Abstract
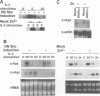
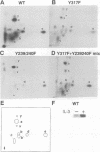
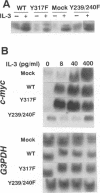
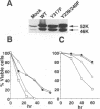
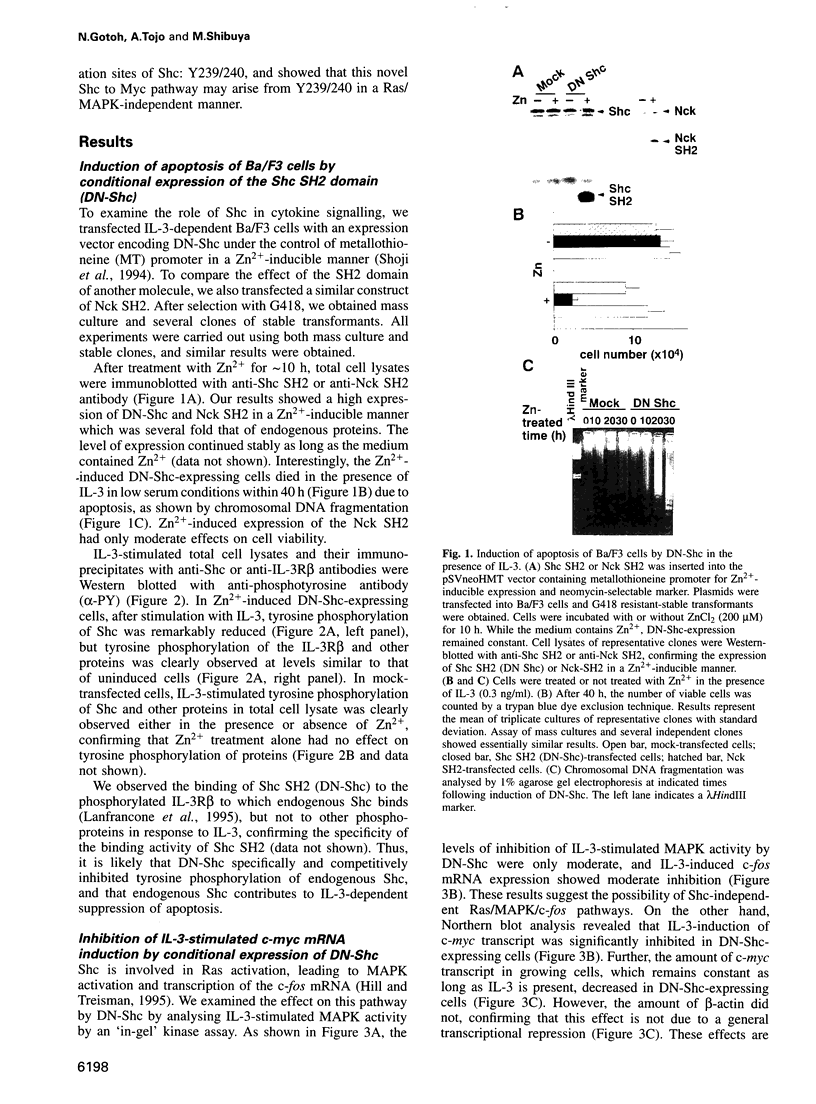
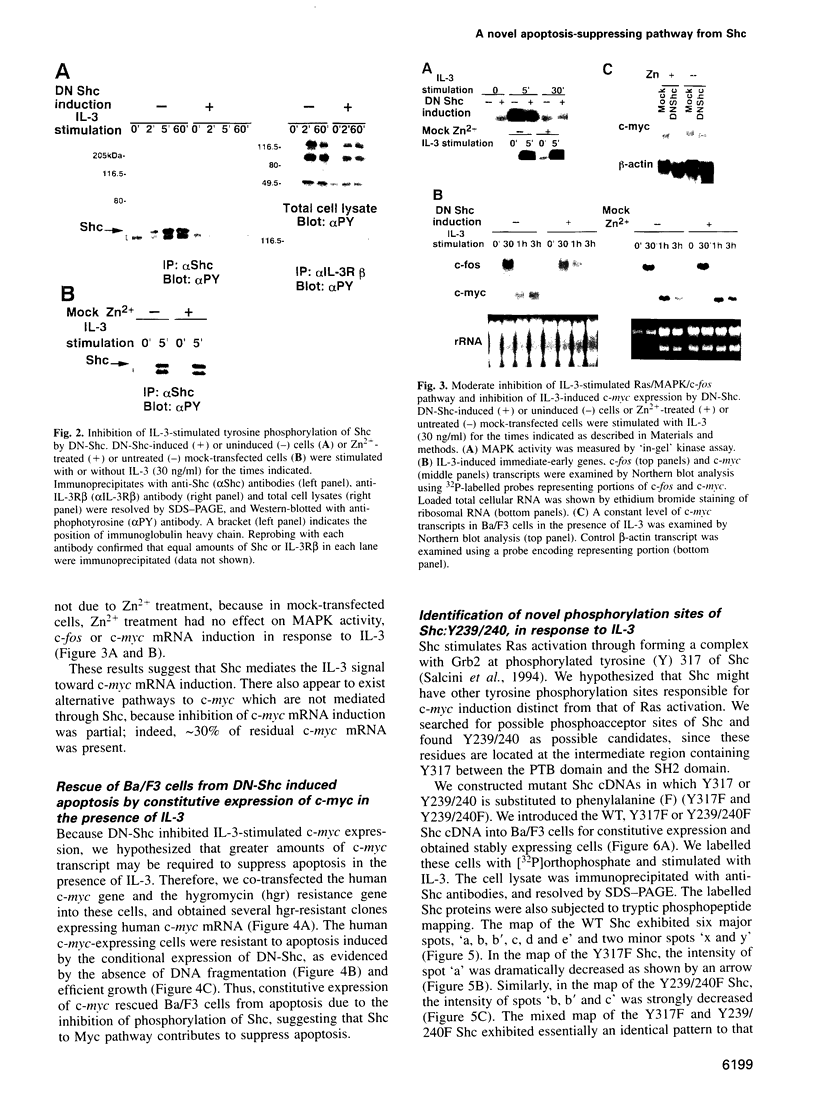
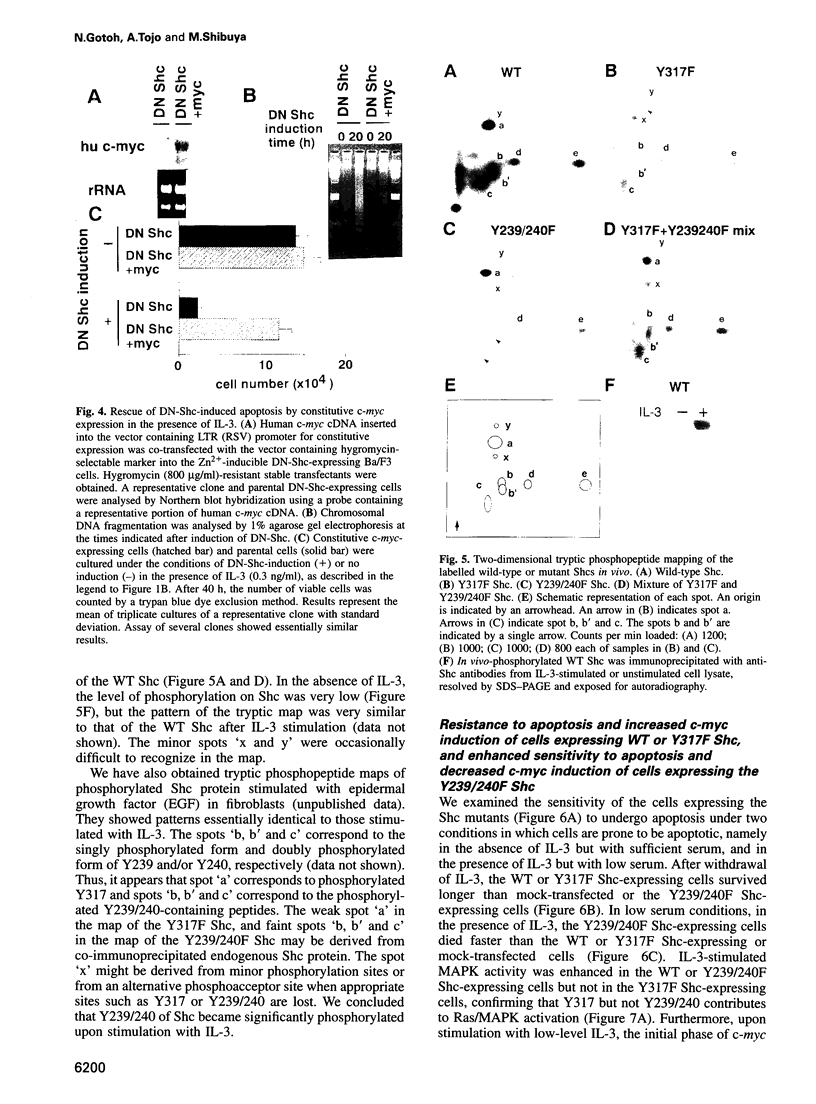
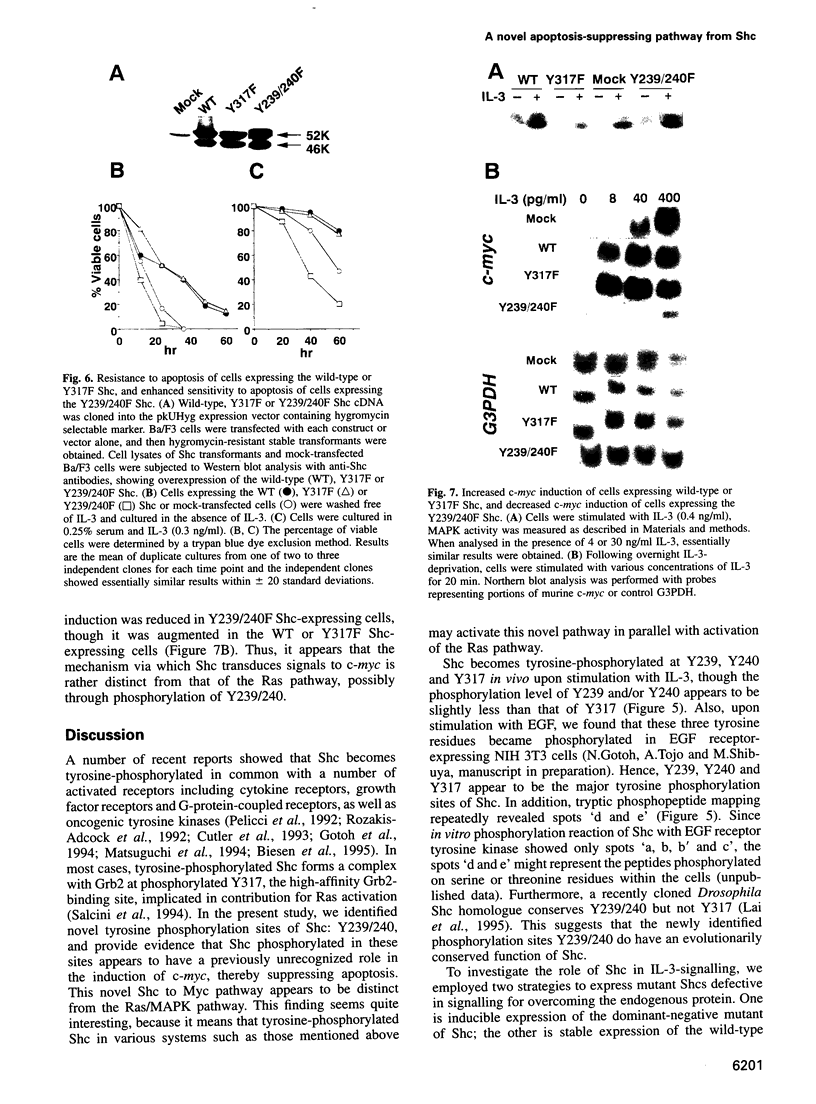
Interleukin 3 (IL-3) not only induces DNA synthesis of haematopoietic cells but also maintains their viability by suppressing apoptosis. IL-3 stimulates tyrosine phosphorylation of the Shc adaptor protein and thereby formation of a complex of Shc with Grb2 at phosphorylated tyrosine (Y) residue 317-Shc. This pathway is implicated in Ras/mitogen-activated protein kinase (MAPK) activation towards c-fos gene expression. We examined the possible involvement of Shc in the antiapoptotic activity of IL-3. Conditional overexpression of the Shc SH2 domain, a dominant-negative mutant of Shc, was found to induce apoptosis of IL-3-dependent Ba/F3 cells along with a reduction of c-myc gene expression. Apoptosis was rescued by the exogenously introduced c-myc gene. Since we identify novel tyrosine phosphorylation sites of Shc: Y239 and Y240, their role on cell survival was tested by mutational analysis. Ba/F3 cells expressing mutant Shc Y317F, which is unable to stimulate efficiently the Ras pathway, still showed resistance to apoptosis. However, cells expressing Shc Y239/240F, which is able to stimulate the Ras pathway, were sensitive to apoptosis. In these cells, induction of the c-myc gene was reduced. These findings suggest that a new signalling pathway for cell survival is generated from Y239/240 of Shc to the nuclei involving c-myc gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Baldari C. T., Pelicci G., Di Somma M. M., Milia E., Giuli S., Pelicci P. G., Telford J. L. Inhibition of CD4/p56lck signaling by a dominant negative mutant of the Shc adaptor protein. Oncogene. 1995 Mar 16;10(6):1141–1147. [PubMed] [Google Scholar]
- Barone M. V., Courtneidge S. A. Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature. 1995 Nov 30;378(6556):509–512. doi: 10.1038/378509a0. [DOI] [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Dean M., Rosenberg N., Wang J. Y., Rapp U. R. Tyrosine kinase oncogenes abrogate interleukin-3 dependence of murine myeloid cells through signaling pathways involving c-myc: conditional regulation of c-myc transcription by temperature-sensitive v-abl. Mol Cell Biol. 1989 Dec;9(12):5685–5695. doi: 10.1128/mcb.9.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Cutler R. L., Liu L., Damen J. E., Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J Biol Chem. 1993 Oct 15;268(29):21463–21465. [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Gotoh N., Muroya K., Hattori S., Nakamura S., Chida K., Shibuya M. The SH2 domain of Shc suppresses EGF-induced mitogenesis in a dominant negative manner. Oncogene. 1995 Dec 21;11(12):2525–2533. [PubMed] [Google Scholar]
- Gotoh N., Tojo A., Muroya K., Hashimoto Y., Hattori S., Nakamura S., Takenawa T., Yazaki Y., Shibuya M. Epidermal growth factor-receptor mutant lacking the autophosphorylation sites induces phosphorylation of Shc protein and Shc-Grb2/ASH association and retains mitogenic activity. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):167–171. doi: 10.1073/pnas.91.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. S., Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995 Jan 27;80(2):199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Kerr I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995 Feb;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Kabarowski J. H., Allen P. B., Wiedemann L. M. A temperature sensitive p210 BCR-ABL mutant defines the primary consequences of BCR-ABL tyrosine kinase expression in growth factor dependent cells. EMBO J. 1994 Dec 15;13(24):5887–5895. doi: 10.1002/j.1460-2075.1994.tb06934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Yokota T., Arai K., Miyajima A. Suppression of apoptotic death in hematopoietic cells by signalling through the IL-3/GM-CSF receptors. EMBO J. 1995 Jan 16;14(2):266–275. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K. M., Olivier J. P., Gish G. D., Henkemeyer M., McGlade J., Pawson T. A Drosophila shc gene product is implicated in signaling by the DER receptor tyrosine kinase. Mol Cell Biol. 1995 Sep;15(9):4810–4818. doi: 10.1128/mcb.15.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfrancone L., Pelicci G., Brizzi M. F., Aronica M. G., Casciari C., Giuli S., Pegoraro L., Pawson T., Pelicci P. G., Arouica M. G. Overexpression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit. Oncogene. 1995 Mar 2;10(5):907–917. [PubMed] [Google Scholar]
- Li W., Nishimura R., Kashishian A., Batzer A. G., Kim W. J., Cooper J. A., Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994 Jan;14(1):509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T., Salgia R., Hallek M., Eder M., Druker B., Ernst T. J., Griffin J. D. Shc phosphorylation in myeloid cells is regulated by granulocyte macrophage colony-stimulating factor, interleukin-3, and steel factor and is constitutively increased by p210BCR/ABL. J Biol Chem. 1994 Feb 18;269(7):5016–5021. [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Liu Z. J., Kawahara A., Minami Y., Yamada K., Tsujimoto Y., Barsoumian E. L., Permutter R. M., Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995 Apr 21;81(2):223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Owen-Lynch P. J., Wong A. K., Whetton A. D. v-Abl-mediated apoptotic suppression is associated with SHC phosphorylation without concomitant mitogen-activated protein kinase activation. J Biol Chem. 1995 Mar 17;270(11):5956–5962. doi: 10.1074/jbc.270.11.5956. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Seder R. A. Lymphocyte responses and cytokines. Cell. 1994 Jan 28;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995 Feb 16;373(6515):573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Giordano S., Zhen Z., Salcini A. E., Lanfrancone L., Bardelli A., Panayotou G., Waterfield M. D., Ponzetto C., Pelicci P. G. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene. 1995 Apr 20;10(8):1631–1638. [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Puil L., Liu J., Gish G., Mbamalu G., Bowtell D., Pelicci P. G., Arlinghaus R., Pawson T. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J. 1994 Feb 15;13(4):764–773. doi: 10.1002/j.1460-2075.1994.tb06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stiles C. D. Serum-inducible genes. Adv Cancer Res. 1989;53:1–32. doi: 10.1016/s0065-230x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Shurtleff S. A., Downing J. R., Sherr C. J. A point mutation at tyrosine-809 in the human colony-stimulating factor 1 receptor impairs mitogenesis without abrogating tyrosine kinase activity, association with phosphatidylinositol 3-kinase, or induction of c-fos and junB genes. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6738–6742. doi: 10.1073/pnas.87.17.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Sachs L., Lotem J. Control of programmed cell death in normal and leukemic cells: new implications for therapy. Blood. 1993 Jul 1;82(1):15–21. [PubMed] [Google Scholar]
- Salcini A. E., McGlade J., Pelicci G., Nicoletti I., Pawson T., Pelicci P. G. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene. 1994 Oct;9(10):2827–2836. [PubMed] [Google Scholar]
- Sato N., Sakamaki K., Terada N., Arai K., Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993 Nov;12(11):4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam L., Gotoh N., Maru Y., Neufeld G., Yamaguchi S., Shibuya M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995 Jan 5;10(1):135–147. [PubMed] [Google Scholar]
- Shoji W., Yamamoto T., Obinata M. The helix-loop-helix protein Id inhibits differentiation of murine erythroleukemia cells. J Biol Chem. 1994 Feb 18;269(7):5078–5084. [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995 Apr 14;268(5208):251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- Terada K., Kaziro Y., Satoh T. Ras is not required for the interleukin 3-induced proliferation of a mouse pro-B cell line, BaF3. J Biol Chem. 1995 Nov 17;270(46):27880–27886. doi: 10.1074/jbc.270.46.27880. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993 Feb;67(2):205–208. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Dickens M., Raingeaud J., Davis R. J., Greenberg M. E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995 Nov 24;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- van Biesen T., Hawes B. E., Luttrell D. K., Krueger K. M., Touhara K., Porfiri E., Sakaue M., Luttrell L. M., Lefkowitz R. J. Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature. 1995 Aug 31;376(6543):781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]