Abstract
Purpose
Physical impairments cause profound functional declines in patients with cancer. Although common rehabilitation measures can address many impairments, the extent of their delivery is unknown. We studied these issues by quantifying physical impairments in patients with metastatic breast cancer and by assessing how they are addressed.
Patients and Methods
A consecutive sample of 163 community-dwelling patients with metastatic breast cancer was stratified by Karnofsky performance score and administered the Medical Outcomes Study Physical Function Subscale and the Older Americans Resource Study Activities of Daily Living subscales. Cancer-related physical impairments were identified through a physical examination, the 6-Minute Walk Test, and the Functional Independence Measure Mobility Subscale. Patients were questioned regarding the nature, type, and setting of treatments for impairments. Physical rehabilitation needs were determined through a consensus process involving physiatrists and physical/occupational therapists specializing in cancer.
Results
Ninety-two percent of patients (150 of 163) had at least one physical impairment. Among 530 identified impairments, 484 (92%) required a physical rehabilitation intervention and 469 (88%) required physical therapy (PT) and/or occupational therapy (OT). Only 30% of impairments requiring rehabilitation services and 21% of those requiring PT/OT received treatment. Impairments detected during hospitalization were overwhelmingly more likely to receive a rehabilitation intervention (odds ratio [OR] = 87.9; 95% CI, 28.5 to 271.4), and PT/OT (OR = 558.8; 95% CI, 187.0 to 1,669.6). Low socioeconomic and minority status were significantly associated with nontreatment.
Conclusion
Remediable physical impairments were prevalent and poorly addressed among patients with metastatic breast cancer, drastically so in the outpatient setting. Undertreatment was particularly prominent among minority and socioeconomically disadvantaged groups.
INTRODUCTION
Although physical impairments threaten the functioning and quality of life (QOL) of patients with advanced cancer, we have little idea about either their frequency or impact, and no report has focused exclusively on patients with metastatic disease. Data suggest that the needs of this population are poorly recognized and inadequately met by the rehabilitation and oncology communities. In a time that now recognizes the value of QOL, indifference toward patients’ ability to function is a striking inconsistency at odds with the Institute of Medicine’s assertion that the purpose of the health care system “is to reduce continually the burden of… disability and to improve the… function of the people of the United States.”1,2
Previous investigations of cancer-related functional problems have typically been disability-based self-report estimates.3–7 As such, they reflect patients’ inability to perform activities but do not address the reasons for that failure. This distinction is important. Although the inability to independently eat or climb steps is a disability, the reason for this inability, such as limb weakness or ataxia, occurs at a simpler level and is defined as an impairment. The distinction between impairment and disability is fundamental to current disablement models,8–11 critical to accurate prognostication, and relevant on a practical basis because impairments lend themselves more directly to treatment than do disabilities.
Cancer-related impairments differ in their nature and causes. Adverse neural, musculoskeletal, and cardiopulmonary effects resulting from metastatic and regional tumor spread are obviously important factors. Cancer treatment also has sequelae. As many as 40% of patients undergoing chemotherapy develop chronic neuropathies.12 Anatomic disruptions consequent to surgeries contribute to a wide range of impairments. The fact that multiple impairments may coexist only complicates the situation. The prognosis and remediation of disability therefore requires a comprehensive determination of relevant impairments.
We undertook this cross-sectional study of patients with metastatic breast cancer to characterize the prevalence of impairments, to determine whether remediable impairments receive appropriate treatment, and to establish where treatment occurred.
PATIENTS AND METHODS
Patients
A consecutive sample of patients receiving outpatient chemotherapy at the Memorial Sloan-Kettering Cancer Center (MSKCC; New York, NY) for metastatic breast cancer was enrolled in July and August 1999. Eligible patients were required to have documented metastatic breast cancer, have Karnofsky performance score (KPS)13,14 between 40 and 90, be 18 years of age or older, have an intact mental status, and be fluent in English. Disease status was determined by medical record review, physician communication, and imaging studies. Potential participants were approached before receiving parenteral chemotherapy or bisphosphonate treatments.
Stratified sampling was used to ensure adequate representation of participants with lower performance status. Participants were grouped into three KPS strata (80 to 90, 60 to 70, and 40 to 50). Two hundred twelve patients were screened during the study period. Of these, 31 had insufficient English fluency and 18 declined to participate. Patients excluded on the basis of English proficiency were more likely to identify themselves as Asian (73% v 2.5%). Excluded patients did not otherwise differ significantly from the final study sample of 163 participants.
Data Collection
Data were collected from the MSKCC electronic medical record (EMR), clinician-administered testing, the study instruments, and a semistructured interview. On instrument review, obviously incorrect or missing responses were verbally clarified or readministered.
EMR
Relevant treatment histories, diagnostic test results, consultation reports, disease status, and KPS were obtained through review of MSKCC’s comprehensive EMR.
Clinician-Administered Testing
Physical examination
The principal investigator performed a standard musculoskeletal and neurologic examination that included goniometric measurements of neck, thoracolumbar spine, shoulder, and hips; limb circumferences; and a detailed neurologic examination involving the cranial nerves, sensation, muscle strength, deep tendon reflexes, gait, and coordination.
Six-minute walk
The 6-Minute Walk Test (6MWT) has well-demonstrated validity and reliability.15,16 Distances were recorded, and participants rated their dyspnea at its completion on a Likert scale.
Functional Independence Measure Mobility Subscale
The Functional Independence Measure (FIM) Mobility Subscale is a valid clinician-rated scale with adequate discrimination in disabled cancer cohorts.17–20 The scale is composed of five items. Participant scores range from 5 to 35, with each item having seven levels that increase from 1 (total dependence) to 7 (total independence).
Identification of Impairments
Impairments were identified on the basis of physical examination findings and the 6MWT. Etiologic determination was supplemented with consultation reports, imaging, and other diagnostic studies. Generalized strength deficits in the absence of a confirmed neurologic or myopathic cause were attributed to deconditioning. Similarly, severe dyspnea after the 6MWT in the absence of objective cardiac or pulmonary pathology was attributed to aerobic deconditioning.
Self-Report Study Instrument
Older Americans Resource Study
The Older Americans Resource Study (OARS) Social/Financial Resources, activities of daily living (ADL), and instrumental ADL (IADL) subscales contain 15, seven, and seven items, respectively and have been shown to be responsive and discriminative in cancer cohorts.21–23
Physical Function-10
The Physical Function-10 (PF-10) is a well-validated subsection of the Medical Outcome Study Short Form-36 that assesses limitations in physical activities.24 The PF-10 and OARS ADL and IADL scales assess overlapping constructs with the PF-10, emphasizing mobility and the OARS self-care.
Distress and interest in rehabilitation
Four nonvalidated questions queried participants about their distress related to problems with mobility and ADL performance (Likert scale) and their willingness to receive help (binary).
Structured Interview
Pre-existing impairments
Patients were asked whether identified impairments were present before their diagnosis with metastatic breast cancer.
Rehabilitation service utilization
Scripted interviews were used to assess rehabilitation service utilization for identified impairments. Separate questions were posed about specific rehabilitation interventions. Photographs of typical orthotics and adaptive equipment were used to improve recall, and participants were asked the setting in which an impairment had been identified.
Determination of Rehabilitation Need
A board-certified physical medicine and rehabilitation (PM&R) physician specializing in cancer rehabilitation (A.L.C.) assessed rehabilitation needs on the basis of impairment-specific treatment standards,25 recommendations in rehabilitation medicine texts,26,27 and treatment algorithms in physical therapy (PT)/occupational therapy (OT) manuals.28–30 Five interventions (assistive devices for ADLs, mobility aids, orthotics and compression garments, PT, and OT) were assessed for each impairment as “needs,” “doesn’t need,” “received,” or “received and still needs.” Table 1 lists evidence-based interventions in the five categories for representative impairments. Determinations were independently reviewed by a second PM&R cancer specialist, with conflicts resolved through a consensus process involving the physicians and PT/OT cancer specialists.
Table 1.
Evidence-Based Physical Rehabilitation Interventions for Prevalent Impairments
| Impairment | PT and/or OT Treatments | Assistive Devices | Orthotics and Compression Garments |
|---|---|---|---|
|
| |||
| Shoulder contracture | Topical or deep heat prior to mobilization, Codman’s exercises, manual techniques (eg, distraction), gentle PROM with progression to AAROM & AROM; education regarding the need for continued daily mobilization, instruction in daily HEP | Canes for AAROM activities, overhead pulleys for home use | Dynamic splints |
|
| |||
| Lymphedema | MLD, fibrous release techniques, multilayer short-stretch bandaging, remedial exercise, skin care, strategies to minimize lymphatic load, weight management, caregiver instruction in MLD and bandaging, primary preventive strategies for cellulitis | Schneider packs/pads for fibrolysis; donning and doffing aids | Compression garment, alternative nocturnal compression device |
|
| |||
| Aerobic deconditioning | Interval aerobic training ± oxygen support with PRN adaptations for bony instability, pain, neurogenic weakness, contractures; education in the rationale for interval conditioning; instruction in perceived exertion scale for self monitoring; support and guidance in establishing long-term OP program | Cane or quad cane as needed, adaptation of aerobic equipment as needed | NA |
|
| |||
| Upper extremity motor deficits | |||
| Proximal | Progressive resistive exercise to residual shoulder stabilizing muscles, core strengthening activities; ADL deconstruction and provision with compensatory strategies; education in strategies for tone reduction PRN | Dressing, bathing, and grooming assistive devices, nonslip mat | Balanced forearm orthotic, sling for painful subluxation |
| Distal | ADL deconstruction and provision with compensatory strategies, PROM as needed with progression to AAROM and AROM; education in strategies for tone reduction PRN | Built up eating utensils; nonslip mat; dressing, bathing, and grooming assistive devices | Universal cuff, wrist extension orthotic with lock, nocturnal resting splints |
NOTE. The five categories of intervention consist of PT, OT, assistive devices for mobility, assistive devices for ADL performance, and orthotics and compression garments.
Abbreviations: PT, physical therapy; OT, occupational therapy; PROM, passive range of motion; AAROM, active assistive range of motion; AROM, active range of motion; HEP, home exercise program; MLD, manual lymphatic drainage; OP, outpatient program; NA, not applicable; ADL, activities of daily living; PRN, as needed.
Responses were collapsed into a binary needs versus received variable. The latter response option included impairments coded both as received and received and still needs. The five treatment categories were condensed into two groupings: the first a composite of all five categories to assess receipt of any physical rehabilitation intervention (APRI), and the second (PT/OT) to assess receipt of PT and/or OT as a marker of physician-directed services. The proportion of participants who received APRI was the main outcome measure.
Statistical Analysis
Descriptive statistics were calculated for participant- and impairment-level data Receipt of treatment at the impairment level was used in logistic regressions because analyses at the participant level would yield spuriously elevated treatment rates. The models were adjusted for clustering of impairments within participants. Independent covariates in the regression models were at both impairment and participant levels.
Univariate logistic regressions were performed with receipt of APRI and PT/OT as outcome variables to assess their association with demographic- and cancer-specific covariates. Standard model-building techniques were applied, including evaluation of interaction effects. Confounding was assessed by evaluating the effect of selectively removing covariates from the model on β coefficients and Wald statistics. The final model was assessed using the Hosmer-Lemeshow goodness-of-fit test.31 Predictive accuracy of the final model was assessed with receiver operating characteristic curves.32 Odds ratios (ORs) are presented with their corresponding 95% CI and significance was assessed using Wald tests. All tests were two tailed. P values less than .05 were considered statistically significant. Analysis was performed with STATA for Windows, version 8.0 (StataCorp, College Station, TX).
RESULTS
Study Patients
Sociodemographics
The KPS-based stratified sampling failed to yield balanced strata as grant termination forced closure of enrollment. The final study cohort of 163 patients (KPS 80 to 90, n = 72; KPS 60 to 70, n = 51; KPS 40 to 50, n = 40) contained an over-representation of higher-performing participants. Demographic and clinical characteristics are presented in Table 2.
Table 2.
Demographic, Cancer, and Functional Characteristics of the Total Study Cohort and the KPS-Based Strata
| KPS
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Total
|
80–90
|
60–70
|
40–50
|
|||||
| Characteristic | No. | % | No. | % | No. | % | No. | % |
|
| ||||||||
| All patients | 163 | 100 | 72 | 44.2 | 51 | 31.3 | 40 | 24.5 |
|
| ||||||||
| Age, years | ||||||||
| Mean | 56.2 | 53.9 | 57.5 | 58.5 | ||||
| Standard deviation | 1.2 | 11.5 | 10.6 | 14.2 | ||||
|
| ||||||||
| Race/ethnicity | ||||||||
| White | 115 | 70.6 | 54 | 75 | 33 | 64.7 | 28 | 70 |
| African American | 31 | 19.0 | 11 | 15.3 | 13 | 25.5 | 7 | 17.5 |
| Asian | 4 | 8.0 | 1 | 1.4 | 2 | 3.9 | 1 | 2.5 |
| Hispanic | 13 | 2.5 | 6 | 8.3 | 3 | 5.9 | 4 | 10 |
|
| ||||||||
| Living situation | ||||||||
| Alone | 32 | 19.6 | 11 | 15.3 | 10 | 19.6 | 11 | 27.5 |
| With husband | 85 | 52.2 | 47 | 65.3 | 23 | 45.1 | 15 | 37.5 |
| Other family | 46 | 28.2 | 14 | 19.4 | 18 | 35.3 | 14 | 35 |
|
| ||||||||
| Employment status* | ||||||||
| Working full time | 49 | 30.2 | 2 | 1.2 | 0 | 0 | 7 | 9.7 |
| Working part time | 13 | 8.0 | 34 | 47.2 | 3 | 5.9 | 7 | 17.9 |
| Unemployed | 37 | 22.7 | 9 | 12.5 | 9 | 17.7 | 1 | 2.6 |
| Retired without disability | 48 | 29.6 | 20 | 27.8 | 20 | 39.2 | 8 | 20.0 |
| Retired with disability | 34 | 21.0 | 16 | 22.2 | 15 | 29.4 | 30.8 | |
|
| ||||||||
| Stage IV disease at diagnosis | 43 | 26.4 | 22 | 30.6 | 12 | 23.5 | 9 | 22.5 |
|
| ||||||||
| Duration of metastatic cancer (months) | ||||||||
| Mean | 30.3 | 23.3 | 37.8 | 33.6 | ||||
| Standard deviation | 27.4 | 22.7 | 31.6 | 27.1 | ||||
|
| ||||||||
| Metastatic sites | ||||||||
| 1–2 | 79 | 48.5 | 45 | 62.5 | 23 | 45.1 | 11 | 27.5 |
| >2 | 84 | 51.54 | 27 | 37.5 | 28 | 54.9 | 29 | 72.5 |
| Location | ||||||||
| Bone | 131 | 80.4 | 50 | 69.4 | 44 | 86.3 | 37 | 92.5 |
| Liver | 88 | 54.0 | 33 | 45.8 | 29 | 56.9 | 26 | 65.0 |
| Lung | 88 | 54.0 | 34 | 47.2 | 28 | 54.9 | 26 | 65.0 |
| Brain | 18 | 11.0 | 3 | 4.2 | 6 | 11.8 | 9 | 22.5 |
|
| ||||||||
| No. of chemotherapy treatment regimens | ||||||||
| Mean | 3.2 | 2.4 | 3.7 | 4 | ||||
| Standard deviation | 2.1 | 1.7 | 2.1 | 2.1 | ||||
|
| ||||||||
| Current regimen | ||||||||
| Hormonal therapy | 24 | 14.7 | 14 | 19.4 | 6 | 11.8 | 4 | 10.0 |
| Taxane | 27 | 16.6 | 11 | 15.3 | 9 | 17.7 | 7 | 17.5 |
| Trastuzumab | 11 | 6.8 | 5 | 6.9 | 2 | 3.9 | 4 | 10.0 |
| Taxane + trastuzumab | 42 | 25.8 | 20 | 27.8 | 18 | 35.3 | 4 | 10.0 |
| Capecitabine | 18 | 11.0 | 6 | 8.3 | 4 | 7.8 | 8 | 20.0 |
| Other | 30 | 18.4 | 11 | 15.3 | 12 | 23.5 | 7 | 17.5 |
| None | 11 | 6.8 | 5 | 6.9 | 0 | 0 | 6 | 15.0 |
|
| ||||||||
| Radiation therapy for metastases† | ||||||||
| None | 87 | 53.4 | 54 | 75 | 25 | 49.0 | 8 | 20 |
| 1 | 42 | 25.8 | 13 | 18.1 | 14 | 27.5 | 15 | 37.5 |
| ≥ 2 | 34 | 20.9 | 5 | 6.9 | 12 | 23.5 | 17 | 42.5 |
|
| ||||||||
| Surgery for breast cancer | 64 | 39.3 | 13 | 18.1 | 21 | 41.2 | 20 | 50 |
| Lumpectomy | 36 | 22.5 | 15 | 21.7 | 8 | 15.75 | 13 | 32.5 |
| MRM | 111 | 69.4 | 50 | 72.5 | 36 | 70.6 | 25 | 62.5 |
| Axillary dissection | 92 | 60.5 | 42 | 63.6 | 30 | 61.2 | 20 | 54 |
|
| ||||||||
| No. of metastatic disease–related surgeries† | 0.7 | 0.4 | 0.5 | 0.2 | 0.7 | 0.5 | 0.9 | 0.7 |
|
| ||||||||
| Pre-existing impairment | 10 | 6.1 | 2 | 2.8 | 4 | 7.8 | 4 | 10 |
|
| ||||||||
| No. of physical impairments | ||||||||
| Mean | 3.3 | 1.8 | 3.9 | 5.3 | ||||
| Standard deviation | 2.0 | 1.2 | 1.4 | 1.6 | ||||
|
| ||||||||
| PF-10 score (0–100) | ||||||||
| Mean | 47.0 | 72.7 | 36.6 | 14.1 | ||||
| Standard deviation | 31.7 | 22.4 | 20.8 | 15.6 | ||||
|
| ||||||||
| Normal strength | 86 | 52.8 | 60 | 83.3 | 19 | 37.3 | 7 | 17.5 |
|
| ||||||||
| No. of ADL problems | ||||||||
| Mean | 1.8 | 0.2 | 1.0 | 4.0 | ||||
| Standard deviation | 0.8 | 0.6 | 1.5 | 2.2 | ||||
|
| ||||||||
| No. of IADL problems | ||||||||
| Mean | 2.4 | 0.9 | 2.8 | 4.8 | ||||
| Standard deviation | 2.1 | 1.2 | 1.8 | 1.3 | ||||
|
| ||||||||
| FIM mobility (7–35) | ||||||||
| Mean | 30.2 | 34.5 | 30.0 | 22.6 | ||||
| Standard deviation | 5.8 | 1.3 | 3.7 | 5.1 | ||||
|
| ||||||||
| Moderate disability† | 53 | 32.3 | 0 | 0 | 17 | 33.3 | 36 | 90.0 |
|
| ||||||||
| For ≥ 1 impairment: | ||||||||
| Needed APRI | 144 | 88.3 | 53 | 73.6 | 51 | 100.0 | 40 | 100.0 |
| Needed PT/OT | 143 | 87.7 | 52 | 72.2 | 51 | 100.0 | 40 | 100.0 |
| Received APRI, if needed | 73 | 50.7 | 15 | 28.3 | 29 | 56.9 | 29 | 72.5 |
| Received PT/OT, if needed | 56 | 39.2 | 16 | 30.8 | 22 | 43.1 | 18 | 45.0 |
Abbreviations: KPS, Karnofsky performance score; MRM, modified radical mastectomy; PF-10, Physical Function-10; ADL, activities of daily living; IADL, instrumental activities of daily living; FIM, Functional Independence Measure; APRI, any physical rehabilitation intervention; PT/OT, physical therapy/occupational therapy.
Participants may endorse more that one so that percentages may not sum to 100.
Moderate disability is defined as PF-10 < 35, FIM Mobility Subscale < 30, ≥ 4 IADL problems.
Cancer characteristics
Participants, by definition, had metastatic disease, with half having metastases in at least two sites. The mean time since diagnosis with metastatic disease was 30.3 months (± 27.4 months). Participants were receiving a variety of treatment regiments, the most common of which was combined trastuzumab-taxane therapy. Current treatment did not significantly vary across KPS strata. Other details are listed in Table 2.
Function characteristics
Physical impairments were identified in 92% of the 163 patients with equal percentages (88%) judged to require either APRI or PT/OT for at least one impairment. Barely half of the participants had normal muscle strength, with the group as a whole demonstrating low levels of physical function (mean PF-10 score, 47.0 ± 31.7) A significant proportion of the cohort reported difficultly with at least one ADL (43%) and IADL (74%). FIM Mobility Subscale score means (30.2 ± 5.8) revealed a generalized compromised mobility. Overall, one third of the participants presented a picture of moderate disability, defined as FIM mobility subscale score less than 30, PF-10 less than 35, and at least 4 problems with IADLs.33 Fifty-one percent of patients had received APRI, and 39.2% of patients had received PT/OT for at least one impairment. For many patients, receipt of APRI (40.3%) and PT/OT (51.8%) was for arm lymphedema.
Impairments
Table 3 lists impairment prevalences and treatment frequencies. All but 8% of the participants (13/163) had at least one physical impairment with 530 (3.3 ± 2.0/participant) occurring in the remaining 150. Forty six (8.7%) impairments were coded “doesn’t need” and required no treatment. APRI was indicated for 484 (91%) of the impairments, with almost as many (469) deemed to require PT/OT. Only 30% of impairments for which APRI was indicated, and only one fifth of those with a PT/OT indication, had received treatment. Less than 10% of patients received treatment for all impairments requiring either APRI or PT/OT.
Table 3.
Impairment Characteristics and Subcategories: Prevalences and Frequencies of APRI and PT/OT if Needed
| Total*
|
Detected During Hospitalization
|
Rehabilitation Needed
|
Received APRI if Needed
|
Therapy Needed
|
Received PT/OT if Needed
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impairment Characteristic | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|
| ||||||||||||
| All patients | 530 | 100 | 68 | 12.8 | 484 | 91.3 | 145 | 30.0 | 469 | 88.5 | 99 | 21.1 |
|
| ||||||||||||
| Impairment detected during hospitalization | 68 | 12.8 | 68 | 100 | 68 | 100 | 64 | 94.1 | 67 | 98.5 | 61 | 91.0 |
|
| ||||||||||||
| Impairment associated with orthotic procedure | 27 | 5.1 | 19 | 70.4 | 27 | 100 | 23 | 85.2 | 27 | 100.0 | 19 | 70.4 |
|
| ||||||||||||
| Lymphedema | ||||||||||||
| Arm | 62 | 11.7 | 0 | 0 | 62 | 100.0 | 30 | 48.4 | 62 | 100.0 | 62 | 48.4 |
| Leg | 29 | 5.5 | 2 | 6.9 | 29 | 100.0 | 6 | 20.7 | 16 | 55.2 | 3 | 10.3 |
|
| ||||||||||||
| Neurogenic weakness, focal PNS* | 24 | 4.5 | 3 | 12.5 | 23 | 95.8 | 4 | 17.4 | 22 | 91.7 | 4 | 18.2 |
|
| ||||||||||||
| Sensory deficit, focal PNS* | 20 | 3.8 | 1 | 5.0 | 19 | 95.0 | 4 | 21.1 | 19 | 95.0 | 1 | 5.3 |
|
| ||||||||||||
| Peripheral neuropathy | ||||||||||||
| Mild | 24 | 4.5 | 0 | 0 | 2 | 8.3 | 1 | 50.0 | 2 | 8.3 | 1 | 50.0 |
| Moderate-severe | 47 | 8.9 | 3 | 6.4 | 45 | 95.7 | 12 | 26.7 | 45 | 95.7 | 3 | 6.7 |
|
| ||||||||||||
| CNS deficit† | ||||||||||||
| Hemiparesis | 8 | 1.5 | 4 | 50.0 | 8 | 100.0 | 4 | 50.0 | 8 | 100.0 | 3 | 37.5 |
| Myelopathy | 13 | 2.5 | 6 | 46.2 | 13 | 100 | 8 | 61.5 | 13 | 100 | 4 | 30.8 |
|
| ||||||||||||
| Cranial nerve deficit | 12 | 2.3 | 0 | 0 | 6 | 50.0 | 0 | 6 | 50.0 | 0 | ||
|
| ||||||||||||
| Ataxia | 36 | 6.8 | 6 | 16.7 | 35 | 97.2 | 12 | 34.3 | 35 | 97.2 | 6 | 17.1 |
|
| ||||||||||||
| Generalized motor weakness (including steroid myopathy) | 62 | 11.7 | 17 | 27.4 | 61 | 98.4 | 26 | 42.6 | 61 | 98.4 | 16 | 26.2 |
|
| ||||||||||||
| Exertional intolerance | ||||||||||||
| Deconditioning | 54 | 10.2 | 5 | 9.3 | 54 | 100.0 | 9 | 16.7 | 54 | 100.0 | 5 | 9.3 |
| Cardiopulmonary | 48 | 9.1 | 3 | 6.3 | 48 | 100.0 | 5 | 10.4 | 48 | 100.0 | 3 | 6.3 |
| Joint contracture | ||||||||||||
| Hip | 10 | 1.9 | 8 | 80.0 | 10 | 100 | 8 | 88.9 | 9 | 90.0 | 8 | 88.9 |
| Shoulder | 32 | 6.0 | 3 | 9.4 | 32 | 100.0 | 4 | 12.5 | 32 | 100.0 | 4 | 12.5 |
| Spine | 13 | 2.5 | 6 | 46.2 | 12 | 92.3 | 9 | 75.0 | 12 | 92.3 | 7 | 58.3 |
|
| ||||||||||||
| Myofascial dysfunction | 18 | 3.4 | 0 | 0 | 15 | 83.3 | 0 | 0 | 15 | 83.3 | 0 | 0 |
Abbreviations: APRI, any physical rehabilitation intervention; PT/OT, physical therapy/occupational therapy; PNS, peripheral nervous system.
Specific impairment types do not add to 530. A category “Other,” comprising impairments with frequencies ≤ 5, is not included in the table.
Rehabilitation delivery rates were much higher for impairments detected during hospitalization, with more than 90% of those patients receiving APRI and PT/OT. Among impairments not identified during hospitalization, less than one fifth of those requiring APRI and less than 10% requiring PT/OT were treated. With the exception of lymphedema, less than 2% of impairments that required PT/OT not detected during hospitalization received treatment.
Physical Impairments, Disability, and Anticancer Treatments
Figure 1 displays a representative sample of impairments listed in Table 3. The PF-10 and OARS ADL scores for participants with specific impairments are displayed relative to sample means. Impairment groups are defined by the inciting pathology (eg, musculoskeletal). Note that with few exceptions, the degree of disability is significant and relatively uniform across impairments.
Fig 1.
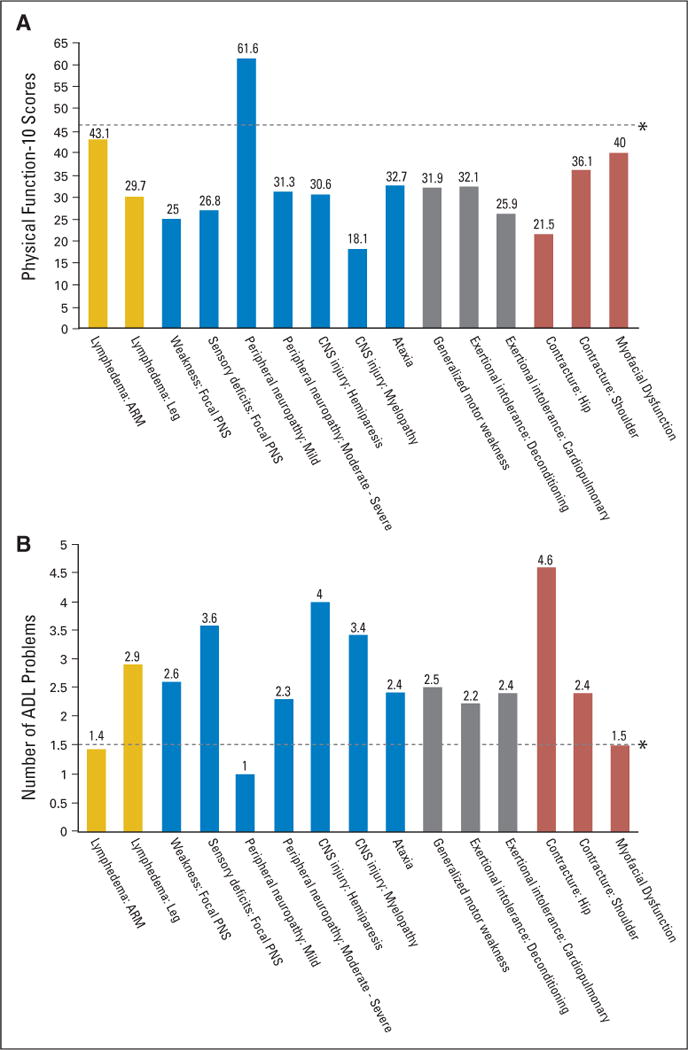
(A) Physical Function-10 and (B) Older Americans Resource Study activities of daily living (ADL) scores for patients with a representative sample of impairments relative to sample means grouped by the inciting pathology (eg, musculoskeletal). PNS, peripheral nervous system. (*) Mean values for the total study sample. Yellow, lymphedema; blue, neurological impairments; gray, generalized stamina/strength deficits; red, musculoskelatal impairments.
The majority of impairment types were a result of imaging-confirmed metastatic lesions. Leg lymphedema and hip contractures generally occurred in participants who had undergone hip replacements or irradiation for symptomatic metastases or impending fracture.
Relationships between cancer, its treatment, and physical impairments were rarely straightforward. Among 46 patients with exertional intolerance attributed to cardio-pulmonary factors, 37 had lung or pericardial metastases, 27 had received a cardiotoxic chemotherapy (18 with multiple gated acquisition scan confirmed cardiomyopathy), 12 had undergone intrathoracic surgery, 25 received radiation encompassing lung fields, and 21 had moderate or worse pleural effusions. More than one factor likely contributed to exertional intolerance in most patients.
Comparable complexity characterized the relationships between impairments and chemotherapy, radiation, and cancer surgeries. Patients had received more than 70 different chemotherapeutic and hormonal regimens. Reducing these to a manageable number yielded no meaningful associations aside from the fact that participants who received second- and third-line regimens or “salvage” chemotherapy had more metastases, impairments, and disability. However, all patients with neuropathies had received at least one taxane-based regimen. Surgical and radiation therapy interventions were equally heterogeneous, and a similar pattern was noted, with patients who received more treatments having more impairments and advanced disease.
Distress and Interest in Receiving Rehabilitation
Table 4 displays the percentages of participants in each KPS substratum and with moderate disability whose mobility and ADL performance distressed them at least moderately, and those interested in receiving treatment. Interest increased with declining KPS scores.
Table 4.
Distress Related to and Desire for Help With ADL Performance and Mobility in All and Moderately Disabled Participants
| All Patients
|
Moderately Disabled Patients*
|
KPS Group
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 80–90
|
60–70
|
40–50
|
||||||||
| Distress and Desire | No. | % | No. | % | No. | % | No. | % | No. | % |
|
| ||||||||||
| Distress associated with performance of ADL rated moderate or worse† | 62 | 38.0 | 39 | 73.6 | 8 | 11.1 | 25 | 49.0 | 29 | 72.5 |
|
| ||||||||||
| Desire for help with ADL | 79 | 54.9 | 44 | 83.0 | 14 | 26.4 | 34 | 66.7 | 31 | 77.5 |
|
| ||||||||||
| Distress associated with mobility rated moderate or worse† | 88 | 54.0 | 44 | 83.0 | 24 | 33.3 | 32 | 62.8 | 32 | 80.0 |
|
| ||||||||||
| Desire help with mobility | 89 | 61.8 | 43 | 81.1 | 22 | 41.5 | 35 | 68.6 | 33 | 82.5 |
Abbreviations: ADL, activities of daily living; KPS, Karnofsky performance score.
Moderate disability is defined as PF-10, Physical Function-10 < 35, Functional Independence Measure Mobility Subscale < 30, > 4 instrumental ADL problems.
Self-reported distress was assessed using a 5-point Likert scale (1 = not at all, 2 = somewhat, 3 = moderately, 4 = quite a bit, 5 = extremely).
Logistic Regression Analyses
Receipt of APRI
Participant- and impairment-level covariates predicted whether impairments requiring APRI were addressed (Table 5). The model correctly predicted receipt of APRI for the majority of impairments with a C statistic of 0.85. Detection of impairments during hospitalization was the strongest predictor of APRI, with an OR of 87.9 (95% CI, 28.5 to 271.4). Impairments associated with orthopedic procedures (OR = 10.1; 95% CI, 4.6 to 22.3) or arm lymphedema (OR = 7.2; 95% CI, CI 3.6 to 14.4) were also addressed more often. White participants (OR = 3.0; 95% CI, CI 1.4 to 6.4) were more likely to have received APRI, whereas those receiving unemployment benefits (OR = 0.2; 95% CI, CI 0.1 to 0.4), or who identified themselves as “unemployed and seeking work” (OR = 0.1; 95% CI, CI 0.0 to 0.1) were significantly less likely to receive APRI.
Table 5.
Multiple Logistic Regression Analyses of Participants and Impairment Characteristics Associated With Receipt of APRI and PT/OT
| Characteristic | Adjusted Odds Ratio | Robust SE* | P | 95% CI |
|---|---|---|---|---|
|
| ||||
| Covariates associated with receipt of APRI if needed† | ||||
| Impairment detected during hospitalization‡ | 87.88 | 50.55 | < .0001 | 28.46 to 271.36 |
| Impairment associated with an orthopedic procedure‡ | 10.12 | 4.08 | < .0001 | 4.59 to 22.30 |
| Impairment = arm lymphedema‡ | 7.21 | 2.55 | < .0001 | 3.61 to 14.43 |
| White race† | 2.99 | 1.16 | .01 | 1.40 to 6.42 |
| Participant receiving unemployment benefits§ | 0.21 | 1.70 | < .0001 | 0.10 to 0.41 |
| Participant unemployed and looking for work§ | 0.05 | 0.02 | < .0001 | 0.02 to 0.13 |
|
| ||||
| Covariates associated with receipt of PT/OT if needed† | ||||
| Impairment detected during hospitalization‡ | 558.75 | 312.06 | < .0001 | 186.99 to 1,669.61 |
| Impairment = arm lymphedema‡ | 69.59 | 32.93 | < .0001 | 27.53 to 175.93 |
| White race§ | 5.68 | 2.78 | < .0001 | 2.18 to 14.82 |
| Participant retired on disability§ | 2.84 | 1.46 | .04 | 1.04 to 7.77 |
Abbreviations: APRI, any physical rehabilitation intervention; PT/OT, physical therapy/occupational therapy.
Adjusted for clustering by participant.
C statistic multiple logistic regression model of APRI = 0.85 and of PT/OT = 0.96.
Impairment-level covariate.
Participant-level covariate.
Receipt of PT/OT
Similar participant- and impairment-level covariates were associated with receipt of PT/OT (Table 5). The full model correctly predicted receipt of PT/OT for the majority of impairments with a C statistic of 0.96. Impairments detected during hospitalization (OR = 558.8; 95% CI, CI 187.0 to 1669.6) or upper extremity lymphedema (OR = 69.6; 95% CI, CI 27.5 to 175.9) were overwhelmingly more likely to be addressed. Impairments occurring in retired participants on disability (OR = 2.8; 95% CI, 1.0 to 7.8) and white patients (OR = 5.7; 95% CI, CI 2.2 to 14.8) were also more likely to receive PT/OT.
The Wald statistics revealed no significant association between receipt of APRI or PT/OT and disability metrics (PF-10, OARS ADL and IADL subscale, and FIM scores), participants’ desire to receive rehabilitation, and cancer treatment-related covariates.
DISCUSSION
This study, to our knowledge, is the first to use clinician-rated, impairment-based assessment to characterize physical rehabilitation needs in patients with any form of cancer. In doing so, it confirmed high prevalence rates and marked undertreatment of physical impairments despite the fact the majority of participants recognized their need for assistance. Particularly striking was the finding that, although more than 90% of the patients appeared to have impairments that would, at least in part, be ameliorated by standard rehabilitation measures, less than a third of these impairments received treatment. Further, participants had metastatic cancer for a mean of 30.3 months (± 27.4 months), suggesting that many may have experienced protracted disability.
Our findings also document an intuitively obvious, but previously unreported, disparity between the delivery of rehabilitation services in the outpatient and inpatient settings. Further, these findings confirm the presence of racial and economic disparities that have been reported for other types of cancer and cancer care.34–36
The striking association between impairment detection and hospitalization (Table 5) may reflect a combination of increased scrutiny in the inpatient setting, the presence of critical pathways, and the absence of access barriers. The role of formalized discharge planning and the provision of postdischarge services may be important.40 The dearth of treatment in the outpatient setting is of particular concern in light of the trend toward greater outpatient cancer management and the fact that impairments are most remediable in their early stages. The fact that arm lymphedema received PT/OT (OR = 69.6) and APRI (OR = 7.2) significantly more other impairments may reflect the convenience afforded by a lymphedema clinic within MSKCC, and suggests that ease of access may be an important determinant in service utilization.
Physical impairments are only a portion of the burden borne by patients with cancer. The ideal scope of cancer rehabilitation encompasses a broad range of vocational, psychological, economic, social, and functional problems. We would have preferred to assess the entire spectrum of needs. However, two factors mitigated against this. First, the task of simultaneously assessing all these factors would be overwhelming. Second, focusing on physical impairments alone reflects conventional PM&R outpatient practice patterns, where cancer specialists address physical impairments and related disabilities. Psychologists and social workers, unaffiliated with rehabilitation medicine, typically address psychosocial issues.
Concerns about the delay between data collection and publication might also be raised. This delay arose from logistic, regulatory, and financial barriers to the interinstitutional transfer of data for analysis. Fortunately, these concerns seem more apparent than real, given that there has been little interval change in the treatment of metastatic breast cancer or in the integration of rehabilitation services. In large rehabilitation departments, the emphasis on cancer has remained stable during the last decade, and reimbursement for rehabilitation services has declined.41 Moreover, there has been no increase in data-driven publications on this problem. It is telling that the National Comprehensive Cancer Network recent consensus guidelines on supportive care do not mention rehabilitation.42
The consensus process used to classify a patient’s rehabilitation needs might also be questioned. However, no reasonable alternative approach seemed possible, and every effort was made to use accepted and objective standards. The high level of functional morbidity in the study cohort argues that rehabilitation was legitimately indicated for the majority of participants. Participant recall bias is a concern, but the use of scripted questions and photographs should have minimized its risk.
This study’s focus on patients’ specific impairments rather than their disabilities complements existing data by providing new insights into causes of functional decline and useful information regarding potential screening and treatment strategies. Our data, however, do not shed light on the reason for the discordance between the presence of impairments and their treatment. The lack of outpatient rehabilitation treatment suggests that functional end points are underemphasized in cancer care. This is of particular concern in light of the growing emphasis on quality survivorship, and warrants further investigation. Initial next steps will include characterizing sources of undertreatment. Our results will serve as the basis for focused interventional trials of screening, directed treatment and educational strategies.
In summary, this study indicates that, although 90% of patients being treated for metastatic breast cancer at a tertiary cancer center would benefit from at least one rehabilitation intervention, fewer than 30% actually received appropriate services. This seems particularly unfortunate in view of the efficacy of standard rehabilitation interventions in treating many of these impairments in patients with cancer, as well as other systemic and debilitating diseases.17,18,43–53
Acknowledgments
We thank Richard Payne, MD, who was instrumental in obtaining the Langeloth Foundation grant that supported initial study design and data collection, and Angela DeMichele, MD, MSCE, for her guidance in identifying sources of confounding and bias in this study.
Supported by a Langeloth Foundation grant, Department of Defense Grant No. DAMD17-03-1-0622, and National Institutes of Health Grant No. KL2 RR024151-01.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Andrea L. Cheville, Andrea B. Troxel, Alice B. Kornblith
Financial support: Andrea L. Cheville
Administrative support: Andrea B. Troxel, Jeffrey R. Basford, Alice B. Kornblith
Provision of study materials or patients: Andrea L. Cheville
Collection and assembly of data: Andrea L. Cheville
Data analysis and interpretation: Andrea L. Cheville, Andrea B. Troxel, Jeffrey R. Basford, Alice B. Kornblith
Manuscript writing: Andrea L. Cheville, Andrea B. Troxel, Jeffrey R. Basford, Alice B. Kornblith
Final approval of manuscript: Andrea L. Cheville, Andrea B. Troxel, Jeffrey R. Basford, Alice B. Kornblith
References
- 1.McGlynn EA, Cassel CK, Leatherman ST, et al. Establishing national goals for quality improvement. Med Care. 2003;41(suppl):I16–I29. doi: 10.1097/00005650-200301001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood) 2002;21:80–90. doi: 10.1377/hlthaff.21.3.80. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann JF, DeLisa JA, Warren CG, et al. Cancer rehabilitation: Assessment of need, development, and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59:410–419. [PubMed] [Google Scholar]
- 4.Heinrich RL, Schag CC, Ganz PA. Living with cancer: The Cancer Inventory of Problem Situations. J Clin Psychol. 1984;40:972–980. doi: 10.1002/1097-4679(198407)40:4<972::aid-jclp2270400417>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Guadagnoli E, Mor V. Daily living needs of cancer outpatients. J Community Health. 1991;16:37–47. doi: 10.1007/BF01340467. [DOI] [PubMed] [Google Scholar]
- 6.Taylor KC, Currow D. A prospective study of patient identified unmet activity of daily living needs among cancer patients at a comprehensive cancer care center. Aust Occup Ther J. 2003;50:79–85. [Google Scholar]
- 7.Hwang SS, Chang VT, Cogswell J, et al. Study of unmet needs in symptomatic veterans with advanced cancer: Incidence, independent predictors and unmet needs outcome model. J Pain Symptom Manage. 2004;28:421–432. doi: 10.1016/j.jpainsymman.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Nagi SZ. A study in the evaluation of disability and rehabilitation potential: Concepts, methods, and procedures. Am J Public Health Nations Health. 1964;54:1568–1579. doi: 10.2105/ajph.54.9.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 10.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. International classification of functioning, disability and health: ICF. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 12.Armstrong T, Almadrones L, Gilbert MR. Chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. 2005;32:305–311. doi: 10.1188/05.ONF.305-311. [DOI] [PubMed] [Google Scholar]
- 13.Crooks V, Waller S, Smith T. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139–M144. doi: 10.1093/geronj/46.4.m139. [DOI] [PubMed] [Google Scholar]
- 14.Extermann M, Overcash J, Lyman GH. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 16.Rostagno C, Olivo G, Comeglio M, et al. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: Comparison with other methods of functional evaluation. Eur J Heart Fail. 2003;5:247–252. doi: 10.1016/s1388-9842(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 17.O’Dell MW, Barr K, Spanier D, et al. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79:1530–1534. doi: 10.1016/s0003-9993(98)90414-2. [DOI] [PubMed] [Google Scholar]
- 18.Huang ME, Cifu DX, Keyser-Marcus L, et al. Functional outcomes in patients with brain tumor after inpatient rehabilitation: Comparison with traumatic brain injury. AM J Phys Med Rehabil. 2000;79(4):327–335. doi: 10.1097/00002060-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Granger CV, Cotter AC, Hamilton BB, et al. Functional assessment scales: A study of persons after stroke. Arch Phys Med Rehabil. 1993;74:133–138. [PubMed] [Google Scholar]
- 20.Granger CV, Cotter AC, Hamilton BB, et al. Functional assessment scales: A study of persons with multiple sclerosis. Arch Phys Med Rehabil. 1990;71:870–875. [PubMed] [Google Scholar]
- 21.Fillenbaum G, Smyer M. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 22.Kornblith AB, Herndon JE, II, Zuckerman E, et al. The impact of docetaxel, estramustine, and low dose hydrocortisone on the quality of life of men with hormone refractory prostate cancer and their partners: A feasibility study. Ann Oncol. 2001;12:633–641. doi: 10.1023/a:1011102619058. [DOI] [PubMed] [Google Scholar]
- 23.Bailey D, Corner J, Addington-Hall J, et al. Older patients’ experiences of treatment for colorectal cancer: An analysis of functional status and service use. Eur J Cancer Care. 2004;13:483–493. doi: 10.1111/j.1365-2354.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 24.Stewart A, Ware J. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 25.Bernas M, Witte C, et al. The diagnosis an treatment of peripheral lymphedema: Draft revision of the 1995 consensus document of the international society of Lymphology executive committee for discussion at the September 3–7, 2001, XVII International Congress of Lymphology in Genoa, Italy. Lymphology. 2001;34:84–91. [PubMed] [Google Scholar]
- 26.Braddom R, Buschbacher R. Physical Medicine and Rehabilitation. Philadelphia, PA: Saunders; 2005. [Google Scholar]
- 27.DeLisa J, Gans B, Walsh NE. Physical Medicine and Rehabilitation: Principles and Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 28.Moyers PA. The guide to occupational therapy practice: American Occupational Therapy Association. Am J Occup Ther. 1999;53:247–322. doi: 10.5014/ajot.53.3.247. [DOI] [PubMed] [Google Scholar]
- 29.Crepau EB, Cohn ES, Boyt Schell BA. Willard and Spackman’s Occupational Therapy. 9. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 30.American Physical Therapy Association. Guide to Physical Therapist Practice. 1. Alexandria, VA: American Physical Therapy Association; 1995. [Google Scholar]
- 31.Lemeshow S, Hosmer D. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 32.Metz C. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 33.Stineman MG, Goin JE, Granger CV, et al. Discharge motor FIM-function related groups. Arch Phys Med Rehabil. 1997;78:980–985. doi: 10.1016/s0003-9993(97)90061-7. [DOI] [PubMed] [Google Scholar]
- 34.Griggs JJ, Sorbero ME, Stark AT, et al. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 35.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the US Department of Defense healthcare system. Cancer. 2003;98:894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 36.Jatoi I, Anderson WF, Rao SR, et al. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–7841. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Reference deleted.
- 39.Reference deleted.
- 40.Frieman M, Breen N. The use of home care by cancer patients: A multivariate analysis. Home Health Care Serv Q. 1997;16:3–19. doi: 10.1300/J027v16n01_02. [DOI] [PubMed] [Google Scholar]
- 41.Braddom RL. Medicare funding for inpatient rehabilitation: How did we get to this point and what do we do now? Arch Phys Med Rehabil. 2005;86:1287–1292. doi: 10.1016/j.apmr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Fort Washington, PA: National Comprehensive Cancer Network; YYYY. [Google Scholar]
- 43.Hicks J. Exercise in patients with inflammatory arthritis and connective tissue disease. Rheum Dis Clin North Am. 1990;16:845–870. [PubMed] [Google Scholar]
- 44.Kraft G, Alquist A. Effect of resistive exercise on physical function in multiple sclerosis (MS) Veterans Aff Rehabil Res Dev Prog Rep. 1996;33:328–329. [Google Scholar]
- 45.Yarasheski K, Roubenoff R. Exercise treatment for HIV-associated metabolic and anthropomorphic complications. Exerc Sport Sci. 2001;29:170–174. doi: 10.1097/00003677-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Courneya KS, Friedenreich CM, Quinney HA, et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 47.Dimeo F, Fetscher S, Lange W, et al. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90:3390–3394. [PubMed] [Google Scholar]
- 48.Dimeo FC, Stieglitz RD, Novelli-Fischer U, et al. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273–2277. [PubMed] [Google Scholar]
- 49.Winningham M, Mac Vicar M. The effect of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. 1988;15:447–450. [PubMed] [Google Scholar]
- 50.Cheville A. Cancer Rehabilitation. In: Braddom R, editor. Physical Medicine and Rehabilitation. 3. Philadelphia, PA: Saunders; 2006. pp. 1228–1256. [Google Scholar]
- 51.Boström C, Harms-Ringdahl K, Karreskog H, et al. Effects of static and dynamic shoulder rotator cuff exercise on women with rheumatoid arthritis: A randomised comparison of impairment, disability, handicap and health. Scand J Rheumatol. 1998;27:281–290. doi: 10.1080/030097498442398. [DOI] [PubMed] [Google Scholar]
- 52.Földi E, Földi M, Weissleder H. Conservative treatment for lymphedema of the limbs. Angiology. 1985;36:171–180. doi: 10.1177/000331978503600306. [DOI] [PubMed] [Google Scholar]
- 53.Ko D, Lerner R, Klose G, et al. Effective treatment of lymphedema of the extremities. Arch Surg. 1998;133:452–458. doi: 10.1001/archsurg.133.4.452. [DOI] [PubMed] [Google Scholar]


