Abstract
Background
We aimed to clarify the possible role of human papillomavirus (HPV) infection in the malignant transformation of sinonasal inverted papilloma (IP).
Methods
Subjects comprised 32 patients with chronic rhinosinusitis (CRS), 17 with IP, 5 with IP and squamous cell carcinoma (IP + SCC), and 16 with primary sinonasal SCC. HPV presence, viral loads, and physical status were investigated using polymerase chain reaction. Retinoblastoma (pRb), p53, and p16INK4a gene products were investigated by immunohistochemistry.
Results
HPV DNA was detected in 6.3 % of cases with CRS, 29.4 % with IP, 40 % with IP + SCC, and 25 % with SCC. IP cases had significantly higher HPV presence than CRS cases (p = 0.04). High-risk HPV-16 was the most frequently encountered subtype (10/13, 76.9 %). HPV-16 viral loads varied from 2.5 to 7953 E6 copies/50 ng genomic DNA. Patients in the SCC and IP + SCC groups had significantly higher viral loads than those in the IP and CRS groups (p < 0.01). All SCC and IP + SCC patients with HPV-16 demonstrated mixed-type integration, whereas 4 of 5 HPV-16 patients in the IP and CRS groups showed episomal type infection (p = 0.04). Positivity to pRb was found in 78.1 % of CRS, 35.3 % of IP, and 68.8 % of SCC cases. The presence of HPV DNA negatively correlated with pRb expression in SCC (p = 0.029) and IP (P = 0.049) groups. Although 62.5 % of SCC cases exhibited p53 positivity, only 5.9 % of IP, and no CRS cases were positive. Regardless of HPV status, p16INK4a positivity was frequently detected in IP cases (82.4 %), less in SCC (12.5 %) cases, and was not detected in the CRS group. Neither the IP nor SCC cohorts showed any correlation between HPV presence and the expression of either p53 or p16INK4a.
Conclusions
HPV infection was more frequent in the IP, IP + SCC, and SCC groups than the CRS group. Higher viral loads and integration observed in the IP + SCC and SCC groups, and an inverse correlation between HPV presence and positive pRb indicated that persistent infection and integration play a part in tumorigenesis and malignant transformation in certain IP cases. However, p16INK4a is not a reliable surrogate marker for HPV infection in IP.
Key words: Human papillomavirus, Cell cycle protein, Inverted papilloma, Malignant transformation, Integration, Viral load, Sinonasal disease
Background
A variety of benign and malignant tumors may arise in the sinonasal cavity. Of these, inverted papilloma (IP), a benign neoplasm, has unique clinical characteristics; of note are its high rates of recurrence and malignant transformation. According to several systematic reviews and meta-analyses, the malignant transformation rate of IP is estimated to be approximately 10 % [3, 17, 20].
Human papillomavirus (HPV) infection in the sinonasal tract is estimated to be present in 37.8 % of IPs [32] and 27 % of squamous cell carcinomas (SCCs) [33]. Although a significant number of these tumors are infected with HPV, especially high-risk HPV types, whether HPV is involved in the malignant transformation of IP or in the pathogenesis of SCC has not been confirmed, as its mechanism and role in malignant transformation remain obscure. There are several of reports on the clinical relationship between IP and HPV infection. Two studies by Beck et al. found that 63 % of IP cases were positive for HPV DNA, and the presence of HPV sequences predicted the recurrence of inverted papilloma [4, 5]. Moreover, these studies found that patients with HPV types 16 or 18 have a higher rate of associated malignancy than patients with HPV 6 or 11 [5]. A systematic review subsequently confirmed that the presence of HPV was significantly associated with the likelihood of IP recurrence [18]. However, there continues to be discussion over whether there is a significant correlation between the development of malignant transformation in IP and HPV types [11, 16, 20].
In our previous study, viral loads and physical status of HPV were examined using fresh frozen samples of IP, SCC, and inflammatory mucosa from the sinonasal tract to clarify the clinical importance of HPV in IP. HPV genomes were detected in 46.1 % of IPs, 27.3 % of SCCs, and 7.6 % of the inflammatory group, respectively [10]. Because the IP group showed significantly higher HPV positivity rates than the inflammatory group, it was concluded that HPV infection is involved in the pathogenesis of IP, and a high viral load and integration of HPV may play important roles in malignant transformation.
The retinoblastoma (pRb), p16INK4a and cyclin D1 genes are components of the pRb cell cycle control pathway. The active hypophosphorylated form of pRb binds and blocks the action of the transcription factor, inhibiting the transition from G1 to S phase. Cyclin D1 stimulates the phosphorylation of pRb by associating itself with cyclin-dependent kinases (CDKs). Binding of p16INK4a to CDK 4 and 6 blocks their association with the D-type cyclins. Another well-known cellular tumor suppressor, p53, is involved in processes such as cell cycle progression, DNA repair, chromatin remodeling, differentiation, apoptosis and senescence. HPV-mediated tumorigenesis is mainly due to the activities of two viral oncoproteins, E6 and E7. HPV E6 can induce the degradation of p53 by direct binding to the ubiquitin ligase E6AP, inhibit p53-dependent signaling upon stress stimuli, and contribute to tumorigenesis [19, 27, 36]. The viral E7 protein binds to and inactivates pRb, activating E2F independent of cyclin dependent kinase. The functional inactivation of host pRb by HPV E7 protein results in overexpression of p16, making p16 a reasonable surrogate marker for the presence of high-risk human papillomavirus.
Several reports concerning cell cycle protein expression in IP, IP + SCC, and SCC have been published [1, 6, 14, 19, 21–23, 25, 27, 28, 36, 37]. However, the simultaneous evaluation of HPV infection and cell cycle protein expression using IP, IP + SCC, and SCC samples, using chronic rhinosinusitis (CRS) as a control, has not yet been reported. Moreover, it is very difficult to compare results because immunohistochemical methods of evaluation differ among studies. The aim of this study was to identify the HPV infection and immunophenotypic features in the malignant transformation of inverted papilloma. In the present study, additional cases of IP, IP + SCC, SCC, and CRS were recruited and HPV status (presence, viral load, and physical status) and cell cycle proteins related to HPV infection— retinoblastoma (pRb), p53, and p16INK4a gene products—were investigated to reinforce our assumptions from the previous study.
Ethics, consent and permissions
The study protocol was approved in advance by the Institutional Review Board of the University of the Ryukyus. This study was conducted to conform to the principles of the Declaration of Helsinki.
Consent to publish
We have obtained consent to publish histologic pictures from the participants.
Results
Patient characteristics (Table 1)
Table 1.
Patient profiles
| IP | IP with SCC | SCC | CRS | |
|---|---|---|---|---|
| Number of cases | 17 | 5 | 16 | 32 |
| Sex, n (%) | ||||
| Male | 11 | 2 | 14 | 22 |
| Female | 6 | 3 | 2 | 10 |
| Age (years) | ||||
| Mean | 57 | 60 | 60 | 45 |
| Range | 44–76 | 55–72 | 40–82 | 17–81 |
| ≤50, n (%) | 7 | 0 | 5 | 19 |
| >50, n (%) | 10 | 5 | 11 | 13 |
| Krouse classification | ||||
| T1 | ||||
| T2 | 3 | |||
| T3 | 13 | |||
| T4 | 1 | 5 | ||
| T classification | ||||
| T1 | ||||
| T2 | 1 | |||
| T3 | 5 | |||
| T4 | 10 | |||
| N classification | ||||
| N0 | 10 | |||
| N1, N2, or N3 | 6 |
According to Krouse classification [17] of IP, T2 was observed in 3 patients, T3 in 13, and T4 in 1. The T4 case had a massive extension to the pterygoid fossa with no malignant lesions. The observation period after our surgeries for the IP group ranged from 18 to 98 months (median 43 months). At the first visit to our clinic, 7 patients had previous history of sinus surgery for IP and 2 cases (11.8 %) had recurrence after surgery.
Metachronous SCC occurred in 3 cases and synchronous SCC in 2 cases of the IP + SCC group. The periods between the initial treatments for IP to malignant transformation in the 3 metachronous SCC cases were 5 months, 6 years, and 9 years. Patients in the IP + SCC group received surgery and subsequent radiotherapy as adjuvant treatments for cancerous lesions. Although 1 case recurred after surgery combined with radiotherapy in the IP + SCC group, salvage endoscopic resection for the recurrent lesion was performed successfully and the lesion has not recurred for 9 years. The patient had HPV-16 infection with mixed integration.
-
2)
HPV detection (Table 2)
Table 2.
HPV presence, HPV type, viral load, and physical status of HPV-16
| Groups | HPV presence | High-risk HPV (numbers) | HPV-16 viral load (E6 copies/50 ng genomic DNA) | E2/E6 of HPV-16 | Integration type |
|---|---|---|---|---|---|
| SCC (n = 16) | 25.0 %, 4 of 16 | HPV-16, 3; HPV-18, 1 | 35 | 0.12 | mixed |
| 79 | 0.1 | mixed | |||
| 594 | 0.85 | mixed | |||
| IP + SCC (n = 5) | 40.0 %, 2 of 5 | HPV16, 2 | 1524 | 0.65 | mixed |
| 7953 | 0.67 | mixed | |||
| IP (n = 17) | 29.4 %, 5 of 17 | HPV16, 3; HPV33, 2 | 2.5 | 1 | episomal |
| 24.2 | 1 | episomal | |||
| 74 | 1 | episomal | |||
| CRS (n = 32) | 6.3 %, 2 of 32 | HPV16, 2 | 5.1 | 0.12 | mixed |
| 6.8 | 1 | episomal |
HPV genomes were detected in 5 of the 17 patients in the IP group (29.4 %, Table 2), compared with 4 of the 16 patients (25.0 %) in the SCC group, 2 of the 5 patients (40 %) in the IP + SCC group, and 2 of the 32 patients (6.3 %) in the CRS group. The IP group showed a significantly higher HPV presence than the CRS group (p = 0.04, Fisher’s exact test).
The HPV types detected in the present study were high-risk types only (i.e. HPV-16, HPV-33, and HPV-18) and multiple HPV infections were not detected. Of the 13 HPV patients, HPV-16 was present in 10, therefore viral loads and physical status of HPV-16 were investigated in the positive samples. Viral loads varied from 2.5 to 7953 E6 copies/50 ng genomic DNA. Patients in the SCC and IP + SCC groups had significantly higher viral loads than the IP and CRS groups (p < 0.01, Mann–Whitney U test). In addition, all patients with HPV-16 infection in the SCC and IP + SCC groups demonstrated mixed type integration, whereas 4 of the 5 patients with HPV-16 infection in the IP and CRS groups showed episomal type infection (p = 0.01, chi-square test).
-
3)
Expression of pRb, p53, and p16INK4a by immunohistochemistry (Table 3)
Table 3.
Expression of cell cycle proteins
| p16INK4a | p53 | pRB | ||||||
|---|---|---|---|---|---|---|---|---|
| Groups | HPV presence | (+) | (−) | (+) | (−) | (+) | (−) | |
| SCC | 25.0 % | HPV (+), n = 4 | 1 | 3 | 2 | 2 | 1 | 3 |
| (n = 16) | HPV (−), n = 12 | 1 | 11 | 8 | 4 | 10 | 2 | |
| IP + SCC | 40.0 % | HPV(+), n = 2 | 2 | 0 | 1 | 1 | 0 | 2 |
| (n = 5) | HPV(−), n = 3 | 0 | 3 | 0 | 3 | 0 | 3 | |
| IP | 29.4 % | HPV (+), n = 5 | 4 | 1 | 0 | 5 | 0 | 5 |
| (n = 17) | HPV (−), n = 12 | 10 | 2 | 1 | 11 | 6 | 6 | |
| CRS | 6.3 % | HPV(+), n = 2 | 0 | 2 | 0 | 2 | 1 | 1 |
| (n = 32) | HPV(−), n = 30 | 0 | 30 | 0 | 30 | 24 | 6 | |
Figure 1 shows the representative cases and expression of pRb, p53, and p16INK4a. The expression of pRb was observed in 78.1 % of the CRS group (Fig. 1d), 35.3 % of the IP group, none of the IP + SCC group, and 68.8 % of SCC group. The presence of HPV DNA showed negative correlation with pRb expression in the SCC group (p = 0.029, chi-square test). A similar relationship between the presence of HPV DNA and pRb expression was observed in the IP group (p = 0.049, chi-square test).
Fig. 1.
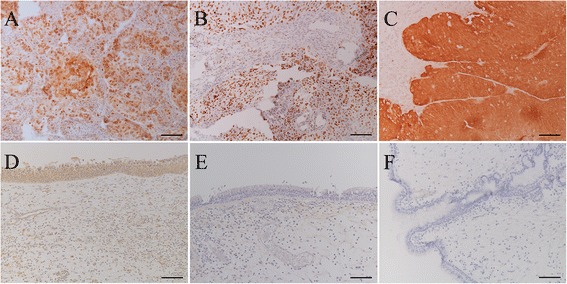
Representative immunohistochemistry results of pRb, p53, and p16INK4a. a: pRb immunohistochemistry of SCC without HPV infection. Bar = 100 μm. b: p53 immunohistochemistry of SCC part of inverted papilloma with SCC. The case showed HPV-16 positive. Bar = 100 μm. c: p16INK4a expression in SCC with HPV-18 positivity. Tumor cells showed strong immunoreaction to p16INK4a in this case. Bar = 100 μm. d: pRb expression in chronic rhinosinusitis. This case was HPV negative. Epithelial cells showed diffuse pRb immunoreaction. Bar = 100 μm. e: p53 expression in chronic rhinosinusitis. This case was also HPV negative. Epithelial cells showed no p53 immunoreaction. Bar = 100 μm. f: p16INK4a expression in chronic rhinosinusitis. This case was HPV negative. The epithelial cells showed no p16INK4a immunoreaction. Bar = 100 μm
The expression of p53 was observed in 10 of the 16 patients (62.5 %) in the SCC group and 1 of the 5 patients (20 %) in the IP + SCC group, whereas it was expressed rarely in the IP (5.9 %) and CRS (0 %, Fig. 1e) groups (Table 3). The expression of p53 in the SCC and IP groups was not significantly related to HPV infection (Table 3).
The expression of p16INK4a was significant in the IP group at 82.4 % compared with 12.5 % in the SCC group and no expression (Fig. 1f) in the CRS group (both p < 0.001, chi-square test). HPV infection in the IP group was not related to p16INK4a expression (Table 3). The case with HPV-33 infection in Fig. 2e showed no expression of p16INK4a, and expression was absent in 2 cases without HPV infection (Table 3, Fig. 2f).
Fig. 2.
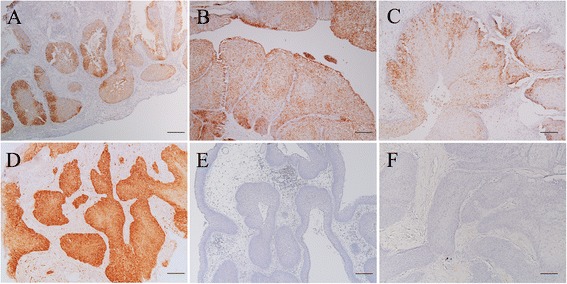
p16INK4a immunohistochemistry in various cases. a: Inverted papilloma with positive p16INK4a reaction. The case had HPV-16 infection with episomal form. Bar = 200 μm. b: Inverted papilloma with positive p16INK4a expression. Human papillomavirus genome was not identified in this case. Bar = 200 μm. c: Inverted papilloma with positive p16INK4a reaction. The case became squamous cell carcinoma later (Fig. 2d). The expression of p16INK4a was more prominent in the basal layer than in the surface layer in inverted papillomas. HPV-16 with mixed integration was detected in this case. Bar = 200 μm. d: Metachronous squamous cell carcinoma that recurred after primary surgery for inverted papilloma (c). The invasive carcinoma cells displayed stronger p16INK4 expression compared with the inverted papilloma (c). HPV-16 was also detected with mixed integration. Bar = 200 μm. e: Inverted papilloma with negative p16INK4a reaction. This case had HPV-33 infection. Bar = 200 μm. f: Inverted papilloma with negative p16INK4a reaction. The human papillomavirus genome was not identified in this case. Bar = 200 μm
In the IP + SCC group, 16INK4a expression was observed in only patients with HPV infection. However, in the SCC group, 1 of the 4 patients with HPV infection showed p16INK4a expression. Cases of p16INK4a positivity in the SCC (Fig. 1c) and IP + SCC groups (Fig. 2d) showed strong p16INK4a immunoreactivity compared with the IP group (Fig. 2a-c). Interestingly, a metachronous IP + SCC case with HPV infection showed relatively low expression of p16INK4a when in IP only (Fig. 2c), whereas intense p16INK4a expression was found in IP + SCC (Fig. 2d).
Discussion
We previously reported that HPV infection is involved in the pathogenesis of IP and that high viral loads and integration of HPV may play important roles in malignant transformation [10]. In the present study, HPV infection was high in IP, IP + SCC, and SCC groups compared with the CRS group. According to a meta-analysis of HPV presence in sinonasal lesions, HPV was detected in 7.0 % of normal sinus mucosa, 4.1 % of nasal polyps, and 38.8 % of papillomas [32]. Furthermore, HPV was detected in 65.3 % of exophytic papillomas, 37.8 % of inverted papillomas, and 22.5 % of cylindrical cell papillomas [32]. According to a systematic review and meta-analysis of HPV prevalence in head and neck cancer, HPV was detected in 27.0–29.3 % of SCCs in sinonasal cancer [13, 33]. In the present study, the prevalence of HPV in CRS, IP, and SCC groups was consistent with previous reports [32, 33]. Although high-risk HPV types were detected in inflammatory diseases as well as in the IP and SCC groups, significant mixed type integration was observed in the SCC and IP + SCC groups. In addition, higher HPV-16 viral loads were detected in the SCC and IP + SCC groups, compared with the IP and CRS groups. These findings suggest that persistent infection and the integration of high-risk HPV types play important roles in the malignant transformation of IP.
Once HPV integration occurs, it leads to disruption of the HPV E2 gene, which controls the expression of the oncogenes E6 and E7 by binding to and repressing their viral promoter, resulting in their abnormal expression [2]. E6 binds to the wild-type p53 via an adenosine triphosphate-dependent step, which leads to p53 degradation and inactivity. The E7 oncoprotein binds to and functionally inactivates pRB, which controls the crucial G1–S phase transition. Functional inactivation of pRb by E7 is known to induce up-regulation of p16INK4a expression [15, 29].
In the present study, pRB expression was frequently observed in CRS, unlike p53, while expression was significantly less in the IP and SCC groups. In contrast, Altavilla et al. reported that all IP cases expressed both pRb and p16INK4a, regardless of HPV infection [1]. However, most of the cases in that study carried low-risk HPV types, whereas in the present study, all HPV cases carried high-risk types. The inverse correlation between HPV presence and pRb expression in the IP and SCC groups indicated that HPV infection in IP and SCC affects cell cycle protein expression, which suggests that HPV infection plays a role in tumorigenesis and malignant transformation.
Although the wild-type p53 is known to be involved in the negative regulation of cell growth, the mutant p53 promotes tumor formation through loss of growth suppression. Since the p53 antibody reacts to both wild- and mutant-type p53 proteins, it is impossible to distinguish between them using formalin-fixed, paraffin-embedded (FFPE) samples. However, the accumulation of p53 to levels detectable by immunohistochemistry is associated with p53 mutations [30]. In the present study, p53 expression was observed in only 1 patient in the IP and CRS groups, whereas the SCC group showed high p53 expression regardless of HPV presence. This finding suggests that the mutant p53 is rarely expressed in IP and CRS. Mirza et al. reported that the majority of HPV-positive cases did not express p53, possibly because of proteolytic degradation and elimination [20]. On the contrary, Schwerer et al. reported that the overexpression of p53 in inverted papilloma compared with normal nasal mucosa [28]. The range of p53 expression varies from zero to approximately one-third in IP [1, 19, 23, 37], whereas relatively high rates of expression are found in IP + SCC [19, 22, 37]. Lin et al. also reported that low expression of p16INK4a and positive staining for p53 are important characteristics in IP + SCC compared with IP [19]. Although the reasons for these discrepancies among the p53 positivity rates between the studies are not clear, different antibodies and evaluation methods might have affected the results.
Expression of the tumor suppressor p16INK4a has been proposed as a surrogate marker for HPV infection [7]. Overexpression of p16 is thought to reflect the presence of biologically active HPV infection. However, there are several contradictory reports on the value of p16INK4a as a biomarker. Smith et al. found no concordance between p16INK4a expression and HPV detection in 20 % of head and neck cancers [31], possibly due to transcriptionally inactive infection or an alternate pathway of p16INK4a activation [34]. In our previous study, the diagnostic sensitivity of p16INK4a overexpression was 53.2 % for the detection of HPV DNA in HNSCC, and was considerably better in oropharyngeal SCC at 80 % [8]. In the present study, p16 INK4a overexpression was identified in only 1 of the 4 SCC cases with HPV, but in more than 80 % of IP cases. These findings are consistent with previous reports [1, 19]. Although the mechanisms behind the high prevalence of p16INK4a in IP are unknown, these results revealed that, in contrast to OPSCC, p16INK4a immunoreactivity is not a surrogate marker for HPV infection in IP.
In summary, the abovementioned immunohistochemical characteristics in specimens from patients with HPV infection in the IP, IP with SCC, and SCC groups were similar to other HPV-related tumors. These immunohistochemical findings and current PCR-based results suggest that HPV infection is involved in inducing malignant transformation of IP through alteration of cell cycle protein expression.
Conclusions
HPV infection was more frequently observed in IP, IP + SCC, and SCC groups than in the CRS group. The higher viral loads and integration observed in the IP + SCC and SCC groups and the inverse correlation between HPV presence and positive pRb in immunohistochemistry indicated that persistent HPV infection and integration are involved in tumorigenesis and malignant transformation in certain IP cases. However, p16INK4a is not a reliable surrogate marker for HPV infection in IP.
Subjects and methods
The subjects in the present study consisted of 17 patients with IP (IP group), 5 with IP associated with SCC (IP + SCC group), 16 with primary sinonasal SCC (SCC group), and 32 with chronic rhinosinusitis (CRS group). Surgical treatments were performed on all patients in the IP and IP + SCC groups. Specimens from 17 patients in the IP group, 1 in the IP + SCC group, 15 in the SCC group, and 32 in the CRS group were collected and stored in liquid nitrogen until analysis. FFPE samples were used for the analysis of only 4 of the 5 cases from the IP + SCC group, and fresh frozen samples were used for the analysis of all other cases in the CRS, IP, and SCC groups, and 1 case in the IP + SCC group.
-
Patient characteristics.
The clinical features in each group, such as age, sex, and tumor stage, were reviewed from medical records.
Detection of HPV genome and identification of HPV types.
Fresh frozen samples were analyzed for HPV and HPV types as previously reported [9]. A Gentra Puregene tissue kit (Qiagen, Maryland) was used to isolate DNA from the specimens according to the manufacturer’s specifications. For the extraction of DNA from the IP-SCC and SCC FFPE samples, 3 10 μm-FFPE sections were placed in a microcentrifuge tube and mixed with 0.5 mL of DEXPAT kit (Takara, Otsu, Japan). After incubation at 100 °C for 10 min, the tube was immediately centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was collected carefully, and 10 μL, including the extracted DNA, was used as a template for a 50 μL polymerase chain reaction (PCR) reaction according to the manufacturer’s protocol. The presence and integrity of the DNA was verified in all samples by PCR β-globin gene amplification using the primers PC04 and GH20 [26]. Negative controls with water and positive controls with the DNA of HPV-16-positive CaSki cell line were included in each amplification series.
The presence of HPV DNA was analyzed by PCR using the general consensus primer sets GP5+/GP6+ and MY09/11 [9]. DNA samples negative for GP5+/GP6+ or MY9/11 PCR were re-amplified in a nested PCR using the GP5+/GP6+ primer pair as previously described [9], which can increase the sensitivity of HPV detection. After purification of positive PCR products, sequence analysis was performed on an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems, CA). The sequences obtained were aligned, and compared with those of known HPV types available from the GenBank database using the BLAST program.
To investigate HPV-16 viral loads and physical status, quantitative real-time PCR using HPV-16 DNA-positive samples was performed to measure the quantities of the E6 and E2 genes of HPV-16 with the ABI Prism 7300 Sequence Detection System (Applied Biosystems) and TaqMan PCR Master Mix II (Roche Molecular Systems, Foster City, CA). The procedures, including experimental conditions, primers and TaqMan probes, were identical to a previous report [9]. Two standard curves for the E6 and E2 genes were generated by amplification of serial 10-fold dilutions (101, 102, 103, 104, 105, and 106 viral copies) of a plasmid pB-actin carrying the complete HPV-16 early region (Addgene, Inc., Cambridge, MA). For cellular DNA quantification, an external standard curve was generated using known serial dilutions (0.3, 3, 30, and 300 ng) of human genomic placental DNA (Sigma-Aldrich, St. Louis, MO), and β-globin was amplified as described by van Duin et al. [35]. The amount of DNA (in ng) was calculated by plotting the Ct (threshold cycle) values against the logarithm of the standard curve. The relative viral load was identified by calculating the copy numbers of specimen E6 in 50 ng cellular DNA. The physical status of HPV-16 was determined according to a previously described method [24]. The amount of integrated E6 was calculated by subtracting the copy numbers of E2 (episomal) from the total copy numbers of E6 (episomal and integrated). Ratios of E2 copy number/total E6 of <1 indicate the presence of both integrated and episomal forms (mixed-type integration). An E2/E6 ratio nearly equal to 1 indicates predominance of the episomal form, whereas a ratio of 0 indicates the presence of only the integrated form.
-
3)
Immunohistochemistry for pRb, p53, and p16INK4a.
Serial 4 μm-thick sections from FFPE samples were deparaffinized in xylene and hydrated in a graded series of alcohol. Epitope retrieval was performed by heating at 95–99 °C for 10 min in Tris/EDTA buffer (pH 9.0). Endogenous peroxidase activity was quenched by incubating the sections in 3 % hydrogen peroxide and 15 mM sodium azide for 5 min. The sections were subsequently incubated overnight at 4 °C with primary monoclonal mouse anti-pRb antibody (1:2000; LifeSpan BioSciences, Seattle WA) for pRb, primary monoclonal mouse anti-p53 antibody (1:500; Progen Biotech GmbH, Heidelberg, Germany) for p53 staining, and primary monoclonal mouse anti-p16INK4a antibody (MTM Laboratories AG, Heidelberg, Germany). After extensive washing in phosphate-buffered saline, the slides were incubated for 30 min at room temperature with a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (MTM Laboratories). Immunolabeling was visualized by incubation in 3-3′-diaminobenzidine and stained slides were counterstained with hematoxylin.
Cases were considered pRb-, p53-, or p16INK4a-positive when intense nuclear and/or cytoplasmic reactivity was present. Positive expression was defined as pRb and p53 staining in more than 25 % [12], or p16INK4a staining in 40 %, of 2000 tumor cells [8].
Acknowledgements
This study was supported by a KAKENHI grant 15K10785 (C) from the Japan Society for the Promotion of Science to Dr. Hasegawa. We also thank the Ryukyu Society for the Promotion of Oto-Rhino-Laryngology for assistance with writing and technical help. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CRS
Chronic rhinosinusitis
- FFPE
Formalin-fixed, paraffin-embedded
- HPV
Human papillomavirus
- IP
Inverted papilloma
- IP + SCC
Inverted papilloma with squamous cell carcinoma
- PCR
Polymerase chain reaction
- pRb
Retinoblastoma gene product
- SCC
Squamous cell carcinoma
Footnotes
Yukashi Yamashita and Masahiro Hasegawa contributed equally to this work.
Competing interests
The authors declare that they have no competing interests related to the present study.
Authors’ contributions
YY and MH contributed to the acquisition of samples and clinical data, supervision of the experiments, and preparation of the manuscript; ZD contributed to the experimental studies and data acquisition; HM, SK, AK, SM, SA, and TU contributed to the acquisition of samples, surgical treatments, and patient follow-ups; HK and TS contributed to the acquisition of samples and clinical data, patient follow-ups, and study design; TI and AG contributed to the experimental studies; and MS contributed to the study design, supervision of experiments, and manuscript review. All authors read and approved the final manuscript.
References
- 1.Altavilla G, Staffieri A, Busatto G, Canesso A, Giacomellil A, Marioni G. Expression of p53, p16INK4A, pRb, p21WAF1/CIP1, p27KIP1, cyclin D1, Ki-67 and HPV DNA in sinonasal endophytic Schneiderian (inverted) papilloma. Acta Oto-Laryngol. 2009;129:1242–49. doi: 10.3109/00016480802620647. [DOI] [PubMed] [Google Scholar]
- 2.Arias-Pulido H, Peyton CL, Joste NE, Vargas H, Wheeler CM. Human papillomavirus type 16 integration in cervical carcinoma in situ and in invasive cervical cancer. J Clin Microbiol. 2006;44:1755–1762. doi: 10.1128/JCM.44.5.1755-1762.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batsakis JG, Suarez P. Schneiderian papillomas and carcinomas: a review. Adv Anat Pathol. 2001;8:53–64. doi: 10.1097/00125480-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Beck JC, McClatchey KD, Lesperance MM, Esclamado RM, Carey TE, Bradford CR. Presence of human papillomavirus predicts recurrence of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:49–55. doi: 10.1016/S0194-5998(95)70144-3. [DOI] [PubMed] [Google Scholar]
- 5.Beck JC, McClatchey KD, Lesperance MM, Esclamado RM, Carey TE, Bradford CR. Human papillomavirus types important in progression of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:558–563. doi: 10.1177/019459989511300506. [DOI] [PubMed] [Google Scholar]
- 6.Bura M, Seiwerth S, Vladika I, Perović D, Nagy P, Ćorić M, et al. Possible prognostic significance of p53 and Ki 67 in inverted sinonasal papilloma. Coll Antropol. 2007;31:545–549. [PubMed] [Google Scholar]
- 7.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–62. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 8.Deng Z, Hasegawa M, Aoki K, Matayoshi S, Kiyuna A, Yamashita Y, et al. A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol. 2014;45:67–76. doi: 10.3892/ijo.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Z, Hasegawa M, Kiyuna A, Matayoshi S, Uehara T, Agena S, et al. Viral load, physical status, and E6/E7 mRNA expression of human papillomavirus in head and neck squamous cell carcinoma. Head Neck. 2013;35:800–808. doi: 10.1002/hed.23034. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M, Deng Z, Maeda H, Yamashita Y, Matayoshi S, Kiyuna A, et al. Human papillomavirus load and physical status in sinonasal inverted papilloma and squamous cell carcinoma. Rhinology. 2012;50:87–94. doi: 10.4193/Rhino.11.106. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann M, Klose N, Gottschlich S, Görögh T, Fazel A, Lohrey C, et al. Detection of human papillomavirus DNA in benign and malignant sinonasal neoplasm. Cancer Lett. 2006;239:64–70. doi: 10.1016/j.canlet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Holzinger D, Flechtenmacher C, Henfling N, Kaden I, Grabe N, Lahrmann B, et al. Identification of oropharyngeal squamous cell carcinomas with active HPV16 involvement by immunohistochemical analysis of the retinoblastoma protein pathway. Int J Cancer. 2013;133:1389–1400. doi: 10.1002/ijc.28142. [DOI] [PubMed] [Google Scholar]
- 13.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6:S104–S120. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katori H, Nozawat A, Tsukuda M. Relationship between p21 and p53 expression, human papilloma virus infection and malignant transformation in sinonasal-inverted papilloma. Clin Oncol. 2006;18:300–5. doi: 10.1016/j.clon.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Khleif SN, DeGregori J, Yee CL, Otterson GA, Kaye FJ, Nevins JR, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A. 1996;93:4350–4354. doi: 10.1073/pnas.93.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft M, Simmen D, Casas R, Pfaltz M. Significance of human papillomavirus in sinonasal papillomas. J Laryngol Otol. 2001;115:709–714. doi: 10.1258/0022215011908955. [DOI] [PubMed] [Google Scholar]
- 17.Krouse JH. Endoscopic treatment of inverted papilloma: safety and efficacy. Am J Otolaryngol. 2001;22:87–99. doi: 10.1053/ajot.2001.22563. [DOI] [PubMed] [Google Scholar]
- 18.Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian Inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008;2:49–59. doi: 10.1007/s12105-008-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin GC, Scheel A, Akkina S, Chinn S, Graham M, Komarck C, et al. MDP16, EGFR, Cyclin D1, and p53 staining patterns for inverted papilloma. Int Forum Allergy Rhinol. 2013;3:885–889. doi: 10.1002/alr.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirza S, Bradley PJ, Acharya A, Stacey M, Jones NS. Sinonasal inverted papillomas: recurrence, and synchronous and metachronous malignancy. J Laryngol Otol. 2007;121:857–864. doi: 10.1017/S002221510700624X. [DOI] [PubMed] [Google Scholar]
- 21.Mumbuc S, Karakok M, Baglam T, Karatas E, Durucu C, Kibar Y. Immunohistochemical analysis of PCNA, Ki67 and p53 in nasal polyposis and sinonasal inverted papillomas. J Int Med Res. 2007;35:237–241. doi: 10.1177/147323000703500208. [DOI] [PubMed] [Google Scholar]
- 22.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head and Neck pathol. 2014;8:269–286. doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oncel S, Cosgul T, Calli A, Calli C, Pinar E. Evaluation of p53, p63, p21, p27, Ki-67 in paranasal sinus squamous cell carcinoma and inverted papilloma. Indian J Otolaryngol Head Neck Surg. 2011;63:172–177. doi: 10.1007/s12070-011-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peitsaro P, Johansson B, Syrjanen S. Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J Clin Microbiol. 2002;40:886–891. doi: 10.1128/JCM.40.3.886-891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saegusa M, Nitta H, Hashimura M, Okayasu I. Down-regulation of p27Kip1 expression is correlated with increased cell proliferation but not expression of p21waf1 and p53, and human papillomavirus infection in benign and malignant tumours of sinonasal regions. Histopathology. 1999;35:55–64. doi: 10.1046/j.1365-2559.1999.00688.x. [DOI] [PubMed] [Google Scholar]
- 26.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 27.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as an ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 28.Schwerer MJ, Sailer A, Kraft K, Baczako K, Maier H. Patterns of p21waf1/cip1 expression in non-papillomatous nasal mucosa, endophytic sinonasal papillomas, and associated carcinomas. J Clin Pathol. 2001;54:871–876. doi: 10.1136/jcp.54.11.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 30.Slade N, Moll UM. Mutational analysis of p53 in human tumors: immunocytochemistry. Methods Mol Biol. 2003;234:231–43. doi: 10.1385/1-59259-408-5:231. [DOI] [PubMed] [Google Scholar]
- 31.Smith EM, Wang D, Kim Y, Rubenstein LM, Lee JH, Haugen TH, et al. P16INK4a expression, human papillomavirus, and survival in head and neck cancer. Oral Oncol. 2008;44:133–42. doi: 10.1016/j.oraloncology.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal papillomas: systemic review and meta-analysis. Laryngoscope. 2013;123:181–192. doi: 10.1002/lary.23688. [DOI] [PubMed] [Google Scholar]
- 33.Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal carcinoma: systemic review and meta-analysis. Hum Pathol. 2013;44:983–91. doi: 10.1016/j.humpath.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Thomas J, Primeaux T. Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol. 2012;16:91–9. doi: 10.1016/j.anndiagpath.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 35.van Duin M, Snijders PJ, Schrijnemakers HF, Voorhorst FJ, Rozendaal L, Nobbenhuis MA, et al. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int J Cancer. 2002;98:590–595. doi: 10.1002/ijc.10232. [DOI] [PubMed] [Google Scholar]
- 36.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 37.Yoon BN, Chon KM, Hong SL, Lee JH, Kim JY, Cho KS, et al. Inflammation and apoptosis in malignant transformation of sinonasal inverted papilloma: the role of the bridge molecules, cyclooxygenase-2, and nuclear factor κB. Am J Otolaryngol. 2013;34:22–30. doi: 10.1016/j.amjoto.2012.07.008. [DOI] [PubMed] [Google Scholar]


