Abstract
We examined the frequency of Parkinson disease with mild cognitive impairment (PD-MCI) and its subtypes and the accuracy of 3 cognitive scales for detecting PD-MCI using the new criteria for PD-MCI proposed by the Movement Disorders Society. Nondemented patients with Parkinson’s disease completed a clinical visit with the 3 screening tests followed 1 to 3 weeks later by neuropsychological testing. Of 139 patients, 46 met Level 2 Task Force criteria for PD-MCI when impaired performance was based on comparisons with normative scores. Forty-two patients (93%) had multi-domain MCI. At the lowest cutoff levels that provided at least 80% sensitivity, specificity was 44% for the Montreal Cognitive Assessment and 33% for the Scales for Outcomes in Parkinson’s Disease-Cognition. The Mini-Mental State Examination could not achieve 80% sensitivity at any cutoff score. At the highest cutoff levels that provided specificity of at least 80%, sensitivities were low (≤44%) for all tests. When decline from estimated premorbid levels was considered evidence of cognitive impairment, 110 of 139 patients were classified with PD-MCI, and 103 (94%) had multi-domain MCI. We observed dramatic differences in the proportion of patients who had PD-MCI using the new Level 2 criteria, depending on whether or not decline from premorbid level of intellectual function was considered. Recommendations for methods of operationalizing decline from premorbid levels constitute an unmet need. Among the 3 screening tests examined, none of the instruments provided good combined sensitivity and specificity for PD-MCI. Other tests recommended by the Task Force Level 1 criteria may represent better choices, and these should be the subject of future research.
Keywords: Parkinson’s disease, parkinsonism, mild cognitive impairment, Montreal Cognitive Assessment
Mild cognitive impairment (MCI) is being recognized increasingly at all stages of Parkinson’s disease (PD). In order to facilitate research in PD-MCI and, ultimately, to guide clinical care, methods that accurately detect individuals with PD-MCI are required. Criteria for diagnosing PD-MCI and defining subtypes have varied across studies. Recently, new criteria were published by the Movement Disorders Society (MDS) Task Force on PD-MCI.1 These criteria provide methods for diagnosing PD-MCI using either an abbreviated assessment (Level 1 criteria) or a comprehensive assessment (Level 2 criteria). The Level 1 criteria suggest using a limited neuropsychological test battery or any of several multidimensional scales of cognitive abilities (the Montreal Cognitive Assessment [www.mocatest.org], the Parkinson’s Disease Cognitive Rating Scale [PD-CRS2], the Scales for Outcomes in Parkinson’s Disease-Cognition [SCOPA-Cog] [http://www.scopa-propark.eu/]. or the Mattis Dementia Rating Scale [MDRS3]). The Level 2 criteria specify how to make use of a more extensive neuropsychological test battery to make the diagnosis. In this study, we examine the frequency of PD-MCI and its subtypes according to the Level 2 criteria and several variations. We also assess the diagnostic accuracy of the Montreal Cognitive Assessment, the Mini-Mental State Examination (MMSE), and the SCOPA-Cog for detecting PD-MCI using the Level 2 criteria as a gold standard. Two prior studies have assessed the ability of the MoCA, MMSE, and/or SCOPA-Cog to detect PD-MCI4; however, to our knowledge, there are no studies examining the performance of these tests using the new criteria.
Patients and Methods
Participants
Nondemented PD patients were enrolled at 6 North American movement disorders centers. Recruitment started in December 2008 and continued through June 2011. Inclusion criteria were a diagnosis of PD according to the United Kingdom PD Society Brain Bank criteria,6 age ≥60 years, English as first language, grade 8 or higher education, and a standard score of at least 80 on the Wechsler Test of Adult Reading (WTAR) (The Psychological Corporation, San Antonio, TX), a standardized estimate of premorbid IQ. Exclusion criteria were evidence of impairments in activities of daily living or instrumental activities of daily living related to cognition on the modified Disability Assessment for Dementia (DAD), neuropsychological testing within the past year, a score 5 or greater on the 15-item Geriatric Depression Scale (GDS), currently unstable psychiatric disorder, and prior or planned neurosurgical intervention (eg deep brain stimulation). Enrolled patients received a clinical evaluation followed 1 to 3 weeks later by formal neuropsychological testing performed by examiners blinded to clinical results.
Each participating institution received local ethics approval before study enrolment. Written informed consent was obtained from all study participants and participating informed contacts (defined as contact at least twice weekly) before formal screening and study visits.
Clinical Assessment
The clinical evaluation included the MoCA, the MMSE, the SCOPA-Cog, the Neurobehavioral Signs and Symptoms Abbreviated Inventory (NBI) (Professional Resources and Technologies, Westtown, PA; a list of 19 cognitively based problems with everyday life), and the MDS Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). Given overlapping items, the order of administration of the MoCA and the MMSE were alternated to avoid bias in performance related to fatigue, learning, or other effects related to order. All investigators were trained in administering these tests and followed a standard protocol. A consenting informed contact completed the NBI and a modified version of the DAD in reference to the participant. The modified DAD added a question regarding whether any reported impairment was related to cognitive difficulties or the physical impairments of PD.
Neuropsychological Testing
Neuropsychological testing was performed 1 to 3 weeks after the clinical evaluation to balance the risks of changes in the intervening time and potential interference from similar tasks on clinical and neuropsychological evaluations. Different individuals administered the clinical evaluation and neuropsychological testing. Two neuropsychological tests were designated to represent each of 5 the following cognitive domains on the basis of having as little reliance as possible on other cognitive domains: attention was tested with the Wechsler Memory Scale-III (WMS-III) letter-number sequencing test7 and the Delis Kaplan Executive Function System (DKEFS) Color Word Interference Color Naming test,8 language was tested with the 30-item Boston Naming Test9 and the DKEFS Verbal Fluency Category Fluency test,8 visuospatial function was tested with the Benton Judgment of Line Orientation (JLO) test10 and the Copy Trial of the Rey Complex Figure Test and Recognition Trial (RCFT),11 memory was tested using the RCFT Delayed Recall and the California Verbal Learning Test-II (CVLT-II) Long Delay Free Recall test,12 and executive function was tested using the Visual Verbal Test abbreviated 10-item version13 and the Trail Making Test B minus A.14 The Trail Making Test is corrected for education; the other tests have no correction for education and, thus, none was applied. Clinical and neuopsychological evaluations were performed at a similar time of day and participants were evaluated in the ON state as judged by the patient’s self-report of the effectiveness of their PD medication at the time of testing.
Diagnosis of PD-MCI
Primary criteria for PD-MCI were based on MDS Task Force criteria for PD-MCI Level 2 criteria (comprehensive assessment) and required (1) a cognitive complaint from the patient or informed contact consisting of 1 or more items endorsed on the NBI, (2) no functions impaired because of cognition as assessed by the modified DAD, and (3) impaired performance on at least 2 of the 10 neuropsychological tests. Impaired performance on a neuropsychological test was defined primarily as a score that was at least 1.5 standard deviations (SDs) below the age-adjusted mean from normative samples. The Task Force criteria also recommend incorporating information on decline from premorbid level using the WTAR or a similar test when available. We calculated decline from premorbid level as the difference between the patient’s age/ethnicity/education-specific WTAR full scale IQ z-score and their neuropsychological test z-score. If the neuropsychological test z-score was less than the WTAR full scale IQ zscore by greater than 1.5, then the neuropsychological test performance was considered impaired. Because the Task Force did not provide any guidelines for use of the WTAR or similar tests of premorbid intellectual functioning, we report the results incorporating this aspect as a secondary analysis. Considering that impairment of insight may in fact be an integral feature of PD-MCI for some patients, we also varied the primary criteria by eliminating the need for a cognitive complaint.
The criteria for PD-MCI were applied at a consensus conference that included a neuropsychologist and 2 neurologists. Individuals who met cognitive criteria for MCI but were deemed to have functional impairment related to cognition (1 or more items on the DAD impaired due to cognition) were classified as having dementia. The medication lists were reviewed for medications with the potential to impair cognition, and a subjective determination was made regarding whether or not the doses, frequency, and combination of medications were likely to impair cognition.
We also assigned subtypes of MCI according to the Task Force criteria.1 An individual was designated as having single-domain PD-MCI if 2 tests from only 1 cognitive domain were impaired. Multi-domain PD-MCI was assigned if at least 1 test from 2 or more domains was impaired.
Statistical Analysis
Nonparametric Wilcoxon rank-sum tests were used to compare continuous measures across groups according to PD-MCI status. The χ2 test was used to compare proportions. Individuals were classified as having PD-MCI or not. Sensitivity, specificity, and positive and negative predictive values of the MoCA, MMSE, and SCOPA-Cog were calculated across all possible cutoff scores below which an individual would be classified as having PD-MCI. The area under the receiver-operator characteristics curve (AUC) was calculated and compared across the 3 tests. AUC confidence intervals (CIs) were generated according to the methods of Delong et al.15 Differences in estimated AUCs were assessed using bootstrap methodologies (2000 bootstrap replicates) implemented using the R library pROC (R Foundation for Statistical Computing, Vienna, Austria).16 The test statistic of interest for pairwise comparison of AUCs is D = (AUC1 − AUC2)/SD(AUC1 − AUC2). The observed value of D was compared with that of a standard normal distribution. Permutation tests were used to compare specificities at a given sensitivity threshold, and vice versa. Logistic generalized estimation equation (GEE) models were used to assess differences in the probability of correct classification between tests within MCI subtypes. A compound symmetry working correlation structure was assumed for the GEE model. The inference was robust to the choice of working correlation structure.
Results
We recruited 139 patients from an initial screening of 169 and excluded 30 for the following reasons: impairment in function because of cognitive impairment (ie, dementia; n = 6), withdrawal of consent (n = 4), positive screen for depression (n = 4), did not meet UK Brain Bank criteria for PD (n = 4), non-native English speaker (n = 4), prior head injury (n = 2), educational attainment less than grade 8 (n = 1), recent neuropsychological testing (n = 1), color blindness (n = 1), age <60 years (n = 1), and unrecorded reasons (n = 2). According to our primary MDS Task Force Level 2 criteria, 46 individuals (33%) were classified as having PD-MCI. Table 1 lists the clinical features of the patients overall and by PD-MCI classification according to those criteria. The groups had similar disease duration, severity, educational level, and age.
TABLE 1.
Characteristics of patients by modified Petersen Parkinson’s disease with mild cognitive impairment diagnosis
| Mean ± SD | ||||
|---|---|---|---|---|
| Characteristic | Total | No PD-MCI | PD-MCI | Pa |
| Total no. | 139 | 93 | 46 | — |
| Age, y | 71.1 (±5.4) | 71.1 (±5.7) | 71.1 ± 4.8 | 0.87 |
| Sex, % men | 67% | 69% | 63% | 0.50 |
| Education, y | 15.82 ± 2.48 | 16.1 ± 2.4 | 15.3 ± 2.7 | 0.08 |
| Time since diagnosis, y | 5.23 ± 4.64 | 5.0 ± 4.0 | 5.8 ± 5.8 | 0.73 |
| Total MDS-UPDRS | 43.19 ± 16.83 | 42.5 ± 16.7 | 44.7 ± 17.1 | 0.42 |
| MDS-UPDRS-III | 26.84 ± 11.27 | 26.7 ± 11.7 | 27.2 ± 10.4 | 0.55 |
| Total LEU, mg | 561 ± 419 | 557 ± 447 | 569 ± 359 | 0.56 |
| Cognitive enhancing medications | 6 | 2 | 4 | 0.09 |
| Potentially cognitive impairing medications | 5 | 2 | 3 | 0.33 |
| Opiate | 2 | 1 | 1 | 1.00 |
| Hypnoticb | 17 | 11 | 6 | 1.00 |
| Antipsychotic | 0 | 0 | 0 | 1.00 |
| Anticholinergicc | 21 | 14 | 7 | 1.00 |
| Estimated premorbid IQ | 113.2 ± 9.0 | 114.8 ± 8.2 | 109.9 (±9.8) | 0.002 |
| MoCA | 25.2 ± 2.9 | 25.9 ± 2.4 | 23.8 ± 3.3 | <0.0001 |
| SCOPA-Cog | 27.51 ± 4.84 | 28.8 ± 3.9 | 24.9 ± 5.5 | <0.0001 |
| MMSE | 28.14 ± 1.98 | 28.6 ± 1.5 | 27.1 ± 2.5 | 0.0006 |
All P values were calculated using the Wilcoxon rank-sum test except sex, for which the χ2 test was used.
These included benzodiazepines, zopiclone, and zaleplon.
These included trihexyphenidyl, ethopropazine, tolterodine, oxybutinin, amitriptyline, and solifenacin.
SD, standard deviation; MCI, mild cognitive impairment; MDS-UPDRS-III, Movement Disorders Society United Parkinson’s Disease Rating Scale, part 3; LEU, levodopa equivalent units; MoCA, Montreal Cognitive Assessment; SCOPA-Cog, Scale for Outcomes in Parkinson’s Disease-Cognition; MMSE, Mini-Mental State Examination.
Table 2 lists the frequencies of PD-MCI and its subtypes according to the different sets of criteria applied. When we included decline from estimated premorbid levels, the proportion of the cohort classified with PD-MCI rose from 33% to 79%. Eliminating the need for a cognitive complaint increased the proportion classified with PD-MCI by a small amount (to 41%). According to the primary criteria, 43 patients (93%) were classified with multi-domain MCI. Of those patients who had multi-domain PD-MCI, 36 of 43 (84%) had impairments in 1 or both executive tasks, 34 of 43 (79%) had impairments in 1 or both visuospatial tasks, 20 of 43 (47%) had impairments in 1 or both memory tasks, 11 of 43 (26%) had impairments in 1 or both attention tasks, and 8 of 43 (19%) had impairments in 1 or both language tasks. Because of the preponderance of multi-domain PD-MCI, we explored 1 additional variation of PD-MCI criteria: requiring 2 tests within a domain to be greater than 1.5 SD below the normative mean to declare impairment on a domain and requiring impairment in at least 1 domain to qualify as PD-MCI. According to this method, the proportion of individuals classified with PD-MCI declined to 22%, and a much higher proportion of patients with PD-MCI had single-domain MCI than when MDS Task Force Level 2 criteria were used (77% vs 7%). Almost equal numbers of individuals were assigned as having visuospatial single- domain PD-MCI (n = 11) and executive single-domain PD-MCI (n = 11). Two patients had single-domain learning/memory PD-MCI.
TABLE 2.
Frequency of Parkinson’s disease with mild cognitive impairment and its subtypes applying different criteria
| Criteria | PD-MCI, no. (% of cohort) |
Single domain PD-MCI: No. (% of MCI) |
Multi-domain PD-MCI: No. (% of MCI) |
|---|---|---|---|
| 1. MDS Task Force level 2 defining impairment based on comparisons to normative scores | 46 (33) | 3 (7) | 43 (93) |
| 2. MDS Task Force level 2 including decline from premorbid level | 110 (79) | 7 (6) | 103 (94) |
| 3. As in 1 and disregarding cognitive complainta | 57 (41) | 5 (9) | 52 (91) |
| 4. As in 1 and requiring 2 tests abnormal per domainb | 31 (22) | 24 (77)c | 6 (19)d |
This requires neuropsychological test scores ≥1.5 standard deviations (SD) below the normative mean to qualify as impaired (regardless of premorbid level) and disregarding the presence or absence of a cognitive complaint.
This requires neuropsychological test scores ≥1.5 SD below the normative mean to qualify as impaired (regardless of premorbid level) and requires 2 tests within a domain to be ≥1.5 SD below the normative mean to declare impairment on a domain. Impairment in at least 1 domain is required to qualify as PD-MCI.
Visuospatial MCI, 11; executive MCI, 11; learning/memory MCI, 2.
Unable to classify 1 patient because of 1 missing neuropsychological test value.
PD-MCI, Parkinson’s disease with mild cognitive impairment; MDS, Movement Disorder Society.
Performance of the MoCA, MMSE, and SCOPA-Cog
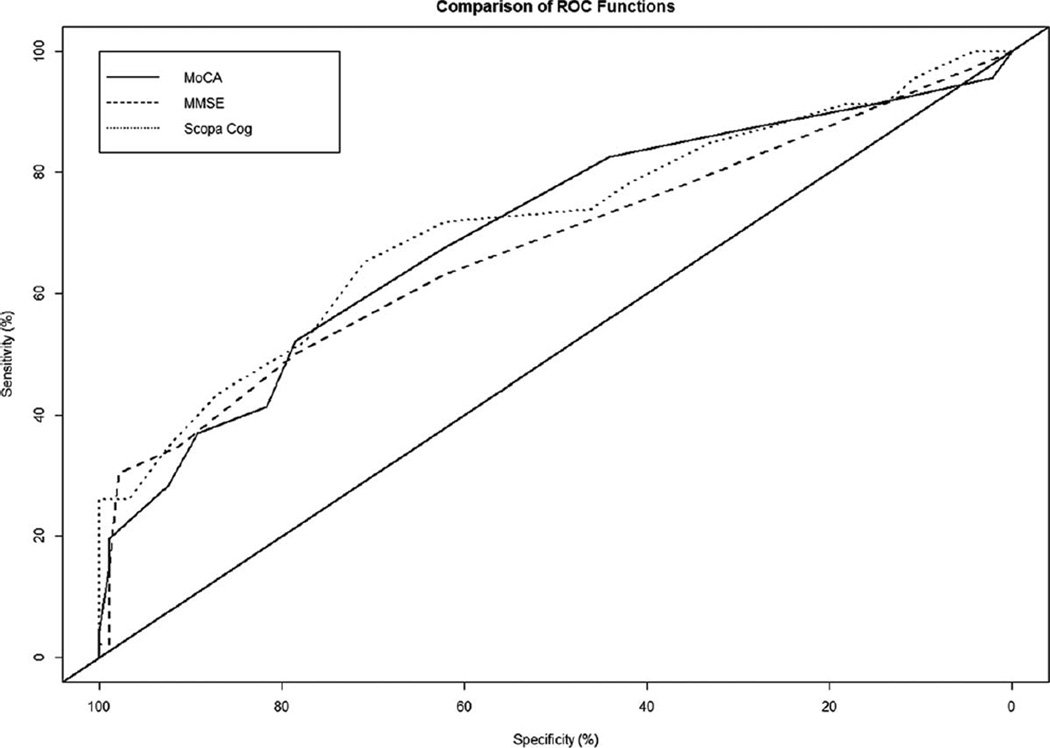
Table 3 shows the performance of the 3 tests for detecting PD-MCI across different cutoff scores using the primary criteria. The receiver-operator characteristics curves are illustrated in Figure 1. The AUC was 0.71 (95% CI, 0.60–0.80) for the MoCA, 0.68 (95% CI, 0.58–0.78) for the MMSE, and 0.72 (95% CI, 0.62–0.81) for the SCOPA-Cog. None of the pair-wise comparisons testing for a non-zero difference in AUC estimates were statistically significant.
TABLE 3.
Performance characteristics of the cognitive screening instruments for detecting Parkinson’s disease with mild cognitive impairment according to Movement Disorders Society Task Force level 2 criteria across a range of possible cutoff scoresa
| Cutoff scoreb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening instrument | 23/24 | 24/25 | 25/26 | 26/27 | 27/28 | 28/29 | 29/30 | 30/31 | 31/32 | 32/33 | 33/34 |
| MoCA | |||||||||||
| Sensitivity | 0.413 | 0.522 | 0.674 | 0.826 | 0.87 | 0.913 | 0.957 | ||||
| Specificity | 0.817 | 0.785 | 0.624 | 0.441 | 0.301 | 0.151 | 0.022 | ||||
| PPV | 0.528 | 0.545 | 0.47 | 0.422 | 0.381 | 0.347 | 0.326 | ||||
| NPV | 0.738 | 0.768 | 0.795 | 0.834 | 0.824 | 0.778 | 0.5 | ||||
| % Correctly diagnosed | 0.683 | 0.698 | 0.64 | 0.568 | 0.489 | 0.403 | 0.331 | ||||
| MMSE | |||||||||||
| Sensitivity | 0.152 | 0.133 | 0.348 | 0.5 | 0.673 | 0.783 | |||||
| Specificity | 0.989 | 0.941 | 0.914 | 0.785 | 0.667 | 0.355 | |||||
| PPV | 0.875 | 0.875 | 0.667 | 0.535 | 0.547 | 0.375 | |||||
| NPV | 0.702 | 0.26 | 0.739 | 0.76 | 0.773 | 0.767 | |||||
| % Correctly diagnosed | 0.712 | 0.755 | 0.727 | 0.691 | 0.626 | 0.496 | |||||
| SCOPA-Cog | |||||||||||
| Sensitivity | 0.435 | 0.522 | 0.652 | 0.717 | 0.739 | 0.783 | 0.848 | 0.87 | 0.913 | 0.913 | |
| Specificity | 0.878 | 0.774 | 0.71 | 0.624 | 0.462 | 0.719 | 0.333 | 0.28 | 0.183 | 0.14 | |
| PPV | 0.625 | 0.533 | 0.526 | 0.485 | 0.405 | 0.4 | 0.386 | 0.374 | 0.356 | 0.344 | |
| NPV | 0.768 | 0.766 | 0.805 | 0.817 | 0.782 | 0.796 | 0.816 | 0.813 | 0.81 | 0.765 | |
| % Correctly diagnosed | 0.727 | 0.691 | 0.691 | 0.655 | 0.554 | 0.54 | 0.504 | 0.475 | 0.424 | 0.396 | |
The scores disregarding premorbid ability are listed.
The maximum possible scores are 30 for the MoCA, 30 for the MMSE, and 43 for the SCOPA-Cog.
MoCA, Montreal Cognitive Assessment; PPV, positive predictive value; NPV, negative predictive value; MMSE, Mini-Mental State Examination; SCOPA-Cog, Scale for Outcomes in Parkinson’s Disease-Cognition.
FIG. 1.
Receiver-operator characteristic (ROC) curves for the 3 cognitive screening tests are illustrated.
Screening Cutoff Scores
No cutoff score on the MMSE provided 80% sensitivity. At the lowest cutoff score that provided 80% sensitivity, the specificity of the MoCA (at 26 or less; ie, classifying individuals who scored 26 or less as having PD-MCI) was 44%, and the specificity of the SCOPA-Cog (at 30 or less) was 33% (P = 0.26 for the difference in specificity). Diagnostic accuracy (the percentage diagnosed correctly) at these cutoff scores was 57% for the MoCA and 50% for the SCOPA-Cog.
Diagnostic Cutoff Scores
The highest cutoff scores that provided at least 80% specificity were 23 or less (ie, classifying individuals who scored 23 or less as having PD-MCI) for the MoCA, 26 or less for the MMSE, and 24 or less for the SCOPA-Cog. These cutoff scores provided sensitivity ranging from 35% to 44% and diagnostic accuracy ranging from 68% to 73%. The sensitivities of the 3 tests did not differ significantly at these cutoff values.
MDS Task Force Level 2 Criteria Considering Premorbid Intellectual Function
Supplemental Table 1 illustrates the performance of the 3 tests when decline from estimated premorbid ability was included in the assessment. Once again, the MMSE was unable to achieve 80% sensitivity at any cutoff score. At optimal screening cutoff scores (the lowest score providing sensitivity of at least 80%), the specificities of the MoCA and SCOPA-Cog were both 17%. At optimal diagnostic cutoff scores (the highest score providing specificity of at least 80%), the sensitivities of the MoCA, MMSE, and SCOPA-Cog ranged from 22% to 35%. The sensitivities of the 3 tests were not significantly different at these cutoff values.
Discussion
The MDS Task Force criteria for PD-MCI resulted in a similar proportion of patients being classified with PD-MCI compared with previous reports in which impaired performance was determined based on comparisons with normative scores. When a decline from the estimated premorbid level was considered evidence of cognitive impairment (as suggested by the MDS Task Force), a much higher proportion of the cohort was classified with PD-MCI than in previous studies, which have reported a frequency of PD-MCI usually in the range of 19% to 25%4,5,17,18 and as high as 53%.19 Cognitive decline from premorbid levels may be important for patients whose expectations are based on their levels of premorbid functioning. For example, for an individual who is gainfully employed, even a slight decline might result in inability to continue at their customary job. It would be important to learn which classification approach (with our without considering premorbid cognitive function) results in a more meaningful separation of patients on the basis of both quality of life and prognosis.
It is rare that a patient comes to evaluation with prior neuropsychological testing that would provide an undisputed baseline. Thus, clinicians rely heavily on history (level of education, occupation, hobbies) and current test scores on measures resistant to neurological insult, such as the WTAR, that can yield an estimate of premorbid function. A gap in the MDS Task Force criteria for PD-MCI is guidance on how to determine an important decline using such measures, particularly for research purposes, where a reproducible method is desirable. In strict application to identify loss of function, the WTAR manual recommends comparing predicted Wechsler Adult Intelligence Scale (WAIS)-III IQ scores and Wechsler Memory Scale (WMS)-III scores with a patient’s current performance (eg WTAR-demographic predicted full-scale IQ). However, use of the WAIS-III and WMS-III test batteries in a PD cognitive research setting is impractical because of long administration times; and, more important, the sensitivity of these global index scores from the WAIS scales is poor for detecting early cognitive changes in association with PD.20,21 Therefore, an alternative method of defining decline using these premorbid estimates is needed.
In the absence of WAIS-III or WMS-III scores, we operationalized decline at 1.5 SD from the WTAR-demographic estimated full-scale IQ and applied this across the domains represented by our chosen core neuropsychological tests. We recognize that this operationalization has its limitations. WTAR scores correlate highly with full-scale IQ and verbal IQ but to a lesser degree with other domains, and the 95% CIs of WTAR-predicted IQ scores are large. However, in patients with high premorbid functioning like those in our cohort, WTAR-predicted scores will tend to underestimate the amount of cognitive deterioration that has occurred. A 1.5-SD criterion for decline is also conservative relative to the definitions of decline based on the WAIS-III and WMS-III. For these reasons, we believe that the 79% PD-MCI we observed in our sample is likely an underestimate of the proportion of individuals who have actually experienced a decline from premorbid cognitive function. The MDS Task Force Level 2 criteria also suggest that decline on serial neuropsychological testing should be used as a criterion for impairment on neuropsychological tests. We did not have any prior neuropsychological testing on these individuals to apply this criterion. Incorporating this aspect could result in an even higher proportion of the cohort classified with PD-MCI according to the new criteria.
When we examined the subtype of PD-MCI in our sample using the MDS Task Force criteria, the vast majority of patients were classified with multi-domain MCI (93%). Using various definitions, prior studies have reported that multi-domain PD-MCI comprised as low as 14% and as high as 61% of the PD-MCI cases in their samples.17–19,22–25 The high proportion of multi-domain MCI in our sample appears to reflect the new MDS Task Force criteria, in that a multi-domain MCI designation only requires 1 abnormal test in each domain in contrast to single-domain MCI, which requires 2 abnormal tests within the affected domain. Other studies have approached this differently, requiring the same number of abnormal tests per domain or the same degree of abnormality per domain regardless of whether a single or multiple domains are affected.17–19,23,24 When we modified the MDS Task Force criteria to require 2 abnormal tests per domain regardless of single-domain or multi-domain designation, the subtype distribution shifted such that the majority of patients were classified with single-domain MCI, and designation of the predominantly affected domains was clear. By using this method, executive and visuospatial functions were the most commonly affected domains in our cohort, in line with several other reports.18,19,23
The performances of the MoCA, MMSE, and SCOPA-Cog were poor when using MDS Task Force criteria whether or not premorbid ability was considered to define cognitive decline. For neither definition of PD-MCI could any of these tests achieve a desirable combined sensitivity and specificity. This calls into question the recommendation of the Task Force, which suggests that either the MoCA or the SCOPA-Cog can be used to diagnose possible PD-MCI (Level 1 criteria). Other studies that have examined 1 or more of these tests for the purpose of identifying individuals with PD-MCI4,5 have concluded that the MoCA performs reasonably well for this purpose and somewhat better than the other tests. In those studies, MoCA cutoff scores that provided sensitivities and negative predictive values above 80% (designated the optimal screening cutoff score) were 25 or less4 and 26 or less.5 At these cutoff scores, specificities ranged from 52% to 75%. The sensitivities of the MMSE and/or SCOPA-Cog generally were lower than for the MoCA in those studies.
The desired characteristics of a diagnostic test depend on the purpose for which it will be used. Currently, PD-MCI does not have specific treatment implications, although ongoing work in this important issue will likely lead to further studies. There has been 1 report from a randomized trial of rasagiline demonstrating positive effects on cognitive tests in PD patients without dementia,26 but no studies have specifically examined patients with MCI. Although a few studies have suggest that PD-MCI has prognostic implications (for a useful review, see Litvan et al.27), currently, the need to identify patients with PD-MCI is largely for the purpose of enrolment in research studies. For this purpose, a test of high specificity is desired to ensure that the population of interest is being studied and to allow meaningful interpretation of the results. High specificity can be achieved using lower cutoff scores but with low sensitivity for all 3 tests, which would result in questionable generalizability of any cohort recruited in this way. The MDS Task Force Level 1 criteria also suggest that the PD-CRS and the MDRS are useful tools for diagnosing PD-MCI using an abbreviated assessment. Assessing the performance of those instruments using the new Level 2 criteria as a gold standard would be a useful next step.
Conclusions
Our exploration of the new MDS Task Force criteria for PD-MCI leads to 2 important observations. First, the proportion of individuals classified as having PD-MCI is much higher than in previous reports when incorporating a decline from estimated premorbid levels into the definitions of impairment, as the Task Force suggests. Whether or not this is appropriate depends on whether or not the new method results in a more meaningful separation of groups on the basis of characteristics like quality of life or prognosis. This should be the subject of future study, as should methods for operationalizing the definition of decline using the WTAR or other estimates of premorbid function. Second, the vast majority of PD-MCI is classified as multi-domain MCI by the new criteria. A minor alteration of the criteria results in a separation of subtypes that may be more useful for research purposes.
We did not find meaningful differences in performance between the MoCA, MMSE, or SCOPA-Cog as a screening or diagnostic tool for PD-MCI. Our results indicate that, to recruit a representative cohort of patients with PD-MCI for research purposes, none of the tests studied would be adequate alone, and any would need to be followed by a second test with higher specificity. Future research should identify a follow-up test that, together with the screen, can constitute an efficient way to identify individuals with PD-MCI. Furthermore, these findings call for a reconsideration of the MDS Task Force Level 1 criteria, which suggest that either the MoCA or the SCOPA-Cog may be used as a brief test for PD-MCI.
Supplementary Material
Acknowledgments
Funding agencies: This study was funded by the Michael J. Fox Foundation for Parkinson’s Research. The Foundation had no role in the conduct, analysis, or interpretation of the study nor in approval of the submitted article. Dr. Marras is also supported by a New Investigator Award from the Canadian Institutes of Health Research.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Relevant conflicts of interest/financial disclosures: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A. Parkinson’s disease-cognitive rating scale: a new cognitive scale specific for Parkinson’s disease. Mov Disord. 2008;23:998–1005. doi: 10.1002/mds.22007. [DOI] [PubMed] [Google Scholar]
- 3.Villeneuve S, Rodrigues-Brazete J, Joncas S, Postuma RB, Latreille V, Gagnon JF. Validity of the Mattis Dementia Rating Scale to detect mild cognitive impairment in Parkinson’s disease and REM sleep behavior disorder. Dement Geriatr Cogn Disord. 2011;31:210–217. doi: 10.1159/000326212. [DOI] [PubMed] [Google Scholar]
- 4.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2011;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 5.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 8.Delis DC, Kaplan E, Kramer JH. Delis–Kaplan Executive Function System (D-KEFS): Examiner’s Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 9.Williams BW, Mack W, Henderson VW. Boston naming test in Alzheimer’s disease. Neuropsychologica. 1989;27:1073–1079. doi: 10.1016/0028-3932(89)90186-3. [DOI] [PubMed] [Google Scholar]
- 10.Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. 2nd ed. New York, NY: Oxford; 1994. [Google Scholar]
- 11.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial—Professional Manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 12.Delis D, Kramer JH, Kaplan E. The California Verbal Learning Test. 2nd ed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 13.Wicklund AH, Johnson N, Weintraub S. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer’s disease and the behavioral presentation of frontotemporal dementia. J Clin Exp Neuropsychol. 2004;26:347–355. doi: 10.1080/13803390490510077. [DOI] [PubMed] [Google Scholar]
- 14.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 15.DeLong E, DeLong D, Clarke-Pearson D. Comparing the area under two or more correlated receiver operating characteristic curves: a non-parametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 16.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves [serial online] BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G Norwegian ParkWest Study G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 18.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 19.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 20.Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106:257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- 21.Downes JJ, Priestley NM, Doran M, Ferran J, Ghadiali E, Cooper P. Intellectual, mnemonic, and frontal functions in dementia with Lewy bodies: a comparison with early and advanced Parkinson’s disease. Behav Neurol. 1998;11:173–183. [PubMed] [Google Scholar]
- 22.Dalrymple-Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson’s disease. Mov Disord. 2011;26:629–636. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- 23.Goldman JG, Weis H, Stebbins G, Bernard B, Goetz CG. Clinical differences among mild cognitive impairment subtypes in Parkinson’s disease. Mov Disord. 2012;27:1129–1136. doi: 10.1002/mds.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sollinger AB, Goldstein FC, Lah JJ, et al. Mild cognitive impairment in Parkinson’s disease: subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16:177–180. doi: 10.1016/j.parkreldis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poletti M, Frosini D, Pagni C, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. Journal of Neurology, Neurosurg Psychiatry. 2012;83:601–606. doi: 10.1136/jnnp-2011-301874. [DOI] [PubMed] [Google Scholar]
- 26.Hanagasi HA, Hakan G, Unsalan P, et al. The Effects of rasagiline on cognitive deficits in Parkinson’s disease patients without dementia: a randomized, double-blind, placebo-controlled, multicenter study. Mov Disord. 2011;26:1851–1858. doi: 10.1002/mds.23738. [DOI] [PubMed] [Google Scholar]
- 27.Litvan I, Aarstland D, Adler CH, et al. MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.