductance regulator
- ENaC
epithelial Na+ channel
- NHE
Na-H exchanger
- NSC
non-selective Na+ channel.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Vincent JL, Akca S, De Mendonca A, et al. The epidemiology of acute respiratory failure in critically ill patients(*) Chest. 2002;121:1602–9. doi: 10.1378/chest.121.5.1602. [DOI] [PubMed] [Google Scholar]
- 2.Mac Sweeney R, McAuley DF, Matthay MA. Acute lung failure. Semin Respir Crit Care Med. 2011;32:607–25. doi: 10.1055/s-0031-1287870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthiaume Y, Broaddus VC, Gropper MA, et al. Alveolar liquid and protein clearance from normal dog lungs. J Appl Physiol. 1988;65:585–93. doi: 10.1152/jappl.1988.65.2.585. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AE, Guyton AC, Bishop VS. Permeability of the alveolar membrane to solutes. Circ Res. 1965;16:353–62. doi: 10.1161/01.RES.16.4.353. [DOI] [PubMed] [Google Scholar]
- 6.Weibel ER. On the tricks alveolar epithelial cells play to make a good lung. Am J Respir Crit Care Med. 2015;191:504–13. doi: 10.1164/rccm.201409-1663OE. [DOI] [PubMed] [Google Scholar]
- 7.Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med. 2001;163:1021–9. doi: 10.1164/ajrccm.163.4.2006116. [DOI] [PubMed] [Google Scholar]
- 8.Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na(+)-K(+)-ATPase. J Appl Physiol. 2002;93:1860–6. doi: 10.1152/japplphysiol.00022.2002. [DOI] [PubMed] [Google Scholar]
- 9.Stone KC, Mercer RR, Gehr P, et al. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–43. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 10.Dobbs LG, Johnson MD, Vanderbilt J, et al. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez R, Yang YH, Griffin C, et al. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol. 2005;288:L179–89. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- 12.Azzam ZS, Sznajder JI. The cellular mechanisms contributing to lung edema clearance. Isr Med Assoc J. 2000;2:235–9. [PubMed] [Google Scholar]
- 13.Matalon S, Benos DJ, Jackson RM. Biophysical and molecular properties of amiloride-inhibitable Na+ channels in alveolar epithelial cells. Am J Physiol. 1996;271:L1–22. doi: 10.1152/ajplung.1996.271.1.L1. [DOI] [PubMed] [Google Scholar]
- 14.Castranova V, Rabovsky J, Tucker JH, et al. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol. 1988;93:472–83. doi: 10.1016/0041-008X(88)90051-8. [DOI] [PubMed] [Google Scholar]
- 15.Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol. 2005;289:L685–95. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ridge KM, Olivera WG, Saldias F, et al. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–60. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 17.Normand IC, Olver RE, Reynolds EO, et al. Permeability of lung capillaries and alveoli to non-electrolytes in the foetal lamb. J Physiol. 1971;219:303–30. doi: 10.1113/jphysiol.1971.sp009663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters DV, Olver RE. The role of catecholamines in lung liquid absorption at birth. Pediatr Res. 1978;12:239–42. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Landolt CC, Staub NC. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol. 1982;53:96–104. doi: 10.1152/jappl.1982.53.1.96. [DOI] [PubMed] [Google Scholar]
- 20.Serikov VB, Grady M, Matthay MA. Effect of temperature on alveolar liquid and protein clearance in an in situ perfused goat lung. J Appl Physiol. 1993;75:940–7. doi: 10.1152/jappl.1993.75.2.940. [DOI] [PubMed] [Google Scholar]
- 21.Smedira N, Gates L, Hastings R, et al. Alveolar and lung liquid clearance in anesthetized rabbits. J Appl Physiol. 1991;70:1827–35. doi: 10.1152/jappl.1991.70.4.1827. [DOI] [PubMed] [Google Scholar]
- 22.Ballard ST, Schepens SM, Falcone JC, et al. Regional bioelectric properties of porcine airway epithelium. J Appl Physiol. 1992;73:2021–7. doi: 10.1152/jappl.1992.73.5.2021. [DOI] [PubMed] [Google Scholar]
- 23.Effros RM, Mason GR, Sietsema K, et al. Pulmonary epithelial sieving of small solutes in rat lungs. J Appl Physiol. 1988;65:640–8. doi: 10.1152/jappl.1988.65.2.640. [DOI] [PubMed] [Google Scholar]
- 24.Rutschman DH, Olivera W, Sznajder JI. Active transport and passive liquid movement in isolated perfused rat lungs. J Appl Physiol. 1993;75:1574–80. doi: 10.1152/jappl.1993.75.4.1574. [DOI] [PubMed] [Google Scholar]
- 25.Sakuma T, Okaniwa G, Nakada T, et al. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 1994;150:305–10. doi: 10.1164/ajrccm.150.2.8049807. [DOI] [PubMed] [Google Scholar]
- 26.Berthiaume Y, Folkesson HG, Matthay MA. Lung edema clearance: 20 years of progress invited review: alveolaredema fluid clearance in the injured lung. J Appl Physiol. 2002;93:2207–13. doi: 10.1152/japplphysiol.01201.2001. [DOI] [PubMed] [Google Scholar]
- 27.Borok Z, Verkman AS. Lung edema clearance: 20 years of progress invited review: role of aquaporin water channels in fluid transport in lung and airways. J Appl Physiol. 2002;93:2199–206. doi: 10.1152/japplphysiol.01171.2001. [DOI] [PubMed] [Google Scholar]
- 28.Matalon S, Lazrak A, Jain L, et al. Invited review: Biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol. 2002;93:1852–9. doi: 10.1152/japplphysiol.01241.2001. [DOI] [PubMed] [Google Scholar]
- 29.Adir Y, Azzam ZS, Lecuona E, et al. Augmentation of endogenous dopamine production increases lung liquid clearance. Am J Respir Crit Care Med. 2004;169:757–63. doi: 10.1164/rccm.200207-744OC. [DOI] [PubMed] [Google Scholar]
- 30.Azzam ZS, Adir Y, Crespo A, et al. Norepinephrine increases alveolar fluid reabsorption and Na,K-ATPase activity. Am J Respir Crit Care Med. 2004;170:730–6. doi: 10.1164/rccm.200308-1127OC. [DOI] [PubMed] [Google Scholar]
- 31.Barnard ML, Olivera WG, Rutschman DM, et al. Dopamine stimulates sodium transport and liquid clearance in rat lung epithelium. Am J Respir Crit Care Med. 1997;156:709–14. doi: 10.1164/ajrccm.156.3.9610013. [DOI] [PubMed] [Google Scholar]
- 32.Barquin N, Ciccolella DE, Ridge KM, et al. Dexamethasone upregulates the Na-K-ATPase in rat alveolar epithelial cells. Am J Physiol. 1997;273:L825–30. doi: 10.1152/ajplung.1997.273.4.L825. [DOI] [PubMed] [Google Scholar]
- 33.Berthiaume Y, Staub NC, Matthay MA. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 1987;79:335–43. doi: 10.1172/JCI112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumasius V, Sznajder JI, Azzam ZS, et al. beta(2)-Adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res. 2001;89:907–14. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]
- 35.Olivera WG, Ciccolella DE, Barquin N, et al. Aldosterone regulates Na,K-ATPase and increases lung edema clearance in rats. Am J Respir Crit Care Med. 2000;161:567–73. doi: 10.1164/ajrccm.161.2.9808050. [DOI] [PubMed] [Google Scholar]
- 36.Saldias F, Lecuona E, Friedman E, et al. Modulation of lung liquid clearance by isoproterenol in rat lungs. Am J Physiol. 1998;274:L694–701. doi: 10.1152/ajplung.1998.274.5.L694. [DOI] [PubMed] [Google Scholar]
- 37.Azzam ZS, Adir Y, Welch L, et al. Alveolar fluid reabsorption is increased in rats with compensated heart failure. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1094–100. doi: 10.1152/ajplung.00180.2005. [DOI] [PubMed] [Google Scholar]
- 38.Olivera W, Ridge K, Wood LD, et al. Active sodium transport and alveolar epithelial Na-K-ATPase increase during subacute hyperoxia in rats. Am J Physiol. 1994;266:L577–84. doi: 10.1152/ajplung.1994.266.5.L577. [DOI] [PubMed] [Google Scholar]
- 39.Guetta J, Klorin G, Tal R, et al. Vasopressin-2 receptor antagonist attenuates the ability of the lungs to clear edema in an experimental model. Am J Respir Cell Mol Biol. 2012;47:583–8. doi: 10.1165/rcmb.2012-0117OC. [DOI] [PubMed] [Google Scholar]
- 40.Azzam ZS, Dumasius V, Saldias FJ, et al. Na,K-ATPase overexpression improves alveolar fluid clearance in a rat model of elevated left atrial pressure. Circulation. 2002;105:497–501. doi: 10.1161/hc0402.102848. [DOI] [PubMed] [Google Scholar]
- 41.Eaton DC, Chen J, Ramosevac S, et al. Regulation of Na+ channels in lung alveolar type II epithelial cells. Proc Am Thorac Soc. 2004;1:10–16. doi: 10.1513/pats.2306008. [DOI] [PubMed] [Google Scholar]
- 42.Dada LA, Chandel NS, Ridge KM, et al. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–64. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briva A, Vadasz I, Lecuona E, et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS One. 2007;2:e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briva A, Santos C, Malacrida L, et al. Adenosine triphosphate-dependent calcium signaling during ventilator-induced lung injury is amplified by hypercapnia. Exp Lung Res. 2011;37:471–81. doi: 10.3109/01902148.2011.598217. [DOI] [PubMed] [Google Scholar]
- 45.Berger G, Guetta J, Klorin G, et al. Sepsis impairs alveolar epithelial function by downregulating Na-K-ATPase pump. Am J Physiol Lung Cell Mol Physiol. 2011;301:L23–30. doi: 10.1152/ajplung.00010.2010. [DOI] [PubMed] [Google Scholar]
- 46.Olivera WG, Ridge KM, Sznajder JI. Lung liquid clearance and Na,K-ATPase during acute hyperoxia and recovery in rats. Am J Respir Crit Care Med. 1995;152:1229–34. doi: 10.1164/ajrccm.152.4.7551375. [DOI] [PubMed] [Google Scholar]
- 47.Lecuona E, Saldias F, Comellas A, et al. Ventilator-associated lung injury decreases lung ability to clear edema in rats. Am J Respir Crit Care Med. 1999;159:603–9. doi: 10.1164/ajrccm.159.2.9805050. [DOI] [PubMed] [Google Scholar]
- 48.Saldias FJ, Azzam ZS, Ridge KM, et al. Alveolar fluid reabsorption is impaired by increased left atrial pressures in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L591–7. doi: 10.1152/ajplung.2001.281.3.L591. [DOI] [PubMed] [Google Scholar]
- 49.Comellas AP, Briva A, Dada LA, et al. Endothelin-1 impairs alveolar epithelial function via endothelial ETB receptor. Am J Respir Crit Care Med. 2009;179:113–22. doi: 10.1164/rccm.200804-540OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao Smith F, Perkins GD, Gates S, et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–35. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–8. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiarella SE, Soberanes S, Urich D, et al. beta(2)-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J Clin Invest. 2014;124:2935–46. doi: 10.1172/JCI75157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ackermann U, Geering K. Mutual dependence of Na,K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 1990;269:105–8. doi: 10.1016/0014-5793(90)81130-G. [DOI] [PubMed] [Google Scholar]
- 54.Bertorello AM, Ridge KM, Chibalin AV, et al. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol. 1999;276:L20–7. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 55.Ridge KM, Dada L, Lecuona E, et al. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-epsilon and −delta. Mol Biol Cell. 2002;13:1381–9. doi: 10.1091/mbc.01-07-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gusarova GA, Dada LA, Kelly AM, et al. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–64. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norlin A, Finley N, Abedinpour P, et al. Alveolar liquid clearance in the anesthetized ventilated guinea pig. Am J Physiol. 1998;274:L235–43. doi: 10.1152/ajplung.1998.274.2.L235. [DOI] [PubMed] [Google Scholar]
- 58.Dobbs LG, Gonzalez R, Matthay MA, et al. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci U S A. 1998;95:2991–6. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]