Abstract
The prevalence of chronic hepatitis C virus (HCV) infection among incarcerated individuals in the United States is estimated to be between 12-31%. HCV treatment during incarceration is an attractive option due to improved access to healthcare and directly observed therapy.
We compared incarcerated and non-incarcerated HCV-infected patients evaluated for treatment at a single academic center between January 1, 2002 and December 31, 2007. During this period, 521 non-incarcerated and 388 incarcerated patients were evaluated for HCV treatment. 319 (61.2%) non-incarcerated patients and 234 (60.3%) incarcerated patients underwent treatment with pegylated interferon and ribavirin. Incarcerated patients were more likely to be male, African-American race, and have a history of alcohol or intravenous drug use. Treated incarcerated patients were less likely to have genotype 1 virus and were less likely to have undergone previous treatment. There was a similar prevalence of co-infection with HIV in both groups. A sustained viral response (SVR) was achieved in 97 (42.9%) incarcerated patients compared to 115 (38.0%) non-incarcerated patients (p=0.304). Both groups had a similar proportion of patients that completed a full treatment course. Stepwise logistic regression was conducted and the final model included full treatment course, non-genotype 1 virus, younger age at treatment start, and negative HIV status. Incarceration status was not a significant predictor when added to this model (p = 0.075).
Conclusion
In a cohort of HCV-infected patients managed in an academic medical center ambulatory clinic, incarcerated patients were as likely to be treated for HCV and as likely to achieve an SVR as non-incarcerated patients.
Keywords: Prisoner, ribavirin, interferon, sustained, response
Background
Chronic hepatitis C (HCV) infection is the leading cause of end-stage liver disease (ESLD) and death from liver disease in the United States.1 The risk of developing cirrhosis from chronic HCV infection ranges from 5% to 25% over 25 to 30 years.2,3 Within the U.S. corrections system, chronic HCV infection, and its consequences in terms of morbidity and mortality, are major public health concerns. It is estimated that 12% to 31% of inmates in the U.S. have chronic HCV infection compared with approximately 1.6% of the general population.4,5 The high prevalence of HCV infection in the prison population is attributable to the frequency of a history of intravenous drug use among inmates. A history of intravenous drug use is estimated in 20% of state inmates and 55% of federal inmates.6 In addition, up to 83% of intravenous drug users will be incarcerated at some point in their lifetime.4 Chronic HCV infection in the prisoner population results in significant morbidity and risk of premature death. In the state of Texas, HCV related mortality increased by rate of 21% per year from 1994-2003.7 Therefore, appropriate management of HCV infection in the prisoner population provides an opportunity to make a significant improvement to public health.
However, therapy for HCV remains challenging, both for the prisoner and general population alike. In the original clinical trials, 10% to 14% of patients discontinued treatment due to side effects.8,9 Psychiatric side effects were reported to occur in up to 31% of patients.8,9 Psychiatric side effects are of particular importance to the prisoner population given that 15% to 24% of inmates in the United States have a severe mental illness and up to half carry at least one psychiatric diagnosis.10 Additionally, psychiatric co-morbidities and lack of access to healthcare may impair treatment availability and adherence.
Published data regarding the success of HCV treatment in the incarcerated population is highly variable and limited to observational studies. In 2003, Allen et al, published the results of 93 inmates treated with standard interferon-α and ribavirin.11 The proportion of patients achieving SVR were comparable to previously published results in the community. Similarly, in 2004, Sterling, et al. reported the outcome of treating 59 inmates in Virginia with standard interferon-α and ribavirin.12 Outcomes were again similar to the published literature. Recently, Chew et al, published the Rhode Island experience treating incarcerated patients with pegylated interferon-α and ribavirin.13 Sustained viral response was achieved in 28% of patients. No comparison was made with the non-incarcerated population. Strock et al, published a series of 268 prisoners that were known to be HCV positive.14 Treatment with pegylated interferon-α and ribavirin was offered to 86 patients and 52% achieved an SVR. It should be noted that this treatment population was predominantly composed of patients infected with genotypes 2 and 3.14 Finally, Maru, et al, published a cohort of 68 patients treated with pegylated interferon-α and ribavirin in the Connecticut Department of Corrections.15 Overall, SVR was achieved in 47.1% of the population with only a 13% discontinuation due to medical or psychiatric issues.15 Although these data suggest that HCV treatment in the prison population is feasible and safe, no study has compared the results HCV treatment with pegylated interferon and ribavirin between contemporaneous incarcerated and non-incarcerated patients treated in the same clinical center.
The primary purpose of this study was to compare the results of HCV treatment between incarcerated and non-incarcerated patients at the University of Wisconsin Hospital and Clinics. Comparing HCV evaluation and treatment between prisoners and a community cohort in the same institution offers several advantages. First, because the same providers are evaluating and treating both HCV cohorts, differences in practice style and individual decision making should be minimized. In addition, the comparison also offers the opportunity to compare demographic differences between patient populations, including factors associated with achieving or failure an SVR. Additionally, limited data exists regarding the proportion of prisoners with HCV that actually proceed to antiviral therapy. Comparing the proportion of patients undergoing treatment between a prisoner population and a community cohort may provide insight into treatment barriers unique to prisons. Consequently, since improved adherence to interferon and ribavirin has been shown to increase the probability of sustained viral response (SVR).16 We sought to identify barriers either to initiating or to completing treatment in HCV-infected prisoners.
Methods
Study Population and Procedures
This study was a retrospective chart review comparing incarcerated patients and patients from the general population being evaluated for chronic HCV infection by the State of Wisconsin Department of Corrections (DOC) and University of Wisconsin Hospital and Clinics between January 1, 2002 and December 31, 2007. Patients were identified from a database of patients evaluated in the University of Wisconsin Hepatology or Infectious Diseases clinic. New patients seen in clinic were asked to fill out a detailed questionnaire including questions about previous history of liver disease and treatment, viral hepatitis risk factors, previous or ongoing substance abuse, and other medical conditions. One of the authors (HT) reviewed this database for patients diagnosed with Hepatitis C. Information obtained from the patient record included patient demographics, incarceration status, medical history, psychiatric history, substance abuse history, HCV genotype, prior HCV treatment if applicable, and liver biopsy results if applicable.
In 2002, the State of Wisconsin adopted a protocol to determine which incarcerated patients should be considered for HCV treatment. To be considered for treatment, incarcerated patients had to have an expected release date after the proposed treatment completion date to avoid premature termination of treatment. For patients with genotype 1 or 4, treatment could be offered only to patients with at least Metavir stage 2 fibrosis on liver biopsy provided the prescribing provider felt that the patient was otherwise medically and psychiatrically appropriate for treatment. All medically and psychiatrically appropriate patients with genotype 2 and 3 were eligible for treatment without a staging biopsy. Among DOC eligible patients, the final decision to offer treatment was made by the provider. If treatment was offered, the patient decided whether or not to accept treatment. A portion of HCV treatment in the prison population was conducted using a telemedicine link between the treating providers at the University of Wisconsin and the providers at the treating correctional facility. A telemedicine link between the University of Wisconsin clinics and the Department of Corrections was established in 2002. Initially, telemedicine was utilized sparingly during prisoner HCV treatment. However, by 2007 almost all HCV treatment conducted in the prison system utilized telemedicine services at some point during therapy. For community patients, the decision to treat was made at the discretion of the provider based on patient wishes along with medical and psychiatric appropriateness for treatment. In total, 909 patients were seen in consultation for HCV infection and 553 patients ultimately underwent treatment for HCV during the aforementioned period.
Patients were treated based on standardized guidelines for the treatment period. Pegylated interferon α-2a or α-2b was prescribed along with ribavirin in all cases. Consensus interferon was not used. The choice of agents and treatment doses were at the discretion of the treating provider. Optimal treatment duration was defined as 48 weeks for genotypes 1 and 4 and 24 weeks for genotype 2 and 3. Decisions to discontinue early were made by patient and provider depending on viral response and tolerance of treatment. Treatment regimen and duration was recorded in the patient database. After completion of treatment, SVR was defined as a negative serum HCV RNA at 24 weeks or longer after treatment discontinuation.
The primary outcome analyzed was proportion of SVR achievement in the treated incarcerated versus community populations. Secondary outcomes of interest included HCV treatment rate and the proportion of SVR achievement in the entire prisoner and community population evaluated for treatment.
The University of Wisconsin Institutional Review Board (IRB) approved this study.
Statistical analysis
Associations among patient demographics, prisoner status and HCV treatment outcome were the focus of the statistical analysis. Patient age, gender, self-reported race and ethnicity, substance abuse history, HCV genotype, prior HCV treatment attempts, HIV status and fibrosis stage and grade when applicable, and proportion achieving SVR were compared between prisoners and non-prisoners.. For those cases where treatment was discontinued, the cited reason is tabulated. The association of prisoner status with the other categorical variables was assessed using Fisher's exact test. The difference in median age at treatment start between prison and non-prison populations was tested using a two-sample Wilcoxon test. Age at treatment was also tested as a classification variable dividing the population into groups age 44 and below and age 45 and above.
For treated individuals, logistic regression was used to assess the association of all other variables with SVR status. Univariate analysis was performed for all predictors and, stepwise logistic regression was conducted to identify the model that minimized the Akaike Information Criteria (AIC) from the set of predictors with no more than 15% missing data. The AIC is a well established method for comparing statistical models that combines a measure of the fidelity of the model to the data and a penalty for the complexity of the model.17 Variables included in this analysis were prisoner status, age at treatment start (as well as two categorical versions of this variable), gender, self-reported race and ethnicity, viral genotype (including two less detailed categorizations), prior treatment failure, HIV infection, history of alcohol abuse, history of IV drug use and cocaine use, and full treatment course. Fibrosis stage and grade were not used in this analysis due to the relatively small proportion of patients that underwent liver biopsy. Including pretreatment biopsy as a variable would have excluded a large number of patients from the model. The model with minimum AIC was found by randomly selecting a random size subset of the variables to use as a starting vector and then using forward and backward selection until the algorithm converged. The addition (and deletion) of all one and two way interaction terms were allowed with the constraint that all models including an interaction must also include both main effects. This process was repeated 2,000 times. The model with the minimum AIC was found 25% percent of the time. All analyses were completed using the R statistical software system.
Definitions
Alcohol Abuse
Defined as the patient answering “yes” to two or more CAGE questions on the standard new patient questionnaire given at the initial evaluation. The CAGE questions are a validated series of four questions designed and validated as a screening test for problem drinking.18 They questions are as follows
Have you ever felt the need to Cut down on drinking?
Have you ever felt Annoyed by criticism of your drinking?
Have you ever had Guilty feelings about your drinking?
Do you ever take a morning Eye-opener?
Intravenous Drug Abuse History
Defined as patient disclosure of previous or ongoing intravenous drug use on the new patient questionnaire.
Full Treatment Course
Defined as completion of 48 weeks treatment with pegylated interferon and ribavirin for HCV genotypes 1 or 4 or 24 weeks treatment for genotypes 2 and 3.
Results
Nine hundred nine patients were evaluated in the University of Wisconsin Hepatology or Infectious Disease clinic with a diagnosis of chronic HCV infection during the study period. Three hundred eighty-eight (42.7%) patients in this population were incarcerated at the time of medical evaluation. There were considerable differences between the incarcerated population and general population (figure 1). The incarcerated patients were more likely to be male (93.0% vs. 64.5, P<0.0001), African-American (25.8% vs. 11.7%, P<0.0001), and have a history of alcohol or drug abuse. Conversely, a greater proportion of the community population had experienced a prior treatment failure (10.4% vs. 1.5%, P<0.0001).
Figure 1.
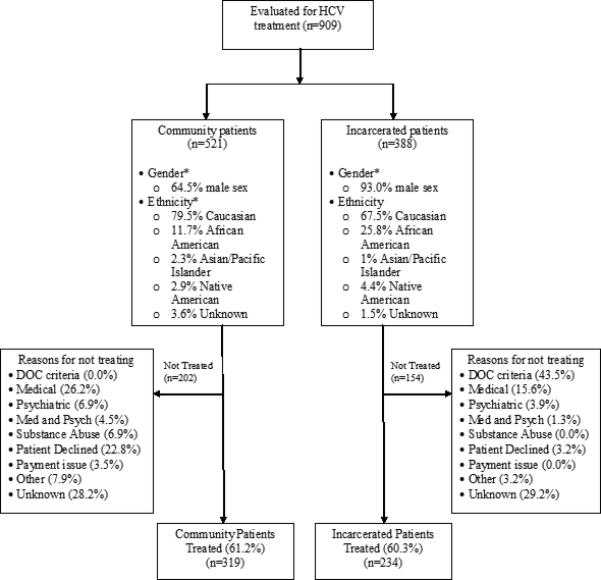
Study design and demographic data for community and incarcerated patients.
Five hundred fifty-three patients were treated for chronic HCV infection with pegylated interferon-α and ribavirin during the study period. Two hundred thirty-four treated patients were incarcerated at the time of treatment. There was no difference in the proportion of patients that underwent treatment between community and incarcerated patients (61.2% vs. 60.3%, P=0.784). The rationale for not starting HCV treatment in each group is provided in figure 1. Only incarcerated patients were excluded from treatment due to failure to meet DOC requirements (43.5% vs. 0.0%, P<0.0001). The most common reason for prisoners to not meet DOC criteria was an expected release date prior to anticipated completion of treatment. Community patients were more likely to not be offered treatment due to medical and psychiatric issues (37.6% vs. 20.8%, P=0.0007) and active substance abuse (6.9% vs. 0.0%, P=0.0004). Community patients were also more likely to decline treatment (22.8% vs. 3.2%, P<0.0001). The exact reason for not initiating HCV treatment was not recorded in a substantial number of patients in each cohort.
The demographics and treatment results are found in Table 1. There were numerous demographic differences between the incarcerated and non-incarcerated populations that underwent treatment. Incarcerated patients were more likely to be younger at time of treatment initiation (median age 44 years versus 50 years, P<0.0001). Incarcerated patients that underwent treatment were more likely than non-incarcerated patients to be male (92.0 vs. 65.2%, P<0.0001), African-American (23.9 vs. 8.5%, P<0.0001), and have a history of previous alcohol or intravenous drug abuse (p<0.001). Non-incarcerated patients were more likely to have previously experienced a treatment failure (11.0 vs. 1.3%, P<0.0001).
Table 1.
Characteristics and outcomes of treated patients by incarceration status
| Incarcerated (n=234) | General population (n=319) | ||
|---|---|---|---|
| Mean age at treatment start | 44.0 +/- 8.1 | 48.5 +/- 8.3 | <0.0001 |
| Male sex | 215 (92.0%) | 208 (65.2%) | <0.0001 |
| Ethnicity | |||
| - Caucasian | 161 (68.8%) | 263 (82.4%) | |
| - African American | 56 (23.9%) | 27 (8.5%) | <0.0001 |
| - Hispanic | 19 (8.1%) | 12 (3.8%) | |
| - Asian/Pacific Islander | 1 (0.4%) | 10 (3.1%) | |
| - Native American | 14 (6.0%) | 9 (2.8%) | |
| Proportion African American | 23.9% | 8.5% | <0.0001 |
| Alcohol abuse history | 139 (59.4%) | 146 (45.7%) | 0.0019 |
| Intravenous drug history | 158 (67.5%) | 143 (44.8%) | <0.0001 |
| HCV Genotype | |||
| - 1a or 1b | 140 (63.4%) | 206 (72.3%) | 0.034 |
| - 2 or 3 | 81 | 79 | |
| - Missing | 13 | 34 | |
| HIV Co-infection | 15 (6.4%) | 19 (6.0%) | 0.859 |
| Previous HCV treatment | 3 (1.3%) | 35 (11.0%) | <0.0001 |
| Metavir stage (where applicable) | |||
| - Stage 0 or 1 | 19 | 49 | <0.0001 |
| - Stage 2,3, or 4 | 110 | 75 | |
| - Not available | 108 | 226 | |
| Percent with stage 0 or 1 fibrosis | 14.7% | 39.5% | <0.0001 |
| Treatment course | |||
| - Full course | 174 (75.0%) | 212 (68.6%) | 0.124 |
| - Partial course | 58 | 97 | |
| - Unknown | 2 | 10 | |
Regarding HCV genotype, there was a greater proportion of genotype 1 in the non-incarcerated treated group (72.3% vs. 63.4%, P = 0.034). Overall, 129 prisoners (55.1%) and 124 community patients (38.9%) had a pre-treatment liver biopsy. Of the patients that had a pre-treatment biopsy, a greater proportion of the treated nonincarcerated arm had no or minimal fibrosis, defined as Metavir stage 0 or 1, on biopsy (39.5 vs. 14.7%, P<0.0001).
Overall, 386 patients (69.8%) completed a full treatment course. The proportion of patients completing a full treatment course was similar between the incarcerated and non-incarcerated populations (75.0 vs. 68.6%, P=0.124). Treatment results are found in figure 2. Sustained viral response (SVR) was ultimately achieved in a similar proportion of incarcerated patients and non-incarcerated patients (42.9 vs. 38.0%, P=0.282). This finding held true for both genotype 1 virus (30.4 vs. 28.2, P=0.644) and genotype 2 and 3 virus (61.3% vs. 64.4%, P=0.749). Among the entire cohort of patients evaluated for HCV treatment, the proportion of patients achieving SVR was also similar between incarcerated and non-incarcerated patients (25.0% versus 22.1%, P=0.304).
Figure 2.
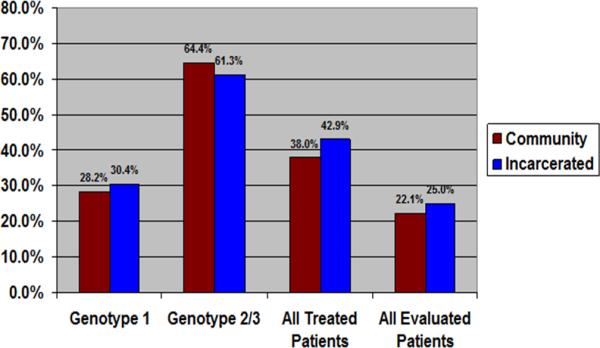
Sustained Viral Response (SVR) by Genotype, Treated Cohort, and Entire HCV Cohort
Univariate logistic regression was used to assess the association between SVR status and the other variables (Table 2). Significant predictors included full treatment course (OR=23, p<0.001), history of cocaine use (OR=1.51, p=0.021), non-genotype 1 virus (OR=4.38, p<0.001), African American race (OR=0.38, p<0.001) and age at treatment start (OR=0.94, p<0.001). Stepwise logistic regression was performed using the data from the 472 treated patients with complete data records of all the variables with less than 15% missing values (table 3). Fibrosis stage was not included due to the low proportion of patients with pre-treatment biopsies. The resulting model (table 3) includes four significant predictors of increased SVR: full treatment course (OR 31.95, P<0.0001), non-genotype 1 virus (OR 3.48, P<0.0001), HIV negativity (OR 2.85, P=0.033) and younger age at treatment start (OR 0.96, P=0.003). Incarceration status, when added to this model, was not associated with the probability of achieving SVR (P=0.075). Because prisoners were younger at treatment start than the non-incarcerated patients and might be standing in for incarceration status, the logistic regression was reanalyzed removing age at treatment start as a variable in the model. Prisoner status was still not a significant predictor of SVR (P=0.326).
Table 2.
Univariate logistic regression analysis for treated patients..
| N | SVR proportion | Beta | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Prisoner Status | 529 | |||||
| No | 303 | 0.380 | Ref | |||
| Yes | 226 | 0.429 | 0.21 | 1.23 | (0.87-1.75) | 0.249 |
| Gender | 529 | |||||
| Female | 125 | 0.408 | Ref | |||
| Male | 404 | 0.399 | −0.04 | 0.96 | (0.64-1.45) | 0.850 |
| Full Treatment Course | 526 | |||||
| No | 146 | 0.048 | Ref | |||
| Yes | 380 | 0.540 | 3.15 | 23.26 | (10.6, 51.02) | <0.001 |
| HIV Positive | 529 | |||||
| No | 496 | 0.411 | Ref | |||
| Yes | 33 | 0.242 | −0.78 | 0.46 | (0.20, 1.04) | 0.061 |
| IV Drug Use History | 529 | |||||
| No | 242 | 0.388 | Ref | |||
| Yes | 287 | 0.411 | 0.09 | 1.1 | (0.78, 1.56) | 0.595 |
| Cocaine Use History | 529 | |||||
| No | 262 | 0.351 | Ref | |||
| Yes | 267 | 0.449 | 1.51 | 1.51 | (1.06, 2.14) | 0.021 |
| Alcohol Abuse History | 529 | |||||
| No | 254 | 0.409 | Ref | |||
| Yes | 275 | 0.393 | −0.07 | 0.93 | (0.66, 1.32) | 0.695 |
| Prior HCV Treatment | 529 | |||||
| No | 494 | 0.405 | Ref | |||
| Yes | 35 | 0.343 | −0.27 | 0.77 | (0.37, 1.58) | 0.471 |
| Genotype | 484 | |||||
| Genotype 1a | 229 | 0.293 | Ref | |||
| Genotype 1b | 101 | 0.327 | 0.16 | 1.17 | (0.71, 1.94) | 0.534 |
| Genotype 2 | 69 | 0.667 | 1.58 | 4.84 | (2.72, 8.60) | <0.001 |
| Genotype 3 | 81 | 0.642 | 1.47 | 4.34 | (2.54, 7.41) | <0.001 |
| Other | 4 | 0.750 | 1.98 | 7.25 | (0.74, 70.99) | 0.089 |
| Genotype condensed | 484 | |||||
| Genotype 1 | 330 | 0.303 | Ref | |||
| Non-Genotype 1 | 154 | 0.649 | 1.48 | 4.38 | (2.92, 6.58) | <0.001 |
| Race | 519 | |||||
| White | 406 | 0.436 | Ref | |||
| Asian | 10 | 0.500 | 0.26 | 1.29 | (0.37, 4.54) | 0.688 |
| African American | 80 | 0.225 | −0.98 | 0.38 | (0.21, 0.66) | 0.006 |
| Native American | 23 | 0.391 | −0.18 | 0.83 | (0.35, 1.97) | 0.675 |
| Ethnicity | 414 | |||||
| Hispanic | 31 | 0.516 | Ref | |||
| Non-Hispanic | 383 | 0.355 | −0.66 | 0.52 | (0.25, 1.08) | 0.078 |
| Biopsy Inflammation Grade | 237 | |||||
| Grade 0 | 6 | 0.500 | Ref | |||
| Grade 1 | 74 | 0.486 | −0.05 | 0.95 | (0.18, 5.00) | 0.949 |
| Grade 2 | 124 | 0.282 | −0.93 | 0.39 | (0.08, 2.04) | 0.267 |
| Grade 3 | 31 | 0.355 | −0.6 | 0.55 | (0.09, 3.2) | 0.506 |
| 2 | 0.000 | - | - | - | - | |
| Grade 4 | ||||||
| Metavir Fibrosis Score | 543 | |||||
| Stage 0 | 22 | 0.500 | Ref | |||
| Stage 1 | 43 | 0.372 | −0.71 | 0.49 | (0.17, 1.4) | 0.185 |
| Stage 2 | 113 | 0.416 | −0.52 | 0.59 | (0.24, 1.49) | 0.266 |
| Stage 3 | 46 | 0.172 | −1.74 | 0.18 | (0.06, 0.55) | 0.003 |
| Stage 4 | 19 | 0.211 | −1.5 | 0.22 | (0.06, 0.89) | 0.033 |
| Age at Treatment Start | 529 | |||||
| 19-25 | 13 | 0.769 | Ref | |||
| 25-35 | 33 | 0.697 | −0.37 | 0.69 | (0.16, 3.06) | 0.625 |
| 35-40 | 60 | 0.533 | −1.07 | 0.34 | (0.09, 1.37) | 0.130 |
| 40-45 | 103 | 0.447 | 1.42 | 0.24 | (0.06, 0.93) | 0.039 |
| 45-50 | 141 | 0.340 | 1.87 | 0.15 | (0.04, 0.59) | 0.006 |
| 50-55 | 109 | 0.312 | −2.00 | 0.14 | (0.04, 0.53) | 0.004 |
| 55-60 | 54 | 0.259 | −2.25 | 0.11 | (0.03, 0.44) | 0.002 |
| 60-73 | 16 | 0.313 | −1.99 | 0.14 | (0.03, 0.72) | 0.020 |
| Age at Treatment Start | 529 | |||||
| 19-35 | 46 | 0.717 | Ref | |||
| 35-45 | 163 | 0.479 | −1.02 | 0.36 | (0.18, 0.74) | 0.005 |
| 45-55 | 250 | 0.328 | −1.65 | 0.19 | (0.1, 0.38) | <0.001 |
| 55-73 | 70 | 0.271 | −1.92 | 0.15 | (0.06, 0.34) | <0.001 |
| Age at Treatment Start | 529 | |||||
| 19-45 | 209 | 0.531 | Ref | |||
| 45-73 | 320 | 0.316 | −0.90 | 0.41 | (0.28, 0.58) | <0.001 |
Table 3.
Results of Stepwise Logistic Regression Analysis Predicting SVR (n=472)
| Predictor | Coefficient | Odds Ratio | 95% CI | P-value |
|---|---|---|---|---|
| Full Treatment Course | 3.46 | 31.95 | (11.37, 89.81) | < 0.0001 |
| Non-genotype 1 | 1.25 | 3.48 | (2.17, 5.6) | < 0.0001 |
| HIV negative | 1.05 | 2.85 | (1.09, 7.48) | 0.0331 |
| Age at treatment start | −0.04 | 0.96 | (0.93, 0.98) | 0.0027 |
| Nagelkerke r2 = 0.41 |
Variables included in regression model: prisoner status, age at treatment start, gender, self-reported race, ethnicity, viral genotype, prior treatment failure, HIV co-infection, history of alcohol abuse, history of IV drug use, history of cocaine use, and full treatment course
Discussion
Chronic HCV infection and its complications remain a major public health challenge. It is especially problematic in the incarcerated population where the prevalence of intravenous drug use, the principle risk factor for acquiring HCV, is very high. Treatment for HCV during periods of incarceration offers the opportunity for directly observed therapy, hopefully ensuring adherence, and the availability of medical staff to monitor and address the well-known side effects of treatment with interferon and ribavirin. In fact, given that medication adherence is a major factor in attaining an SVR and the impact of socioeconomic impediments to community-based treatment in this population, the prison setting may be an optimal time to treat HCV infection.16
Our study compared the effectiveness of HCV treatment between a cohort of incarcerated patients in the Department of Corrections of the state of Wisconsin and a contemporaneous cohort of non-incarcerated patients in the outpatient practice at the University of Wisconsin Hospital and Clinics. We found no difference between the general population and incarcerated population in terms of the proportion starting HCV treatment or ultimately achieving SVR. Although previous studies have demonstrated that HCV treatment in the prison setting may be successful, the present study is the first to compare the effectiveness of anti-viral therapy among contemporaneous incarcerated and community-based patients attending the same clinical practice.12-16 Thus, this study is the first to offer a true control cohort by which to compare prisoner treatment and outcomes. Using a control cohort provides the opportunity to explore obstacles to effective HCV treatment that may be unique to the prison population when comparing to the community. In addition, by using a cohort of community patients in the same practice, we have minimized differences in practice style and decision-making between the prison population and the community.
Our study offers additional advantages over the published literature. First, our study is the largest published cohort of prisoners being considered for therapy and treated for HCV. The size of the cohort allowed not only for the comparison of outcomes of treatment with pegylated interferon and ribavirin, but also consideration of obstacles to successful treatment. Several studies have demonstrated that one of the major obstacles to successful HCV treatment is the actual initiation of treatment itself. A recent study by Feuerstadt, et al, revealed that only 15% of patients achieved SVR in a low-income urban clinic and cited social issues and inadequate access to medical care as two principle obstacles to effective HCV treatment.19 Equally concerning, only 3.3% of the patients screened with HCV eventually obtained an SVR, in large part due to the fact that over 84% of the patients screened failed to meet acceptable criteria for outpatient treatment.19 In addition, in a recent cohort of almost 100,000 VA patients with HCV viremia, Kramer, et al, showed that only 11.6% of the cohort received HCV treatment.20 Active alcohol and drug usage and poorly controlled depression were the principle reasons for not initiating treatment in this population. In our population, greater than 60% of HCV positive prisoners underwent HCV treatment, far greater than the proportion of patients receiving treatment in these two published cohorts. In addition, only 3.9% of the prisoner population in our cohort was denied treatment due to active psychiatric issues and no patients were denied treatment due to substance abuse. Therefore, our study reiterates the critical importance that psychiatric and substance abuse treatment play in the successful eradication of HCV. Prison potentially offers access to appropriate HCV care to a population with numerous socioeconomic, psychiatric, and substance abuse risk factors. Our study also demonstrated an impressive adherence to treatment among the prisoner population. We suggest that the use of telemedicine helped forge a therapeutic link between incarcerated patients, their care-givers in the Department of Corrections and the medical professionals in the academic medical center which maximized treatment completion rates, an observation recently made by Arora and colleagues also.21 Because of the barriers to outpatient treatment in this high- risk population, current guidelines recommend offering HCV identification, education, and treatment when appropriate for incarcerated patients.4,6 Our study validates this policy of using periods of incarceration as an opportunity to treat HCV and the substance abuse and mood disorders that often are co-morbid in this population.
There are several limitations to our study. First, this study was a retrospective chart review. Because patients were not enrolled in a protocol and therefore choice of interferon alpha, dosage regimen, medication adjustments, data entry points and collection were not standardized. Third, there were baseline differences in the two populations that may have affected the probability of achieving an SVR. With the exception of a greater proportion of genotype 2 and 3 virus in the treated incarcerated population, these demographic differences are not unexpected when comparing incarcerated populations to the community. The treated incarceration population was younger at time of treatment initiation and more frequently male, of African-American race, and treatment naïve. Multivariable logistic regression was performed to determine if these demographic differences had an effect on the probability of achieving SVR. Not surprisingly, treatment of genotype 2 and 3 virus predicted SVR. The higher proportion of genotype 2 and 3 virus in the treated incarcerated population represents a limitation of the study and may bias the SVR results in favor of the incarcerated population. This finding likely represents a reflection of DOC protocol, which permits treatment of genotype 2 and 3 virus regardless of fibrosis stage on biopsy. In addition, there are several variables known to predict for SVR that were not collected in this study and thus could have had an impact on study findings. Pretreatment HCV RNA values were not consistently drawn nor necessarily analyzed with the same assay. As such, they were not included in the study. Differences in pretreatment HCV RNA have been shown to impact probability of achieving SVR.22 Similarly, no assessment of patient pretreatment body weight measurement or insulin resistance was performed. Higher body weight and the presence of insulin resistance both negatively impact the probability of SVR.23,24 Patients in our study were enrolled before IL28B genotype status was known to be a powerful predictor of treatment response to interferon and ribavirin in patients with chronic HCV genotype 1 infection.25 We are unable to comment on the influence of IL28 B genotypes, other than speculate in relation to the ethnic provenance of the two groups. Finally, the frequency of liver biopsy was low in the entire population and could not be considered in the stepwise regression. Given that advanced fibrosis stage is a negative predictive factor for SVR, it is possible that differences in stage of fibrosis at the time of treatment initiation could have impacted the study findings. The reasons for the low biopsy are probably multifactorial. In the case of patients infected with genotype 2 and 3 virus, it is likely that the provider felt that treatment was indicated regardless of biopsy findings. In the case of genotype 1 infection, nearly the entire incarcerated cohort underwent a liver biopsy per state protocol. In community patients, there are many reasons why a staging liver biopsy may not have been performed. These include patients not wishing to undergo an invasive and potentially painful procedure and clinician discretion regarding utility of histopathology in making treatment decisions for the individual patient.
While our study did not include an assessment of the cost of treating HCV infection in the prison setting, Tan, et al published a cost-effectiveness analysis and found that HCV treatment with pegylated interferon and ribavirin during incarceration improved quality of life and was cost-effective.26 However, with recent budgetary pressures on local and state governments in the United States, funding for HCV treatment may well be cut. In addition, treatment cost for HCV genotype 1 is only going to increase. Two expensive additions to the armamentarium, boceprevir and telaprevir, have been approved by the FDA for treatment of patients with chronic HCV genotype 1 infection, when given in conjunction with pegylated interferon and ribavirin.27,28 The increasing cost of HCV treatment will raise ethical considerations about how to best treat HCV in correctional institutions in a cost effective manner. Consequently, we suggest cost-effectiveness studies with the use of protease inhibitors in the incarcerated population be performed.
Finally, it should be noted that there are concerns regarding risk of re-infection after treatment for HCV. Bate, et al published a series in which 5 prisoners treated successfully for HCV were re-infected after release from incarceration.29 This study highlights the importance of drug and alcohol addiction treatment as an integral component of HCV treatment, in the incarcerated population, both while in prison and after release into the community.
In summary, our study reinforces, in a large study population, the message that anti-viral treatment of the HCV-infected incarcerated population is not only effective, but can be as successful as HCV treatment in the general population. Given the dismal results of outpatient HCV treatment reported in high risk populations, we conclude that incarceration may be an optimal setting for treatment.19,20 Furthermore, given the scale of the prevalence of HCV infection in the incarcerated population, we suggest that anti-viral treatment while in prison is the optimal time for treatment to reverse a public health crisis.
Acknowledgments
Disclosure/Funding Source: This research was supported by the American Cancer Society Research Scholar Grant (07-077-01) to R.S., by the University of Wisconsin Institute for Clinical and Translational Research, funded through an NIH Clinical and Translational Science Award, grant number 1UL1RR025011, and by the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health, grant number 1UL1RR025011. The funders had no role design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest: Dr. Lucey is or has been site principal investigator in clinical trials of anti-viral treatment for HCV infection sponsored by the following pharmaceutical companies: Hyperion; Human Genome Sciences; Bristol-Myers Squibb, Gilead, Vertex, Abbott, Salix and Novartis. The remaining authors report no conflicts of interest.
References
- 1.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 3.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132(4):296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Weinbaum C, Lyerla R. Margolis HS; Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–36. [PubMed] [Google Scholar]
- 5.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 6.Spaulding AC, Weinbaum CM, Lau DT, Sterling R, Seeff LB, Margolis HS, et al. A framework for management of hepatitis C in prisons. Ann Intern Med. 2006;144(10):762–9. doi: 10.7326/0003-4819-144-10-200605160-00010. Review. [DOI] [PubMed] [Google Scholar]
- 7.Harzke AJ, Baillargeon J, Paar DP, Pulvino J, Murray OJ. Chronic liver disease mortality among male prison inmates in Texas, 1989-2003. Am J Gastroenterol. Jun. 2009;104(6):1412–9. doi: 10.1038/ajg.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Baillargeon J, Binswanger IA, Penn JV, Williams BA, Murray OJ. Psychiatric disorders and repeat incarcerations: the revolving prison door. Am J Psychiatry. 2009;166(1):103–9. doi: 10.1176/appi.ajp.2008.08030416. [DOI] [PubMed] [Google Scholar]
- 11.Allen SA, Spaulding AC, Osei AM, Taylor LE, Cabral AM, Rich JD. Treatment of chronic hepatitis C in a state correctional facility. Ann Intern Med. 2003;138(3):187–90. doi: 10.7326/0003-4819-138-3-200302040-00010. Erratum in: Ann Intern Med. 2003 Oct 7;139(7):605. [DOI] [PubMed] [Google Scholar]
- 12.Sterling RK, Hofmann CM, Luketic VA, Sanyal AJ, Contos MJ, Mills AS, et al. Treatment of chronic hepatitis C virus in the Virginia department of corrections: can compliance overcome racial differences to response? Am J Gastroenterol. 2004;99(5):866–72. doi: 10.1111/j.1572-0241.2004.30310.x. [DOI] [PubMed] [Google Scholar]
- 13.Chew KW, Allen SA, Taylor LE, Rich JD, Feller E. Treatment outcomes with pegylated interferon and ribavirin for male prisoners with chronic hepatitis C. J Clin Gastroenterol. 2009;43(7):686–91. doi: 10.1097/MCG.0b013e31818dd94c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strock P, Mossong J, Hawotte K, Arendt V. Access to treatment of hepatitis C in prison inmates. Dig Dis Sci. 2009;54(6):1325–30. doi: 10.1007/s10620-008-0483-8. [DOI] [PubMed] [Google Scholar]
- 15.Maru DS, Bruce RD, Basu S, Altice FL. Clinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populations. Clin Infect Dis. 2008;47(7):952–61. doi: 10.1086/591707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. International Hepatitis Interventional Therapy Group. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123(4):1061–9. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 17.Akaike Hirotugu. A new look at the statistical model identification”. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- 18.Beresford TP, Blow FC, Hill E, Singer K, Lucey MR. Comparison of CAGE questionnaire and computer-assisted laboratory profiles in screening for covert alcoholism. Lancet. 1990;336:482–5. doi: 10.1016/0140-6736(90)92022-a. [DOI] [PubMed] [Google Scholar]
- 19.Feuerstadt P, Bunim AL, Garcia H, Karlitz JJ, Massoumi H, Thosani AJ, et al. Effectiveness of hepatitis C treatment with pegylated interferon and ribavirin in urban minority patients. Hepatology. 2010;51(4):1137–43. doi: 10.1002/hep.23429. [DOI] [PubMed] [Google Scholar]
- 20.Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J of Hepatology. 2011 doi: 10.1016/j.jhep.2011.05.032. in-press. [DOI] [PubMed] [Google Scholar]
- 21.Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139(1):120. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Eslam M, Aparcero R, Kawaguchi T, Del Campo JA, Sata M, Khattab MA, et al. Meta-analysis: insulin resistance and sustained virological response in hepatitis C. Aliment Pharmacol Ther. 2011;34(3):297. doi: 10.1111/j.1365-2036.2011.04716.x. [DOI] [PubMed] [Google Scholar]
- 24.Reddy KR, Shiffman ML, Rodriguez-Torres M, Cheinquer H, Abdurakhmanov D, Bakulin I, et al. Induction pegylated interferon alfa-2a and high dose ribavirin do not increase SVR in heavy patients with HCV genotype 1 and high viral loads. Gastroenterology. 2010;139(6):1972. doi: 10.1053/j.gastro.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 25.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. Epub 2009 Aug 16. [DOI] [PubMed] [Google Scholar]
- 26.Tan JA, Joseph TA, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology. 2008;48(5):1387–95. doi: 10.1002/hep.22509. [DOI] [PubMed] [Google Scholar]
- 27.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):1827–38. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 29.Bate JP, Colman AJ, Frost PJ, Shaw DR, Harley HA. High prevalence of late relapse and reinfection in prisoners treated for chronic hepatitis C. J Gastroenterol Hepatol. 2010;25(7):1276–80. doi: 10.1111/j.1440-1746.2010.06295.x. [DOI] [PubMed] [Google Scholar]


