Abstract
The phylum Arthropoda contains the largest number of described living animal species, with insects and crustaceans dominating the terrestrial and aquatic environments, respectively. Their successful radiations have long been linked to their rigid exoskeleton in conjunction with their specialized endocrine systems. In order to understand how hormones can contribute to the evolution of these animals, here, we have categorized the sesquiterpenoid and ecdysteroid pathway genes in the noninsect arthropod genomes, which are known to play important roles in the regulation of molting and metamorphosis in insects. In our analyses, the majority of gene homologs involved in the biosynthetic, degradative, and signaling pathways of sesquiterpenoids and ecdysteroids can be identified, implying these two hormonal systems were present in the last common ancestor of arthropods. Moreover, we found that the “Broad-Complex” was specifically gained in the Pancrustacea, and the innovation of juvenile hormone (JH) in the insect linage correlates with the gain of the JH epoxidase (CYP15A1/C1) and the key residue changes in the binding domain of JH receptor (“Methoprene-tolerant”). Furthermore, the gain of “Phantom” differentiates chelicerates from the other arthropods in using ponasterone A rather than 20-hydroxyecdysone as molting hormone. This study establishes a comprehensive framework for interpreting the evolution of these vital hormonal pathways in these most successful animals, the arthropods, for the first time.
Keywords: juvenile hormone, methyl farnesoate, ecdysteroid, Myriapoda, Chelicerata, Arthropoda
Introduction
The arthropods or joint-legged animals, in terms of described living and fossil species numbers, are certainly the most successful animals on the earth. They exhibit a diverse range of body plans and can be found in the widest possible range of habitats worldwide. One of the reasons linked to their success is their hardened chitinous exoskeleton, which provides them with the protection from their often harsh environment and ecosystems (Minelli et al. 2013). In order to grow and increase in body size, arthropods have evolved a complex endocrine cassette. These hormones include sesquiterpenoids (such as methyl farnesoate [MF] and juvenile hormone [JH]) and the ecdysteroids (such as 20-hydroxyecdysone [20E]), which coordinately play vital roles in regulating the maturation, growth, and development of arthropods. Their actions and metabolism have been particularly well-investigated in the Insecta, in which high titer of JH generally restrains insects from undergoing metamorphosis, and high titer of 20E triggers molting/ecdysis (Jindra et al. 2013). Nevertheless, their functions and regulation of production and degradation in noninsect arthropods are poorly understood (Hui et al. 2013; Kenny et al. 2013). With the advent of affordable next-generation sequencing, genomes of noninsect arthropods are now available, including the crustaceans (water flea and shrimp) (Colbourne et al. 2011; Kenny et al. 2014), myriapods (centipede and millipede) (Chipman et al. 2014; Kenny, Shen, et al. 2015), and chelicerates (scorpion, horseshoe crab, mite, and tick) (Grbic et al. 2011; Cao et al. 2013; Chan et al. 2014; Sanggaard et al. 2014; Kenny, Chan, et al. 2015). Here, we utilized these noninsect arthropod genomes to systemically reveal the genetic componentry of the sesquiterpenoid and ecdysteroid systems across the Arthropoda for the first time.
Materials and Methods
Identification of Sesquiterpenoid and Ecdysteroid Pathway Genes
Amino acid sequences of known components in sesquiterpenoid and ecdysteroid systems from insect species including Drosophila melanogaster, Bombyx mori, and Tribolium castaneum were retrieved from FlyBase (http://flybase.org/, last accessed July 4, 2015), SilkDB database (http://silkworm.genomics.org.cn/, last accessed July 4, 2015), and BeetleBase (http://beetlebase.org/, last accessed July 4, 2015) and used as queries to identify putative gene homologs in noninsect arthropod genomes via TBLASTN search (Altschul et al. 1990) with the cutoff E-value of 1 × 10−6. The noninsect arthropod genomes included those of the water flea (Daphnia pulex; http://www.jgi.doe.gov/, last accessed July 4, 2015) (Colbourne et al. 2011), shrimp (Neocaridina denticulata) (Kenny et al. 2014), centipede (Strigamia maritima; http://www.hgsc.bcm.tmc.edu/, last accessed July 4, 2015) (Chipman et al. 2014), millipede (Trigoniulus corallinus) (Kenny, Shen, et al. 2015), tick (Ixodes scapularis; https://www.vectorbase.org, last accessed July 4, 2015), spider mite (Tetranychus urticae; http://bioinformatics.psb.ugent.be/genomes/, last accessed July 4, 2015) (Grbic et al. 2011), dust mite (Dermatophagoides farinae) (Chan et al. 2014), and three species of horseshoe crabs (Carcinoscorpius rotundicauda, Limulus polyphemus, and Tachypleus tridentatus) (Nossa et al. 2014; Kenny, Chan, et al. 2015). Gene sequences were transcribed into protein sequence using the standard translational code and subjected to reciprocal blast using BLASTX against the nr database (http://blast.ncbi.nlm.nih.gov/Blast.cgi, last accessed July 4, 2015) for initial confirmation of identity.
Phylogenetic Confirmation of Gene Identity
To confirm the orthology of retrieved gene sequences, phylogenetic analyses using both maximum-likelihood and Bayesian inference methods were carried out. Gene sequences were first translated into amino acid sequences and aligned to sequences with known identity using MAFFT (Katoh and Standley 2013) under appropriate models as noted in figure legends. Maximum-likelihood phylogeny was carried out by MEGA 6 (Tamura et al. 2013) using the LG + 4G + I model and 1,000 bootstrap replicates. Bayesian inference was performed on alignments using MrBayes (Ronquist and Huelsenbeck 2003). The model jumping command in MrBayes was implemented, which selected substitution models in proportion to their posterior probability. A Markov chain Monte Carlo search was initiated with random trees and run for 1,000,000 generations with sampling every 100 generations (or as noted when generation number differed). Convergence was indicated when the average standard deviation of split frequencies was less than 0.01 (Ronquist and Huelsenbeck 2003). Convergence was checked by plotting the likelihood scores against generations and the first 25% of the generations were discarded as burn-in. Trees were visualized in FigTree for annotation and display.
Results and Discussion
Sesquiterpenoid Biosynthetic and Degradative Pathway Genes in Noninsect Arthropods
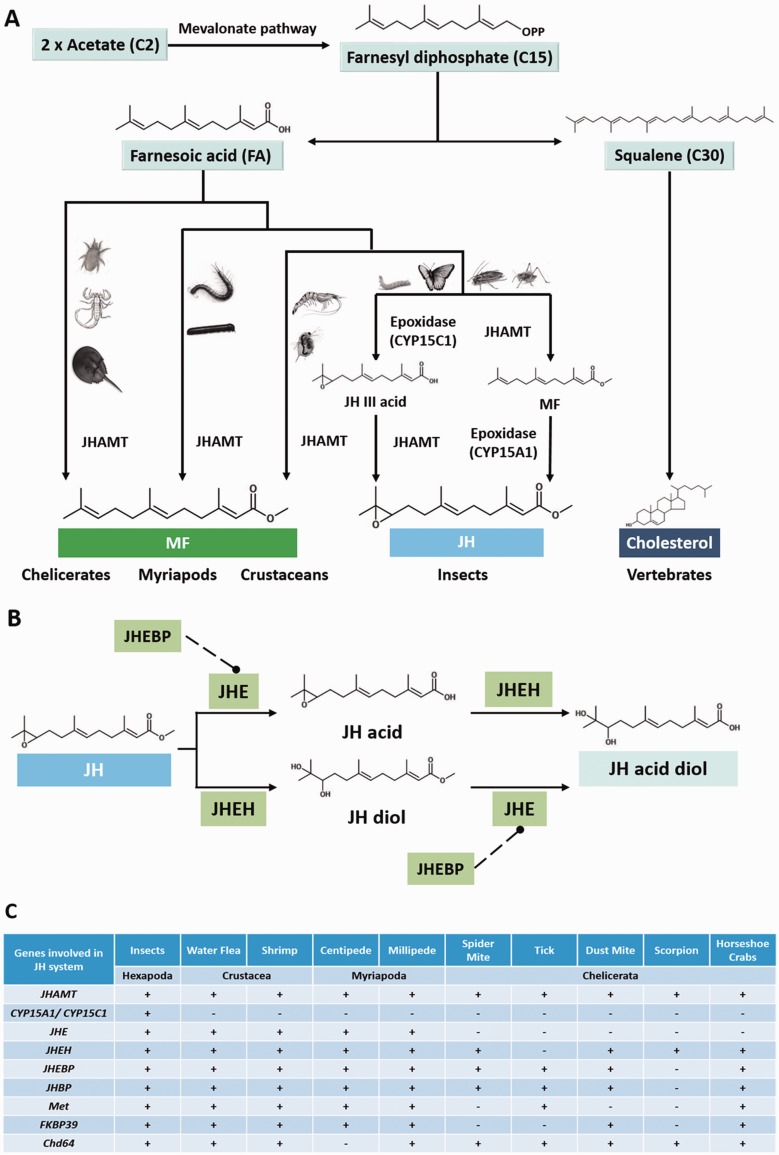
Sesquiterpenoids are a class of hydrocarbon/terpenes found in arthropods and plants; in arthropods, they are derived from the conversion of acetate via the mevalonate pathway (Tobe and Bendena 1999; Belles et al. 2005; Hui et al. 2010, 2013). In insects, there are two alternative pathways for the biosynthesis of JH: Either through the conversion of farnesoic acid (FA) to JH-III acid (JHA) by an epoxidase (CYP15C1) followed by methylation by JH acid methyltransferase (JHAMT) in the lepidopterans; or methylation of FA to MF by JHAMT followed by oxidation by another epoxidase (e.g., CYP15A1) as found in cockroaches and locust (Helvig et al. 2004; Belles et al. 2005; Marchal et al. 2011; Daimon et al. 2012; fig. 1A). In crustaceans, MF is synthesized through methylation of FA (Tobe et al. 1989). In addition to playing defensive roles in some social insects such as ants and bees, sesquiterpenoids including MF and JH are endogenously produced as master controllers of molting and sexual maturity in crustaceans and insects, respectively (Nagaraju 2007; Riddiford 2008). Although JH has never been identified in noninsect arthropods, JH mimics have been observed to play a role in controlling sex development and predator response in D. pulex (Olmstead and Leblanc 2002; Tatarazako et al. 2003; Miyakawa, Gotoh, et al. 2013). Recently, it has also been noted that the genes involved in JH production and degradation pathways, previously thought to be insect specific, are identified in water flea, shrimp, and centipede (Chipman et al. 2014; Sin et al. 2014). Here, we have identified JHAMT orthologs in the genomes of all investigated arthropods (fig. 1C and supplementary figs. S1 and S20, Supplementary Material online), suggesting the existence of a sesquiterpenoid biosynthetic system in the last common ancestor of all arthropods. However, no homologs of insect JH epoxidase (CYP15A1 or C1) could be identified in any of these noninsect arthropod genomes (fig. 1C). On the basis of this finding, we conclude, for the first time, that JH epoxidase was specifically gained in the insect lineage.
Fig. 1.
— Schematic diagrams showing (A) biosynthetic and (B) degradative pathways of sesquiterpenoid hormones in arthropods (Hui et al 2010, 2013; Sin et al 2014; for details, refer to the main text); (C) summary of the presence of the sesquiterpenoid biosynthetic, degradative, and signaling pathway genes in the investigated arthropod genomes. “+” denotes presence and “−” represents the absence of supporting evidence.
In addition to the biosynthetic pathway genes for insect JH, we could also identify genes encoding enzymes (JH esterase [JHE] and JH epoxide hydrolase [JHEH]) and associated protein (JH esterase binding protein [JHEBP]) involved in insect JH degradative pathway in these investigated arthropod genomes (fig. 1C and supplementary figs. S2–S4, Supplementary Material online). In insects, JHE and JHEH contribute to the reduction of JH titer and induce metamorphosis (fig. 1B) (Share and Roe 1988; Anand et al. 2008), and JHEBP functions in JHE transportation and degradation that can indirectly modulate JH titer (Hao et al. 2013).
The esterases are a diverse and rapidly evolving class of genes in arthropods, and their roles in regulation of hormonal action can be vastly different between species. In insects, dipterans and hymenopterans possess “Dipteran-type” JHE genes, whereas lepidopterans use another type of JHE (Mackert et al. 2008). In our sequence and phylogenetic analyses, Dipteran-type JHEs are found in crustaceans including N. denticulata (Sin et al. 2014) and Pandalopsis japonica (Lee et al. 2011), and myriapods such as the centipede S. maritima (Chipman et al. 2014) and the millipede T. corallinus (supplementary fig. S21, Supplementary Material online). Although bootstrap support is weak at the base of the Dipteran-type JHE node in this tree, these genes share the diagnostic residues that suggest JHE identity (Mackert et al. 2008) and a clear lack of affinity to any other esterase gene present in the Arthropoda (represented in supplementary fig. S21, Supplementary Material online, by Dr. melanogaster sequences). Although there is a tremendous diversity in esterase gene number and sequence in all sequenced chelicerate genomes to date, there are no genes which share strong phylogenetic signal with the JHE genes of the Dipteran-like clade, suggesting that chelicerates may use another type of esterase.
JHEBP was first described in Manduca sexta (Shanmugavelu et al. 2000) and has since been found in a number of insects, crustaceans, and a centipede (Chipman et al. 2014; Sin et al. 2014). Of the genomes examined here, only Mesobuthus martensii lacks a clear JHEBP homolog, although sequence MMa33864 may represent a highly divergent member of this clade. This sequence contains the Timm-44-like domain at the C terminus (supplementary fig. S4, Supplementary Material online; Liu et al. 2007; Hao et al. 2013).
JHEH plays an important role in regulating insect JH titer by conversion of JH into JH diol or JHA into JHA diol (Mackert et al. 2010). It was first cloned from M. sexta, and belongs to the microsomal epoxide hydrolase (EC 3.3.2.9) family (Wojtasek and Prestwich 1996; Zhou et al. 2014). Here, we identified JHEH orthologs in both myriapods and chelicerates (fig. 1C and supplementary fig. S3, Supplementary Material online), suggesting that JHEH may have evolved in the last common ancestor of arthropods.
Sesquiterpenoid Signaling Genes
A range of gene homologs involved in the JH signaling pathway can be identified in the investigated arthropod genomes. These include “Methoprene-tolerant” (Met), “cytosolic juvenile hormone binding protein” (cytosolic JHBP), FKBP39, and Chd64 (fig. 1C and supplementary figs. S5–S8, Supplementary Material online).
Met is a member of the basic Helix-Loop-Helix Per-Arnt-Sim family that is assumed to function as a JH intracellular receptor, binding with JH via its C-terminal Per-Arnt-Sim (PAS) domain; this activates downstream gene transcription in insects (Konopova and Jindra 2007; Charles et al. 2011; Li et al. 2011). Met orthologs have been previously identified in crustaceans such as the water flea (LeBlanc et al. 2013; Miyakawa, Toyota, et al. 2013) and the shrimp (Sin et al. 2014). A single amino acid mutation in the PAS domain (change from T to V) of daphnid Met significantly changed the JH responsiveness, and the authors suggested that this mutation contributed to the evolution of the insect JH receptor system (Miyakawa, Toyota et al. 2013). In our sequence analysis, we found that a Met ortholog is also present in myriapods and chelicerates, and the conservation of the valine position could only be detected in the insect lineage, consistent with the previous suggestion that the valine substitution in the insect ancestor coevolved with JH production (fig. 1C and supplementary figs. S6 and S23, Supplementary Material online).
Cytosolic JHBP genes have been described in the shrimp N. denticulata (Sin et al. 2014), centipede S. maritima (Chipman et al. 2014), and in a number of other arthropod species, particularly in the Lepidoptera (Touhara and Prestwich 1993; Touhara et al. 1994; Carter et al. 2013). However, to date little has been done to describe the evolutionary origin of these genes. In our analyses, we have identified cytosolic JHBP in a range of noninsect arthropods which do not produce JH (fig. 1C; Sin et al. 2014). Initial examination of its domain structure and sequences suggests strong affinity with the “glyoxalase domain containing 4” (GLOD4) genes which are known to bind to a range of ligands (supplementary fig. S5, Supplementary Material online). Because protein with binding affinity toward MF has been identified in the hemolymph of crustaceans (Li and Borst 1991; King et al. 1995; Tamone et al. 1997), we suggest that these cytosolic JHBP orthologs in the noninsect arthropods could potentially function as FA or MF binding proteins.
FKBP39 is a member of the FK506 binding protein family that serves as protein folding chaperones in insects; this protein binds to juvenile hormone response elements (JHRE) that regulate the cross-talk between ecdysteroids and JH (Li et al. 2007). We have also identified FKBP39 orthologs in almost all of the noninsect arthropods investigated here, which share the two characteristic residue signatures including a 3-nucleotide indel and a six residue “GMKVGG” domain (supplementary fig. S7, Supplementary Material online). Chd64 is another protein previously known to bind to the JHRE and modulates the responses to JH exposure (Li et al. 2007). Although Chd64 possesses a clear calponin homology (CH) domain (pfam id: pfam00307), its relationship to the wider family of other CH-containing genes is largely uncataloged (Kozłowska et al. 2014). In our analysis, we have also identified putative Chd64 orthologs in the noninsect arthropods (supplementary fig. S24, Supplementary Material online). Our identification of JHRE-related genes in noninsect arthropods provides new potential targets for dissecting the regulation of sesquiterpenoid action.
The Ecdysteroid Pathway Genes of Noninsect Arthropods
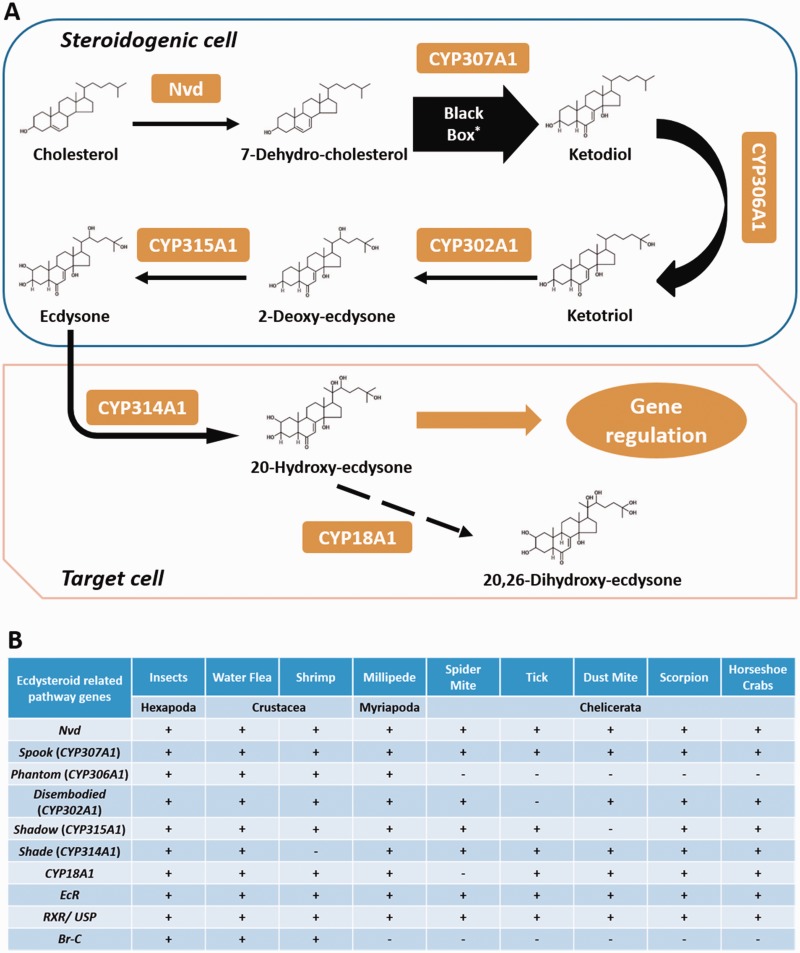
Ecdysteroids play a variety of roles in regulating the growth (in particular molting) and sexual maturation of insects (Parthasarathy et al. 2010; Tennessen and Thummel 2011) and crustaceans (Chávez et al. 2000; Warren et al. 2002, 2004; Petryk et al. 2003; Niwa et al. 2004; Mykles 2011). Other than in insects, their biosynthetic pathway has only been described in two crustaceans (Rewitz and Gilbert 2008; Sin et al. 2014) and a mite (Cabrera et al. 2015). In both insects and crustaceans, the ecdysteroid 20E is synthesized from dietary cholesterol via cytochrome P450 enzymes encoded by the Halloween genes (Rewitz et al. 2007; Rewitz and Gilbert 2008; Sin et al. 2014; fig. 2A). Initially, the conserved Rieske-like oxygenase neverland converts cholesterol into 7-dehydrocholesterol which is then processed into bioactive 20E through a series of reactions catalyzed by Spook/Spookier (CYP307A1/2), Phantom (CYP306A1), Disembodied (CYP302A1), Shadow (CYP315A1), and Shade (CYP314A1), respectively (fig. 2A). Here, we have identified the majority of these gene homologs in all investigated arthropod genomes (fig. 2B and supplementary figs. S9–S16, 25, and 26, Supplementary Material online), suggesting that myriapods and chelicerates could also synthesize ecdysteroids. Nevertheless, we could not identify any CYP306A1 ortholog in any chelicerate genomes. Although it has been previously suggested that the ortholog of CYP306A1 is present in the scorpion (Cao et al. 2013), our phylogenetic analysis strongly supports the view that the gene sequence belongs to the CYP18A1 family instead (supplementary figs. S16 and S26, Supplementary Material online). We therefore suggest that chelicerates probably use ponasterone A (25-deoxy-20E) as the major form of molting hormone rather than the typical arthropod 20E, which is consistent with the identification of 25-deoxy-20E in the spider mite (Grbic et al. 2011).
Fig. 2.
— (A) Schematic diagram showing the biosynthetic and degradative pathways of ecdysteroids in arthropods (Sin et al 2014; for details, refer to the main text); (B) summary of the presence of ecdysteroid biosynthetic, inactivation, and signaling pathway genes in the investigated arthropods. “+” denotes presence and “−” represents the absence of supporting evidence. Nvd: neverland.
In insects, the inactivation pathway of ecdysteroids is poorly understood and CYP18A1 is the only key enzyme that has previously been shown to be involved in its degradation (Guittard et al. 2011; Li et al. 2014). We have also identified CYP18A1 in all noninsect arthropods (fig. 2B and supplementary figs. S15 and 16, Supplementary Material online), suggesting that a common core set of genes for controlling ecdysteroid titer existed in the last common ancestor of arthropods.
Ecdysone receptor (EcR) proteins and retinoid X receptor (RXR, or Ultraspiracle/Usp in lepidopterans and dipterans) form a heterodimer to which ecdysteroids bind and which control a variety of downstream processes related to development and reproduction in insects (Riddiford et al. 2000; Riddiford 2008; Nakagawa and Henrich 2009). The presence of EcR in noninsect arthropods has been noted previously (Thomson et al. 2009; Chipman et al. 2014), and we have also identified EcR and RXR homologs in all investigated arthropods (fig. 2B and supplementary figs. S17, S18, and S27, Supplementary Material online). Given that MF shows higher binding affinity to Usp than JH (Jones et al. 2006), and the findings of a correlation between RXR expression and MF biosynthetic rate in female lobster during the reproductive cycle (Tiu et al. 2012) and differential RXR expression during shrimp ovarian maturation (Cui et al. 2013), RXR may potentially play a role in the cross-talk between ecdysteroids and sesquiterpenoid hormones during arthropod development and reproduction.
Broad-Complex (Br-C) or broad genes are well known to play a diverse range of roles in ecdysone response during oogenesis (Tzolovsky et al. 1999) and metamorphosis (Bayer et al. 1997). The expression of Br-C is also affected by JH and it may coordinate holometabolan metamorphosis by acting downstream of Met (Reza et al. 2004; Konopova and Jindra 2008). However, the evolutionary origin of these genes remains unclear. Broad genes possess the characteristic BTB (Bric a brac, Tramtrack, and Broad) domain, and have been reported in two crustaceans, the shrimp N. denticulata (Sin et al. 2014) and Penaeus monodon (Buaklin et al. 2013). In our phylogenetic analysis, the well-characterized Broad proteins in insects and crustaceans form a monophyletic group, and there is no evidence of a broad gene ortholog in the noninsect/crustacean arthropod genomes examined here (supplementary fig. S28, Supplementary Material online). We therefore suggest that the broad gene is a pancrustacean innovation.
Concluding Remarks: The Panarthropod Sesquiterpenoid and Ecdysteroid Pathways
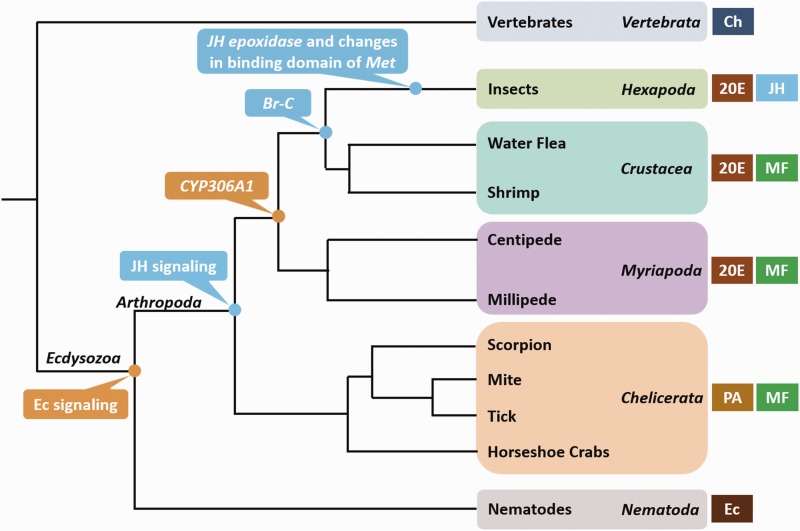
In this study, we utilized newly available noninsect arthropod genomes to gain the first global picture of the componentry of the sesquiterpenoid and ecdysteroid pathways in arthropods. Major gene orthologs involved in the biosynthetic, degradative, and signaling pathways of sesquiterpenoids and ecdysteroids can be found in crustaceans (shrimp and water flea), myriapods (centipede and millipede), and chelicerates (mite, tick, scorpion, and horseshoe crab) revealing the existence of these hormonal systems in the common ancestor of arthropods (fig. 3). Lineage-specific gene gains that probably contributed to their successful evolution have also been identified, including Br-C in the Pancrustacea, JH epoxidase (CYP15A1/C1), and key residue changes in the binding domain of the JH receptor (Met) in the insect common ancestor, and the appearance of “Phantom” in the mandibulate lineage after the split of chelicerates from other arthropods, resulting in the appearance of different molting hormones. Considering that previous studies showed the existence of functional ecdysteroid responsive systems in nematodes (Parihar et al. 2010; Liu et al. 2012), it is likely that the ecdysteroid system coevolved with the emergence of molting in the Ecdysozoa (fig. 3). The comprehensive framework established in this study will allow further dissection of the functional roles played by these crucial pathways in vivo in noninsect arthropods.
Fig. 3.
— Arthropod phylogenetic tree with vertebrate and nematode outgroups showing the possible evolution of sesquiterpenoid and ecdysteroid systems. The evolutionary relationships are based on Dunn et al. (2008), Mallatt et al. (2004), and Regier et al. (2010). PA: ponasterone A; Ec: ecdysteroids; Ch: cholesterol.
Supplementary Material
Supplementary figures S1–S28 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
Z.Q. was supported by a postgraduate studentship awarded by The Chinese University of Hong Kong. This study was supported by the Lo Kwee-Seong Biomedical Research Fund and Lee Hysan Foundation (8300023) (H.M.L. and J.H.L.H.), Collaborative Research Fund of the Research Grants Council, HKSAR Government (C4042-14G) (K.H.C., T.F.C., and J.H.L.H.), and a direct grant (4053034) of The Chinese University of Hong Kong (J.H.L.H.).
Literature Cited
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215:403–410. [DOI] [PubMed] [Google Scholar]
- Anand A, Crone EJ, Zera AJ. 2008. Tissue and stage-specific juvenile hormone esterase (JHE) and epoxide hydrolase (JHEH) enzyme activities and Jhe transcript abundance in lines of the cricket Gryllus assimilis artificially selected for plasma JHE activity: implications for JHE microevolution. J Insect Physiol. 54:1323–1331. [DOI] [PubMed] [Google Scholar]
- Bayer CA, von Kalm L, Fristrom JW. 1997. Relationships between protein isoforms and genetic functions demonstrate functional redundancy at the Broad-Complex during Drosophila metamorphosis. Dev Biol. 187(2):267–282. [DOI] [PubMed] [Google Scholar]
- Belles X, Martin D, Piulachs MD. 2005. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol. 50:181–199. [DOI] [PubMed] [Google Scholar]
- Buaklin A, Sittikankaew K, Khamnamtong B, Menasveta P, Klinbunga S. 2013. Characterization and expression analysis of the Broad-complex (Br-c) gene of the giant tiger shrimp Penaeus monodon. Comp Biochem Physiol B Biochem Mol Biol. 164(4):280–289. [DOI] [PubMed] [Google Scholar]
- Cabrera AR, et al. 2015. Three Halloween genes from the Varroa mite, Varroa destructor (Anderson & Trueman) and their expression during reproduction. Insect Mol Biol. 24:277–292. [DOI] [PubMed] [Google Scholar]
- Cao Z, et al. 2013. The genome of Mesobuthus martensii reveals a unique adaptation model of arthropods. Nat Commun. 4:2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JM, et al. 2013. Unscrambling butterfly oogenesis. BMC Genomics 14(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TF, et al. 2014. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol. 135(2):539–548. [DOI] [PubMed] [Google Scholar]
- Charles J-P, et al. 2011. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci U S A. 108:21128–21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez VM, et al. 2000. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 127:4115–4126. [DOI] [PubMed] [Google Scholar]
- Chipman AD, et al. 2014. The first myriapod genome sequence reveals conservative arthropod gene content and genome organisation in the centipede Strigamia maritima. PLoS Biol. 12:e1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne JK, et al. 2011. The ecoresponsive genome of Daphnia pulex. Science 331:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Wu L, Chan SM, Chu K. 2013. cDNA cloning and mRNA expression of retinoid-X-receptor in the ovary of the shrimp Metapenaeus ensis. Mol Biol Rep. 40:6233–6244. [DOI] [PubMed] [Google Scholar]
- Daimon T, et al. 2012. Precocious metamorphosis in the juvenile hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet. 8:e1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CW, et al. 2008. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452:745–749. [DOI] [PubMed] [Google Scholar]
- Grbic M, et al. 2011. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guittard E, et al. 2011. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev Biol. 349:35–45. [DOI] [PubMed] [Google Scholar]
- Hao W, Zhang Y, Xu YS. 2013. Identification of a juvenile hormone esterase binding protein gene and its developmental and hormone regulation in the silkworm, Bombyx mori. J Insect Physiol. 59:906–912. [DOI] [PubMed] [Google Scholar]
- Helvig C, Koener JF, Unnithan GC, Feyereisen R. 2004. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci U S A. 101:4024–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui JH, Benena WG, Tobe SS. 2013. Future perspectives for research on the biosynthesis of juvenile hormones and related sesquiterpenoids in Arthropod endocrinology and ecotoxicology. In: Devillers J, editor. Juvenile hormone and juvenoids: moldeling biological effects and environmental fate. New York: CRC Press; p. 15–30. [Google Scholar]
- Hui JHL, Hayward A, Bendena WG, Takahashi T, Tobe SS. 2010. Evolution and functional divergence of enzymes involved in sesquiterpenoid hormone biosynthesis in crustaceans and insects. Peptides 31:451–455. [DOI] [PubMed] [Google Scholar]
- Jindra M, Palli SR, Riddiford LM. 2013. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 58:181–204. [DOI] [PubMed] [Google Scholar]
- Jones G, Jones D, Teal P, Sapa A, Wozniak M. 2006. The retinoid-X receptor ortholog, ultraspiracle, binds with nanomolar affinity to an endogenous morphogenetic ligand. FEBS J. 273:4983–4996. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny NJ, Chan KW, et al. 2015. Ancestral whole genome duplication in the marine chelicerate horseshoe crabs. Heredity (under review). [Google Scholar]
- Kenny NJ, Quah S, Holland PW, Tobe SS, Hui JH. 2013. How are comparative genomics and the study of microRNAs changing our views on arthropod endocrinology and adaptations to the environment?. Gen Comp Endocrinol. 188:16–22. [DOI] [PubMed] [Google Scholar]
- Kenny NJ, Shen, et al. 2015. Genome of the rusty millipede, Trigoniulus corallinus, illuminates diplopod, myriapod and arthropod evolution. Genome Biol Evol. 7:1280–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny NJ, et al. 2014. Genomic sequence and experimental tractability of a new decapod shrimp model, Neocaridina denticulata. Mar Drugs. 12:1419–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LE, Ding Q, Prestwich GD, Tobe SS. 1995. The characterization of a haemolymph methyl farnesoate binding protein and the assessment of methyl farnesoate metabolism by the haemolymph and other tissues from Procambrus clarkii. Insect Biochem Mol Biol. 25:495–501. [Google Scholar]
- Konopova B, Jindra M. 2007. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci U S A. 104:10488–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopova B, Jindra M. 2008. Broad-Complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development 135:559–568. [DOI] [PubMed] [Google Scholar]
- Kozłowska M, et al. 2014. Calponin-like Chd64 is partly disordered. PLoS One 9(5):e96809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc GA, Wang YH, Holmes CN, Kwon G, Medlock EK. 2013. A transgenerational endocrine signaling pathway in Crustacea. PLoS One 8:e61715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, et al. 2011. Two juvenile hormone esterase-like carboxylesterase cDNAs from a Pandalus shrimp (Pandalopsis japonica): cloning, tissue expression, and effects of eyestalk ablation. Comp Biochem Physiol B Biochem Mol Biol. 159(3):148–156. [DOI] [PubMed] [Google Scholar]
- Li H, Borst DW. 1991. Characterization of a methyl farnesoate binding protein in hemolymph from Libinia emarginata. Gen Comp Endocrinol. 81:335–342. [DOI] [PubMed] [Google Scholar]
- Li M, Mead EA, Zhu J. 2011. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci U S A. 108:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Z, Robinson GE, Palli SR. 2007. Identification and characterization of a juvenile hormone response element and its binding proteins. J Biol Chem. 282(52):37605–37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. 2014. CYP18A1 regulates tissue-specific steroid hormone inactivation in Bombyx mori. Insect Biochem Mol Biol. 54:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Enright T, Tzertzinis G, Unnasch TR. 2012. Identification of genes containing ecdysone response elements in the genome of Brugia malayi. Mol Biochem Parasit. 186:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ho L, Bonning B. 2007. Localization of a Drosophila melanogaster homolog of the putative juvenile hormone esterase binding protein of Manduca sexta. Insect Biochem Mol Biol. 37(2):155–163. [DOI] [PubMed] [Google Scholar]
- Mackert A, do Nascimento AM, Bitondi MMG, Hartfelder K, Simões ZLP. 2008. Identification of a juvenile hormone esterase-like gene in the honey bee, Apis mellifera L.—expression analysis and functional assays. Comp Biochem Physiol B Biochem Mol Biol. 150(1):33–44. [DOI] [PubMed] [Google Scholar]
- Mackert A, Hartfelder K, Bitondi MMG, Simões ZLP. 2010. The juvenile hormone (JH) epoxide hydrolase gene in the honey bee (Apis mellifera) genome encodes a protein which has negligible participation in JH degradation. J Insect Physiol. 56:1139–1146. [DOI] [PubMed] [Google Scholar]
- Mallatt JM, Garey JR, Shultz JW. 2004. Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Mol Phylogenet Evol. 31:178–191. [DOI] [PubMed] [Google Scholar]
- Marchal E, et al. 2011. Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol. 41:219–227. [DOI] [PubMed] [Google Scholar]
- Minelli A, Boxshall G, Fusco G. 2013. Arthropod biology and evolution. Berlin Heidelberg (Germany): Springer. [Google Scholar]
- Miyakawa H, Gotoh H, Sugimoto N, Miura T. 2013. Effect of juvenoids on predator-induced polyphenism in the water flea, Daphnia pulex. J Exp Zool A Ecol Genet Physiol. 319:440–450. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Toyota K, et al. 2013b. A mutation in the receptor Methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nat Commun. 4:1856. [DOI] [PubMed] [Google Scholar]
- Mykles DL. 2011. Ecdysteroid metabolism in crustaceans. J Steroid Biochem Mol Biol. 127:196–203. [DOI] [PubMed] [Google Scholar]
- Nagaraju GPC. 2007. Is methyl farnesoate a crustacean hormone?. Aquaculture 272:39–54. [Google Scholar]
- Nakagawa Y, Henrich VC. 2009. Arthropod nuclear receptors and their role in molting. FEBS J. 276:6128–6157. [DOI] [PubMed] [Google Scholar]
- Niwa R, et al. 2004. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem. 279:35942–35949. [DOI] [PubMed] [Google Scholar]
- Nossa C, et al. 2014. Joint assembly and genetic mapping of the Atlantic horseshoe crab genome reveals ancient whole genome duplication. GigaScience 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead AW, Leblanc GA. 2002. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J Exp Zool. 293:736–739. [DOI] [PubMed] [Google Scholar]
- Parihar M, et al. 2010. The genome of the nematode Pristionchus pacificus encodes putative homologs of RXR/Usp and EcR. Gen Comp Endocrinol. 167:11–17. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Sheng Z, Sun Z, Palli SR. 2010. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 40:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk A, et al. 2003. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A. 100:13773–13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier JC, et al. 2010. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463:1079–1083. [DOI] [PubMed] [Google Scholar]
- Rewitz KF, Gilbert LI. 2008. Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: evolutionary implications. BMC Evol Biol. 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz KF, O'Connor MB, Gilbert LI. 2007. Molecular evolution of the insect Halloween family of cytochrome P450s: phylogeny, gene organization and functional conservation. Insect Biochem Mol Biol. 37:741–753. [DOI] [PubMed] [Google Scholar]
- Reza AM, et al. 2004. Hormonal control of a metamorphosis-specific transcriptional factor Broad-Complex in silkworm. Comp Biochem Physiol B Biochem Mol Biol. 139:753–761. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. 2008. Juvenile hormone action: a 2007 perspective. J Insect Physiol. 54:895–901. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW. 2000. Ecdysone receptors and their biological actions. Vitam Horm. 60:1–73. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. [DOI] [PubMed] [Google Scholar]
- Sanggaard KW, et al. 2014. Spider genomes provide insight into composition and evolution of venom and silk. Nat Commun. 5:3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugavelu M, Baytan AR, Chesnut JD, Bonning BC. 2000. Bonning a novel protein that binds juvenile hormone esterase in fat body tissue and pericardial cells of the tobacco hornworm Manduca sexta L. J Biol Chem. 275(3):1802–1806. [DOI] [PubMed] [Google Scholar]
- Share MR, Roe RM. 1988. A partition assay for the simultaneous determination of insect juvenile hormone esterase and epoxide hydrolase activity. Anal Biochem. 169:81–88. [DOI] [PubMed] [Google Scholar]
- Sin YW, et al. 2014. Identification of putative ecdysteroid and juvenile hormone pathway genes in the shrimp Neocaridina denticulata. Gen Comp Endocrinol. 214:167–176. [DOI] [PubMed] [Google Scholar]
- Tamone SL, Prestwich GD, Chang ES. 1997. Identification and characterization of methyl farnesoate binding proteins from the crab, Cancer magister. Gen Comp Endocrinol. 105:168–175. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarazako N, Oda S, Watanabe H, Morita M, Iguchi T. 2003. Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere 53:827–833. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Thummel CS. 2011. Coordinating growth and maturation—insights from Drosophila. Curr Biol. 21:R750–R757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SA, Baldwin WS, Wang YH, Kwon G, LeBlanc GA. 2009. Annotation, phylogenetics, and expression of the nuclear receptors in Daphnia pulex. BMC Genomics 10(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiu SH, Hult EF, Yagi KJ, Tobe SS. 2012. Farnesoic acid and methyl farnesoate production during lobster reproduction: possible functional correlation with retinoid X receptor expression. Gen Comp Endocrinol. 175:259–269. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Bendena WG. 1999. The regulation of juvenile hormone production in arthropods: functional and evolutionary perspectives. Ann N Y Acad Sci. 897:300–310. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Young DA, Khoo HW. 1989. Production of methyl farnesoate by the mandibular organs of the mud crab, Scylla serrata: validation of a radiochemical assay. Gen Comp Endocrinol. 73:342–353. [DOI] [PubMed] [Google Scholar]
- Touhara K, Prestwich GD. 1993. Juvenile hormone epoxide hydrolase: photoaffinity labeling, purification, and characterization from tobacco hornworm eggs. J Biol Chem. 268(26):19604–19609. [PubMed] [Google Scholar]
- Touhara K, Soroker V, Prestwich GD. 1994. Photoaffinity labeling of juvenile hormone epoxide hydrolase and JH-binding proteins during ovarian and egg development in Manduca sexta. Insect Biochem Mol Biol. 24(6):633–640. [Google Scholar]
- Tzolovsky G, Deng WM, Schlitt T, Bownes M. 1999. The function of the broad-complex during Drosophila melanogaster oogenesis. Genetics 153(3):1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JT, et al. 2002. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A. 99:11043–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JT, et al. 2004. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol. 34:991–1010. [DOI] [PubMed] [Google Scholar]
- Wojtasek H, Prestwich GD. 1996. An insect juvenile hormone-specific epoxide hydrolase is related to vertebrate microsomal epoxide hydrolases. Biochem Biophys Res Commun. 220:323–329. [DOI] [PubMed] [Google Scholar]
- Zhou K, et al. 2014. Crystal structure of juvenile hormone epoxide hydrolase from the silkworm Bombyx mori. Proteins 82:3224–3229 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.