Abstract
A high affinity, tridentate metal ion site has been constructed previously by His substitutions in an antagonist binding site located between transmembrane segment (TM)-V and TM-VI in the substance P NK-1 receptor. Here, an attempt is made to probe helix-helix interactions systematically in the NK-1 receptor by engineering of bis-His Zn(II) sites. His residues were introduced at selected positions individually and in combinations in the exterior segments of TM-II, III and V in both the wild-type background and after Ala substitution of naturally occurring His residues, and the increase in the affinity for Zn(II) was monitored in competition binding experiments with iodinated substance P or a tritiated non-peptide antagonist. In this way, two high affinity bis-His sites were constructed between position 193 in TM-V (Glu193, G1uV:01) and position 109 in TM-III (Asn1O9, AsnIII:05) as well as between the neighboring, naturally occurring His108 in TM-III (HisIII:04) and position 92 in TM-II (Tyr92, TyrII:24), respectively. Functionally, the coordination of zinc ions at these two sites blocked the receptor as it antagonized the substance P-induced increase in phosphatidylinositol turnover. It is concluded that the bis-His zinc sites from the central TM-III helix to TM-II and -V, respectively, together with the interconnected, previously constructed tridentate site between TM-V and -VI, constitute a basic network of distance constraints for the molecular models of receptors with seven transmembrane segments which, for example, strongly support an anti-clockwise orientation of the seven helical bundle as viewed from the extracellular space.
Full text
PDF

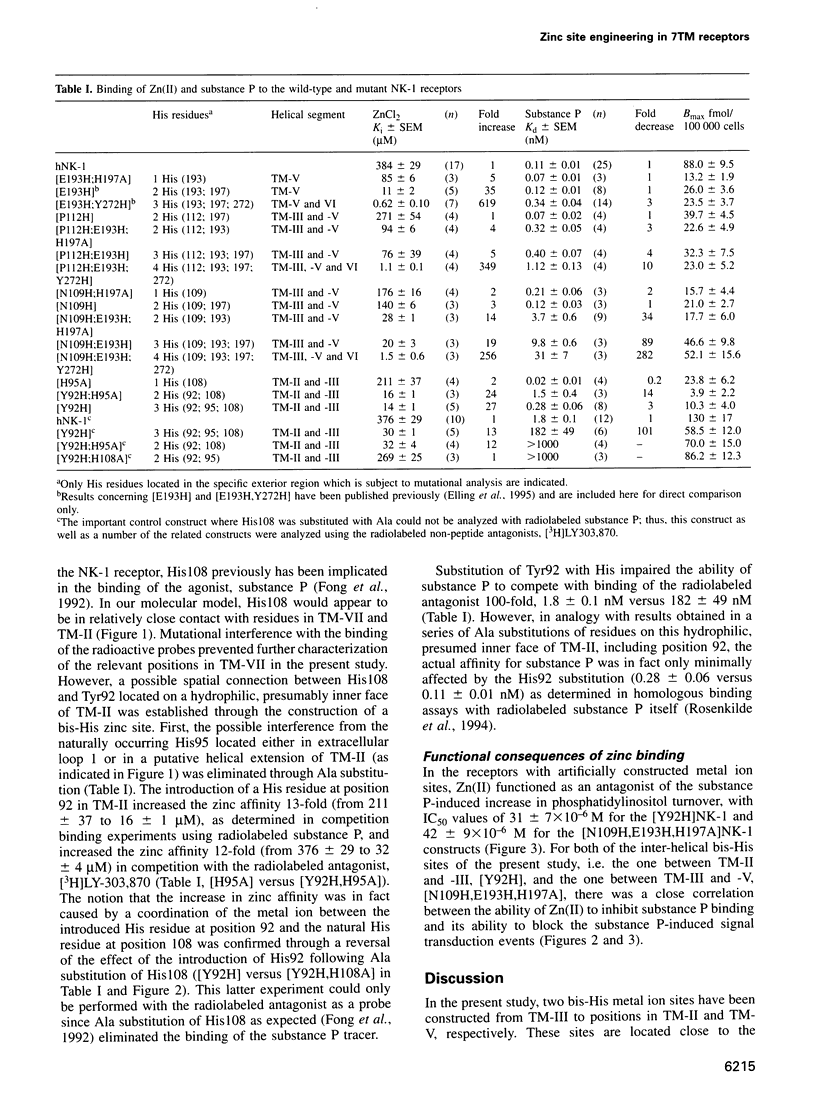
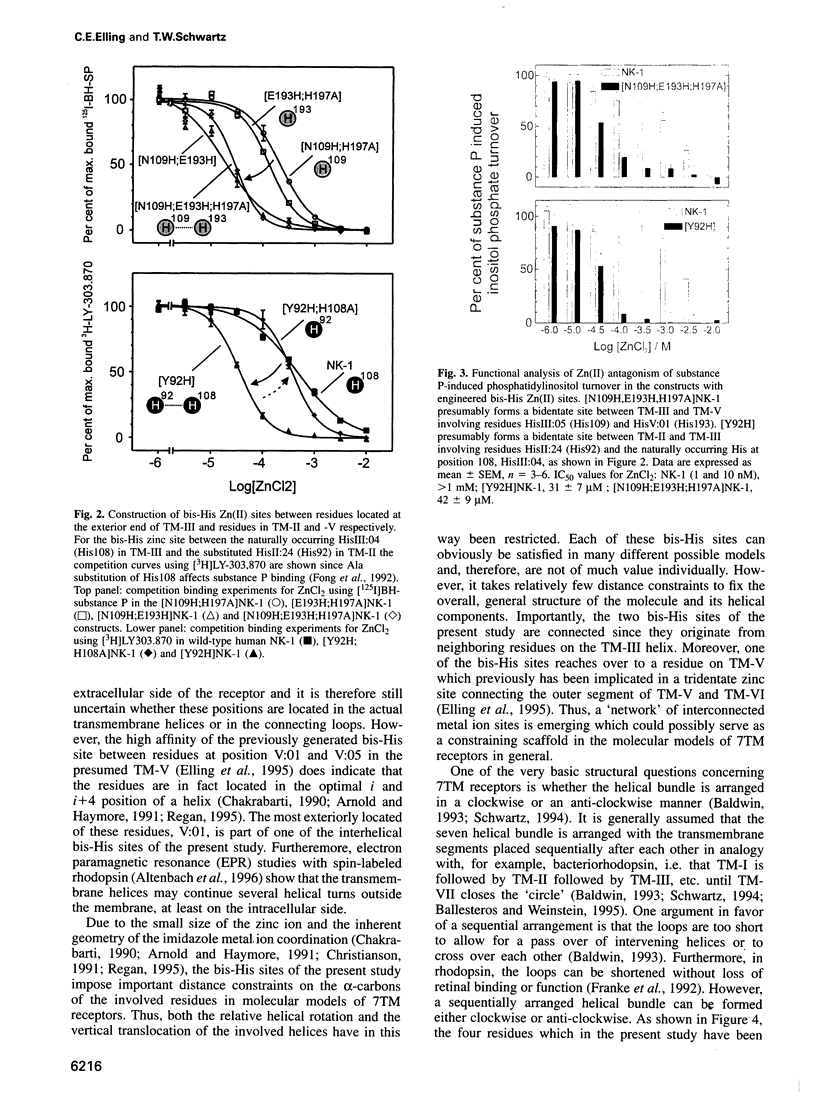
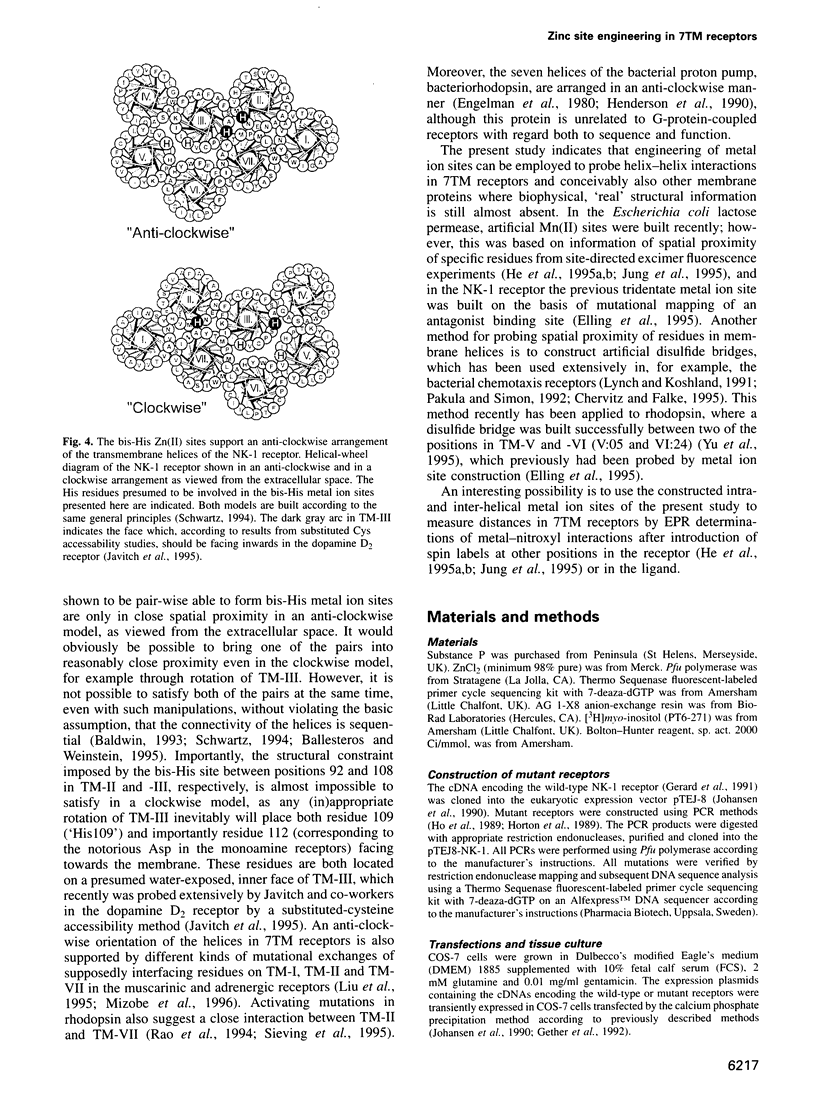


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold F. H., Haymore B. L. Engineered metal-binding proteins: purification to protein folding. Science. 1991 Jun 28;252(5014):1796–1797. doi: 10.1126/science.1648261. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993 Apr;12(4):1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P. Geometry of interaction of metal ions with histidine residues in protein structures. Protein Eng. 1990 Oct;4(1):57–63. doi: 10.1093/protein/4.1.57. [DOI] [PubMed] [Google Scholar]
- Chervitz S. A., Falke J. J. Lock on/off disulfides identify the transmembrane signaling helix of the aspartate receptor. J Biol Chem. 1995 Oct 13;270(41):24043–24053. doi: 10.1074/jbc.270.41.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson D. W. Structural biology of zinc. Adv Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- Deng H., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., Di Marzio P., Marmon S., Sutton R. E., Hill C. M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996 Jun 20;381(6584):661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Dragic T., Litwin V., Allaway G. P., Martin S. R., Huang Y., Nagashima K. A., Cayanan C., Maddon P. J., Koup R. A., Moore J. P. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996 Jun 20;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Elling C. E., Nielsen S. M., Schwartz T. W. Conversion of antagonist-binding site to metal-ion site in the tachykinin NK-1 receptor. Nature. 1995 Mar 2;374(6517):74–77. doi: 10.1038/374074a0. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Broder C. C., Kennedy P. E., Berger E. A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 May 10;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Cascieri M. A., Yu H., Bansal A., Swain C., Strader C. D. Amino-aromatic interaction between histidine 197 of the neurokinin-1 receptor and CP 96345. Nature. 1993 Mar 25;362(6418):350–353. doi: 10.1038/362350a0. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Huang R. R., Strader C. D. Localization of agonist and antagonist binding domains of the human neurokinin-1 receptor. J Biol Chem. 1992 Dec 25;267(36):25664–25667. [PubMed] [Google Scholar]
- Fong T. M., Yu H., Cascieri M. A., Underwood D., Swain C. J., Strader C. D. The role of histidine 265 in antagonist binding to the neurokinin-1 receptor. J Biol Chem. 1994 Jan 28;269(4):2728–2732. [PubMed] [Google Scholar]
- Franke R. R., Sakmar T. P., Graham R. M., Khorana H. G. Structure and function in rhodopsin. Studies of the interaction between the rhodopsin cytoplasmic domain and transducin. J Biol Chem. 1992 Jul 25;267(21):14767–14774. [PubMed] [Google Scholar]
- Gerard N. P., Garraway L. A., Eddy R. L., Jr, Shows T. B., Iijima H., Paquet J. L., Gerard C. Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry. 1991 Nov 5;30(44):10640–10646. doi: 10.1021/bi00108a006. [DOI] [PubMed] [Google Scholar]
- Gether U., Johansen T. E., Snider R. M., Lowe J. A., 3rd, Nakanishi S., Schwartz T. W. Different binding epitopes on the NK1 receptor for substance P and non-peptide antagonist. Nature. 1993 Mar 25;362(6418):345–348. doi: 10.1038/362345a0. [DOI] [PubMed] [Google Scholar]
- Gether U., Marray T., Schwartz T. W., Johansen T. E. Stable expression of high affinity NK1 (substance P) and NK2 (neurokinin A) receptors but low affinity NK3 (neurokinin B) receptors in transfected CHO cells. FEBS Lett. 1992 Jan 27;296(3):241–244. doi: 10.1016/0014-5793(92)80295-r. [DOI] [PubMed] [Google Scholar]
- Gether U., Nilsson L., Lowe J. A., 3rd, Schwartz T. W. Specific residues at the top of transmembrane segment V and VI of the neurokinin-1 receptor involved in binding of the nonpeptide antagonist CP 96,345 [corrected]. J Biol Chem. 1994 Sep 30;269(39):23959–23964. [PubMed] [Google Scholar]
- He M. M., Voss J., Hubbell W. L., Kaback H. R. Use of designed metal-binding sites to study helix proximity in the lactose permease of Escherichia coli. 1. Proximity of helix VII (Asp237 and Asp240) with helices X (Lys319) and XI (Lys358). Biochemistry. 1995 Dec 5;34(48):15661–15666. doi: 10.1021/bi00048a009. [DOI] [PubMed] [Google Scholar]
- He M. M., Voss J., Hubbell W. L., Kaback H. R. Use of designed metal-binding sites to study helix proximity in the lactose permease of Escherichia coli. 2. Proximity of helix IX (Arg302) with helix X (His322 and Glu325). Biochemistry. 1995 Dec 5;34(48):15667–15670. doi: 10.1021/bi00048a010. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., Fu D., Chen J., Karlin A. Mapping the binding-site crevice of the dopamine D2 receptor by the substituted-cysteine accessibility method. Neuron. 1995 Apr;14(4):825–831. doi: 10.1016/0896-6273(95)90226-0. [DOI] [PubMed] [Google Scholar]
- Johansen T. E., Schøller M. S., Tolstoy S., Schwartz T. W. Biosynthesis of peptide precursors and protease inhibitors using new constitutive and inducible eukaryotic expression vectors. FEBS Lett. 1990 Jul 16;267(2):289–294. doi: 10.1016/0014-5793(90)80947-h. [DOI] [PubMed] [Google Scholar]
- Jung K., Voss J., He M., Hubbell W. L., Kaback H. R. Engineering a metal binding site within a polytopic membrane protein, the lactose permease of Escherichia coli. Biochemistry. 1995 May 16;34(19):6272–6277. doi: 10.1021/bi00019a003. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Rhodopsin, photoreceptor of the rod cell. An emerging pattern for structure and function. J Biol Chem. 1992 Jan 5;267(1):1–4. [PubMed] [Google Scholar]
- Liu J., Schöneberg T., van Rhee M., Wess J. Mutational analysis of the relative orientation of transmembrane helices I and VII in G protein-coupled receptors. J Biol Chem. 1995 Aug 18;270(33):19532–19539. doi: 10.1074/jbc.270.33.19532. [DOI] [PubMed] [Google Scholar]
- Lynch B. A., Koshland D. E., Jr Disulfide cross-linking studies of the transmembrane regions of the aspartate sensory receptor of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10402–10406. doi: 10.1073/pnas.88.23.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobe T., Maze M., Lam V., Suryanarayana S., Kobilka B. K. Arrangement of transmembrane domains in adrenergic receptors. Similarity to bacteriorhodopsin. J Biol Chem. 1996 Feb 2;271(5):2387–2389. doi: 10.1074/jbc.271.5.2387. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. R., Cohen G. B., Oprian D. D. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994 Feb 17;367(6464):639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- Regan L. Protein design: novel metal-binding sites. Trends Biochem Sci. 1995 Jul;20(7):280–285. doi: 10.1016/s0968-0004(00)89044-1. [DOI] [PubMed] [Google Scholar]
- Rosenkilde M. M., Cahir M., Gether U., Hjorth S. A., Schwartz T. W. Mutations along transmembrane segment II of the NK-1 receptor affect substance P competition with non-peptide antagonists but not substance P binding. J Biol Chem. 1994 Nov 11;269(45):28160–28164. [PubMed] [Google Scholar]
- Schertler G. F., Hargrave P. A. Projection structure of frog rhodopsin in two crystal forms. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11578–11582. doi: 10.1073/pnas.92.25.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertler G. F., Villa C., Henderson R. Projection structure of rhodopsin. Nature. 1993 Apr 22;362(6422):770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- Schwartz T. W. Locating ligand-binding sites in 7TM receptors by protein engineering. Curr Opin Biotechnol. 1994 Aug;5(4):434–444. doi: 10.1016/0958-1669(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Sealfon S. C., Chi L., Ebersole B. J., Rodic V., Zhang D., Ballesteros J. A., Weinstein H. Related contribution of specific helix 2 and 7 residues to conformational activation of the serotonin 5-HT2A receptor. J Biol Chem. 1995 Jul 14;270(28):16683–16688. doi: 10.1074/jbc.270.28.16683. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Discrimination of molecular signals by the olfactory receptor neuron. Neuron. 1994 Oct;13(4):771–790. doi: 10.1016/0896-6273(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Sieving P. A., Richards J. E., Naarendorp F., Bingham E. L., Scott K., Alpern M. Dark-light: model for nightblindness from the human rhodopsin Gly-90-->Asp mutation. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):880–884. doi: 10.1073/pnas.92.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader C. D., Candelore M. R., Hill W. S., Sigal I. S., Dixon R. A. Identification of two serine residues involved in agonist activation of the beta-adrenergic receptor. J Biol Chem. 1989 Aug 15;264(23):13572–13578. [PubMed] [Google Scholar]
- Strader C. D., Gaffney T., Sugg E. E., Candelore M. R., Keys R., Patchett A. A., Dixon R. A. Allele-specific activation of genetically engineered receptors. J Biol Chem. 1991 Jan 5;266(1):5–8. [PubMed] [Google Scholar]
- Thirstrup K., Elling C. E., Hjorth S. A., Schwartz T. W. Construction of a high affinity zinc switch in the kappa-opioid receptor. J Biol Chem. 1996 Apr 5;271(14):7875–7878. doi: 10.1074/jbc.271.14.7875. [DOI] [PubMed] [Google Scholar]
- Unger V. M., Schertler G. F. Low resolution structure of bovine rhodopsin determined by electron cryo-microscopy. Biophys J. 1995 May;68(5):1776–1786. doi: 10.1016/S0006-3495(95)80354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kono M., McKee T. D., Oprian D. D. A general method for mapping tertiary contacts between amino acid residues in membrane-embedded proteins. Biochemistry. 1995 Nov 21;34(46):14963–14969. doi: 10.1021/bi00046a002. [DOI] [PubMed] [Google Scholar]
- Zhou W., Flanagan C., Ballesteros J. A., Konvicka K., Davidson J. S., Weinstein H., Millar R. P., Sealfon S. C. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol. 1994 Feb;45(2):165–170. [PubMed] [Google Scholar]
- Zoffmann S., Gether U., Schwartz T. W. Conserved HisVI-17 of the NK-1 receptor is involved in binding of non-peptide antagonists but not substance P. FEBS Lett. 1993 Dec 28;336(3):506–510. doi: 10.1016/0014-5793(93)80865-r. [DOI] [PubMed] [Google Scholar]