Abstract
Context
Exercise benefits patients with cancer, but studies of home-based approaches, particularly among those with Stage IV disease, remain small and exploratory.
Objectives
To conduct an adequately powered trial of a home-based exercise intervention that can be facilely integrated into established delivery and reimbursement structures.
Methods
Sixty-six adults with Stage IV lung or colorectal cancer were randomized, in an eight-week trial, to usual care or incremental walking and home-based strength training. The exercising participants were instructed during a single physiotherapy visit and subsequently exercised four days or more per week; training and step-count goals were advanced during bimonthly telephone calls. The primary outcome measure was mobility assessed with the Ambulatory Post Acute Care Basic Mobility Short Form. Secondary outcomes included ratings of pain and sleep quality as well as the ability to perform daily activities (Ambulatory Post Acute Care Daily Activities Short Form), quality of life (Functional Assessment of Cancer Therapy-General), and fatigue (Functional Assessment of Cancer Therapy-Fatigue).
Results
Three participants dropped out and seven died (five in the intervention and two in the control group, P = 0.28). At Week 8, the intervention group reported improved mobility (P = 0.01), fatigue (P = 0.02), and sleep quality (P = 0.05) compared with the usual care group, but did not differ on the other measures.
Conclusion
A home-based exercise program seems capable of improving the mobility, fatigue, and sleep quality of patients with Stage IV lung and colorectal cancer.
Keywords: Cancer, fatigue, exercise, mobility, function, quality of life
Introduction
Stage IV cancer care has, in addition to the treatment of the cancer itself, a goal of limiting disability. Although significant attention is directed toward pain and symptom control, little emphasis is placed on increasing a patient’s physical activity and capabilities,1,2 which may be a mistake. A growing body of evidence suggests that physical activity-directed interventions are not only safe and low cost but also improve the function, quality of life (QOL), and independence of people with cancer.3–5 In fact, mounting evidence suggests that even those with Stage IV cancer can derive meaningful benefits.6–9
The influence of these findings on clinical care has thus far been limited. Two factors seem particularly significant. First, many of the studies have been center-based and resource intensive—their widespread introduction into the outpatient setting would impose unacceptable financial and resource demands on the patient and the health care system. Second, most health care systems in the U.S. lack a formal infrastructure for either implementation or reimbursement, and consequently, exercise programs for patients with cancer seldom exist outside the research environment.
We recently developed and evaluated a Rapid, Easy, Strength Training (REST) exercise program as part of a multidisciplinary, center-based intervention in two randomized controlled trials (RCTs),10,11 involving patients undergoing outpatient radiation treatments of late-stage cancer. Participants not only enjoyed and tolerated the sessions well but also reported significant improvements in QOL, physical well-being, and symptom burden. Unfortunately, the intervention’s generalizability is limited by a lack of similar programs in many community settings. We have found that patients can be instructed in REST during a single physiotherapy visit in a manner that will permit them to carry out a home program. However, it remains unclear whether home-based strengthening programs can be as effective in people in the later stages of cancer as they have been shown to be in other populations.12 This study estimates the effect of a limited intensity and duration REST and pedometer-based walking program on the primary outcome, self-reported mobility, and secondary outcomes, pain, QOL, fatigue, sleep quality, and the ability to perform daily activities, when delivered to debilitated patients with Stage IV lung and colorectal cancers during a single session with weekly telephone follow-up.
Methods
Study Participants
Patients with pathology-confirmed Stage IV lung and colorectal cancers being followed in the Mayo Clinic Outpatient Oncology Clinic were recruited between May 15 and July 15, 2010. Cancer stage was established by the presence of metastatic disease confirmed by imaging, with uncertainties resolved by consulting the treating oncologist. Potentially eligible patients were invited to participate via telephone calls. Recruitment alternated in groups of six between those with lung or colorectal cancer to ensure balanced participation. Exclusion criteria included a Folstein Mini-Mental State Examination score of 25 or less, inadequate English proficiency, hospice enrollment, or an average pain numeric rating scale score of ≥ 6 of 10. Eligible participants also were required to have Ambulatory Post Acute Care (AM-PAC) Computer Adaptive Test (CAT) (described subsequently) scores between 50 and 75, as participants with scores in this range are generally capable of performing REST and have the greatest likelihood of benefiting.13–15 Medical comorbidities and current or past cancer treatments did not affect eligibility. The study was approved by the Mayo Clinic, Rochester Institutional Review Board, and was registered on ClinicalTrials.gov (NCT01334983).
Randomization and Blinding
Patients agreeing to participate were evaluated by a research coordinator who obtained their informed consent, supervised completion of the baseline assessment instruments, and opened the sealed envelope to reveal a participant’s randomization status. Participants were randomly assigned to the intervention or control groups in blocks of six using an unstratified allocation sequence that was generated by the study statistician. Blinding of participants, physical therapists (PTs), and the research coordinator to group assignment was not possible because of the nature of the intervention. Data entry and statistical analyses were performed by blinded personnel.
Intervention
The intervention comprised an initial one-on-one, 90-minute instructional session in REST as well as a pedometer-based walking program and was followed by bimonthly telephone calls directed toward reviewing and advancing the participants’ programs as possible. The intervention was delivered by two PTs (J. K. and J. V.). During the instructional session, participants were provided with an illustrated REST instruction manual, five color-coded incremental resistance exercise bands, a perceived exertion rating scale, and a calendar to log REST sessions and step counts.
The REST program (Table 1) comprised two sets of five-exercise routines, one targeting the upper and the other the lower body. Most exercises included a rapid initial motion of the extremities, a slower truncal motion, a three-count hold, and a controlled return to the starting position. Patients graded their effort using a color-coded version of the Borg category-ratio (CR10)16 scale and used the exercise band that forced them to exert moderate force but with which they could maintain control throughout the exercises. Those who found prolonged standing difficult were permitted to exercise in a seated position. Participants were instructed to perform 10 repetitions of each REST exercise in the upper and lower body routines at least twice a week for a total of four sessions (two upper and two lower body) and to log these on their calendars. More frequent REST performance was encouraged, although participants were instructed not to exceed performance of the upper and lower body routines once per day. Participants gradually increased their repetitions to 15. REST was advanced to the next level of band resistance when a participant could perform 15 repetitions of an exercise with less than moderate exertion.
Table 1.
Rapid, Easy, Strength Training Exercises Targeting Major Muscle Groups of the Trunk and Extremities
| REST | Exercise | Description of Movements | Limb Muscle Group(s) | Truncal Muscle Group(s) |
|---|---|---|---|---|
| Upper Body | Biceps curl |
|
Biceps, brachialis, supinator | Thoracic spinal extensors |
| Rowing |
|
Biceps, brachialis, supinator | Middle trapezius, rhomboids | |
| Pull down |
|
Triceps, scapular retractors, spinal extensors | Middle and lower trapezius, rhomboids, latissimus dorsi | |
| Bat swing |
|
Deltoid, rotator cuff | Oblique and transverse abdominals | |
| Chest press |
|
Triceps | Pectoralis major and minor, supraspinatus | |
| Lower Body | Squats |
|
Quadriceps | Gluteus maximus |
| Calf raise |
|
Gastrocnemius, soleus | ||
| Straight leg steps: Front |
|
Quadriceps | Iliopsoas, abdominals, spinal extensors | |
| Straight leg steps: Side |
|
Tensor facia lata | Gluteus medius and minimus, abdominals, spinal extensors | |
| Straight leg steps: Back |
|
Quadriceps | Gluteus maximus, abdominals, spinal extensors |
Participants were provided with Omron® HJ-720ITC Pocket Pedometers (Omron Healthcare, Inc., Lake Forest, IL) and instructed to wear them at least four days per week. Participants were instructed to walk briskly at a pace of roughly one mile per 20 minutes (approximately 3.5 metabolic equivalents), when possible, and to log their counts on the study calendars.
Subjects were contacted by telephone on Weeks 1, 3, 5, and 7 by the PTs who had provided their initial instruction. A five-minute or less scripted interview was used to screen for concerning signs or symptoms and to advance participants’ programs. The PTs queried participants regarding new or progressive pain and neurological deficits; altered mental status; altered bowel or bladder function; palpitations, light-headedness, or other acute changes with activity; and progressive fatigue or anorexia. If participants endorsed any of these problems, or volunteered others, the PTs communicated this information to the physician principal investigator (PI) (A. L. C), who reviewed the participants’ electronic medical records (EMRs). Adverse events were captured in this manner and by the PI’s monthly review of all participants’ EMRs. During the Week 1 call, PTs calculated a mean daily step count and set a goal 10% higher than this value for the following two weeks. A pattern of alternate week advancement when participants met their goals was continued throughout the study.
Control Condition
Participants in the control group were neither directed to exercise, nor was their activity monitored. They were, however, offered REST instruction at the conclusion of the study. Non-enrollment contact with the study personnel included a telephone call at Week 4 thanking them for their participation and a call at Week 8 alerting them to expect a mailing with the outcome measures.
Data Collection Schedule
All study participants provided the following demographic information: age, gender, ethnicity, and level of education and completed baseline patient-reported outcomes (PROs) immediately after providing informed consent. All PROs were recorded using pen and paper. Proxy responses were not accepted. Follow-up PRO booklets were mailed to participants at the conclusion of the eight-week trial. Nonresponders were telephoned and mailed a second PRO booklet 10 days after the initial mailing. Up to five attempts were made to contact nonresponding participants by telephone.
PROs
Function: AM-PAC CAT and AM-PAC Mobility and Activities Short Forms
The AM-PAC CAT is an item response theory-based measure of function with established validity and reliability in individuals with chronic disease.17–19 It has been previously tested in neurologic, orthopedic, and medically complex conditions.20 The AM-PAC CAT includes three domains (applied cognition, personal care and instrumental activities, as well as physical and movement activities), established through Rasch analysis, confirmatory factor analysis, and modified parallel analysis.13,18 Two AM-PAC Short Forms (SFs) were used to assess the personal care and instrumental activity and physical and movement activity domains: an 18-item Mobility SF and a 15-item Activity SF generated from the Rasch-modeled AM-PAC item banks.13 The Mobility and Activity SFs yield slightly narrowed score ranges, Mobility 16.2–95.8 and Activity 27.4–100, compared with the AM-PAC-CAT but display similar psychometric properties.21 On both SFs, higher scores indicate better function.
Quality of Life: Functional Assessment of Cancer Therapy-General
The well-validated Functional Assessment of Cancer Therapy-General (FACT-G) includes 27 items rated on five-point (0–4) scales, which are summed to produce a total score.22,23 QOL domains assessed by the FACT-G include physical, social/family, emotional, and functional well-being. Scores range from 0 to 108, with higher scores reflecting better QOL.
Fatigue: FACT-Fatigue Subscale
The FACT-Fatigue subscale (FACT-F) is a valid and reliable measure of fatigue intensity. The scores from its thirteen items are combined to render a total score that ranges from 0 to 52, with higher scores indicating less fatigue.24
Pain and Sleep Quality: Symptom Numeric Rating Scales
Eleven-point numeric rating scales are validated for cancer-related symptom assessment and were used to assess pain and sleep quality, with higher scores indicating greater pain intensity and better sleep quality.25,26
Non-PROs
Adherence
Intervention group participants returned their completed REST and pedometer step-count logs at Weeks 4 and 8. Subjects were considered to be participating adequately if they performed REST four times or more per week, logged step counts four times or more per week, and increased their step counts by 10% or more at four-week or less intervals.
Vital Status
Participant vital status was followed for eight months after the completion of PRO collection via the Mayo Clinic Tumor Registry and EMR.
Statistical Analysis
The selection of the primary endpoint, mobility, was based on our prior finding that patients with Stage IV cancer are most concerned by loss of the ability to get around under their own volition.27 A four-point change on the AM-PAC Mobility SF, twice the minimally important difference,28 was considered a meaningful intergroup difference. AM-PAC Basic Mobility scores between 50 and 75 have an SD of 5.3.28 Therefore, 29 subjects per group provided 0.8 power to detect a four-point difference with an α of 0.05 (two-sided). Anticipating a 15% drop-out rate, 33 individuals were recruited per group.
Descriptive statistics were calculated for baseline variables, with t-tests and Fisher’s exact tests used, as appropriate, to assess group equivalences. Fisher’s exact tests also were used to compare the proportion of deaths and dropouts between the groups. Analyses were based on intention-to-treat in all randomized participants. Wilcoxon rank-sum tests were used to assess intergroup PRO differences at baseline and Week 8, as well as the magnitude of change between baseline and Week 8. Week 8 intergroup comparisons were performed with raw and imputed data. Missing data were imputed for surviving participants, as well as for all participants with missing data for Week 8, using a regression model incorporating baseline covariates (age, gender, cancer type, and baseline PRO values) that properly incorporated patient-specific variability. Differences in Week 8 and baseline AM-PAC Mobility SF scores were calculated, and their association with REST program adherence, demographic variables, and cancer type was examined using univariate linear regression analyses. All statistical analyses were performed with Stata for Windows, version 9.2 (StataCorp LP, College Station, TX).
Results
The CONSORT diagram in Fig. 1 shows the flow of study participants. Ninety-three patients were screened, 76 met the eligibility criteria, and 66 were enrolled. Seven participants (21%) from the REST group and three (9%) from the control group withdrew from the study prior to completion or were lost to follow-up. Neither the proportion of noncompleters (P = 0.55) nor the death of participants (P = 0.23) differed significantly between the groups. Noncompleters had significantly higher baseline levels of pain (P = 0.009) and fatigue (P = 0.002), as well as greater disability as reflected in lower Mobility SF (P = 0.009) and Activity SF (P = 0.02) scores. The groups were well balanced with respect to demographic, cancer type, and treatment characteristics (Table 2).
Fig. 1.
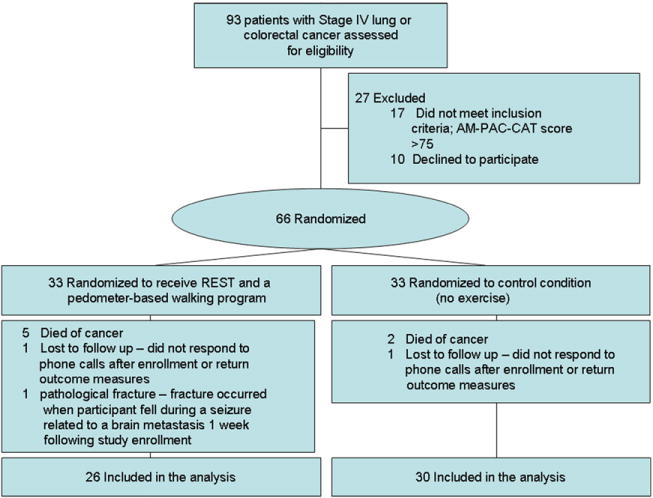
CONSORT diagram.
Table 2.
Study Participants’ Demographic and Cancer Characteristics, and Exercise-Related Attitudes
| Intervention
|
Control
|
||
|---|---|---|---|
| Demographics | n = 33 | n = 33 | P-value |
| Age (yrs), mean (SD) | 63.8 (12.5) | 65.5 (8.9) | 0.52 |
| Male, N (%) | 16 (48.5) | 19 (57.6) | 0.46 |
| Caucasian ethnicity | 33 (100) | 33 (100) | |
| Level of education | 0.48 | ||
| ≤ High school | 5 (15.2) | 4 (12.1) | |
| Some college | 5 (15.2) | 9 (27.3) | |
| ≥ College | 23 (69.7) | 20 (60.3) | |
| Cancer characteristics | |||
| % Colon cancer | 17 (51.5) | 15 (45.5) | 0.62 |
| Receiving radiation at enrollment | 3 (9.1) | 2 (6.1) | 0.64 |
| Receiving radiation at completiona | 1 (3.8) | 2 (6.9) | 0.57 |
| Receiving chemotherapy at enrollment | 15 (45.5) | 14 (42.4) | 0.32 |
| Receiving chemotherapy at completiona | 9 (34.6) | 11 (36.7) | 0.91 |
| Type of chemotherapy | 0.71 | ||
| Biologics | 5 (15.1) | 2 (6.0) | |
| Chemotherapy: single agent | 1 (3.0) | 2 (6.1) | |
| Colorectal cancer chemotherapy combination | 2 (6.1) | 1 (3.0) | |
| Colorectal cancer: bevacizumab based | 7 (21.2) | 7 (21.2) | |
| Lung cancer: platinum based | 1 (3.0) | 1 (3.0) | |
| Lung cancer: bevacizumab based | 1 (3.0) | 1 (3.0) | |
| Otherb | 1 (3.0) | 5 (15.1) | |
| Exercise-related attitudes and beliefs | |||
| Self-efficacy for exercise | 3.2 (1.0) | 3.3 (0.8) | 0.76 |
| Outcome expectations for exercise | 3.8 (0.7) | 4.0 (0.8) | 0.47 |
Percentage based on completers: n = 26 for the intervention group and n = 30 for the control group.
Irnotecan + cetuximab, carboplatin + paclitaxel + radiation, carboplatin + gemcitabine, cisplatin +5 fluorouracil.
Adherence Among the Intervention Group Participants
Twenty (76.9%) of the 26 participants in the intervention group who completed the trial met the requirements for participation. Fig. 2a–c illustrates the participants’ mean weekly step counts, the mean number of days per week that participants logged step counts, and the mean number of REST sessions performed per week, respectively.
Fig. 2.
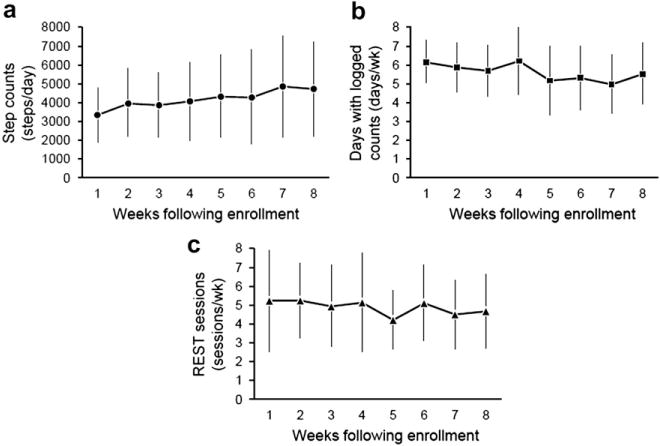
Adherence among the intervention group during the study interval; (a) mean weekly step counts, (b) mean number of days per week that participants logged step counts, and (c) performed REST routines.
Survival
Fig. 3 shows a Kaplan-Meier survival curve for participants in the study groups. Survival did not differ significantly between the groups (hazard ratio of 0.92 for control group, P = 0.75).
Fig. 3.
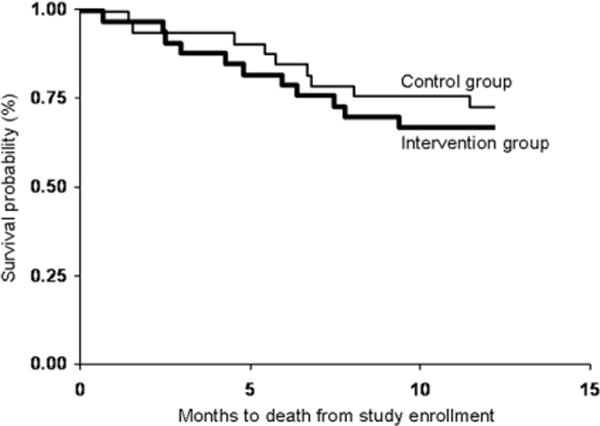
Kaplan-Meier plot illustrating the survival of the study groups up to 12 months of follow-up.
PRO Reports
Fig. 4 demonstrates intergroup differences in mean PRO scores for baseline, Week 8 raw, and Week 8 data imputed from surviving subjects. None of the PROs differed significantly between the groups at baseline. At Week 8, the intervention group had higher Mobility SF (P =0.01) and FACT-F (P =0.02) scores, as well as better sleep quality (P = 0.05) but did not differ significantly in their other PROs. Mean changes between the intervention and control groups in mobility (4.88 ± 4.66 vs. 0.23 ± 5.22, P = 0.002), fatigue (4.46 ± 8.65 vs. −0.79 ±9.11, P =0.03), and sleep quality (1.46 ±1.88 vs. −0.109 ±1.71, P = 0.002) between Week 8 and baseline also significantly differed between the groups (Table 3). All significant findings were robust to imputation of missing data for surviving and deceased participants.
Fig. 4.
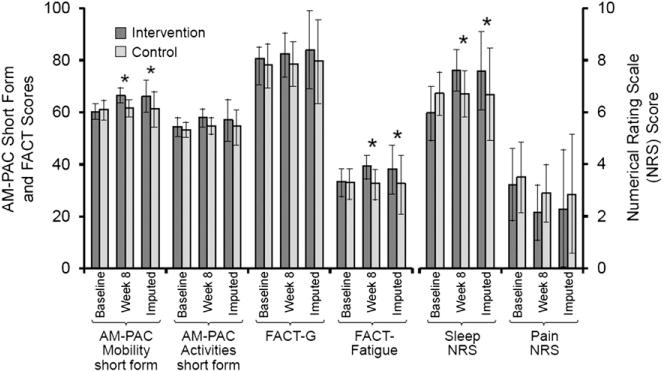
Histogram showing differences in patient-reported outcomes between the study groups at baseline, Week 8 raw data, and Week 8 imputed data among surviving subjects. *P < 0.05.
Table 3.
Mean Change in Patient-Reported Outcome Scores Between Week 8 and Baseline by Study Group
| Intervention (n = 26)
|
Control (n = 29)
|
||||||
|---|---|---|---|---|---|---|---|
| Patient-Reported Outcome Measure | Mean Difference | SD | 95% CI | Mean Difference | SD | 95% CI | P-valuea |
| Mobility Short Form | 4.88 | 4.66 | 2.96, 6.80 | 0.23 | 5.22 | −1.76, 2.22 | 0.002 |
| Activity Short Form | 1.56 | 5.53 | −0.72, 3.84 | 0.94 | 5.91 | −1.26, 3.14 | 0.74 |
| FACT-G | 1.07 | 11.60 | −5.97, 3.83 | 0.12 | 10.22 | −3.19, 4.74 | 0.54 |
| FACT-F | 4.46 | 8.65 | 0.81, 8.11 | −0.79 | 9.11 | −4.26, 2.67 | 0.03 |
| Sleep | 1.46 | 1.88 | 0.70, 2.22 | −0.10 | 1.71 | −0.74, 0.54 | 0.002 |
| Pain | −0.62 | 2.59 | −1.66, 0.43 | −0.50 | 2.01 | −1.25, 0.25 | 0.87 |
FACT-G = Functional Assessment of Cancer Therapy-General; FACT-F = FACT-Fatigue.
Intergroup difference assessed with Wilcoxon rank-sum test.
Associations Between Adherence and Improved Mobility
Among the intervention group participants, cancer type, age, and gender were not associated with the change in Mobility SF score. The total number of REST sessions performed during the study (β coefficient 0.16, P = 0.02) and the number of weeks that participants logged step counts on four or more days (β coefficient 1.07, P = 0.05) were associated with the change in Mobility SF score.
Adverse Events
Although more participants died in the intervention group, this difference was not significant (P = 0.28), and no adverse events occurred during or within hours of performing the REST exercises or the walking program.
Discussion
We report what is, to the best of our knowledge, the first adequately powered trial to assess the benefits of a home-based exercise intervention among patients with Stage IV cancer. Patients with lung and colon cancers were chosen because reports suggest that exercise benefits these populations4,5 and different patterns of metastatic spread (i.e., bone and brain vs. viscera) ensure that these malignancies encompass the disparate patterns of physical decompensation in Stage IV cancer. Our findings seem significant because they show that we were able to enhance the participants’ mobility, improve their sleep quality, and reduce their fatigue with a gentle progressive home program. The intervention’s simplicity, as well as its limited time and travel demands, may facilitate its integration with current care delivery structures.
The positive effects of exercise on fatigue and physical function among patients with late stage cancer have been suggested by a number of small, center-based, exploratory studies. However, these largely single-arm pilot studies lacked the size and methodological rigor to support solid inferences regarding clinical benefit.6,29–32 A variety of other studies have recruited mixed stage cohorts; however, their completion rates and benefits specific to patients with Stage IV disease are difficult to obtain.10,33–35 Only one study examined an exclusively home-based program.7 Although it suggested benefit, conventional intergroup comparisons were not used, making the magnitude of any effect size uncertain. Recently, Oldervoll et al.8 reported on a study of 231 participants with late-stage cancer enrolled in a much more definitive two-arm randomized trial. The twice weekly center-based intervention, although it improved physical functioning, did not improve participants’ fatigue.8 In summary, our findings add certainty to the evidence that exercise can improve the aspects of physical functioning of patients with late-stage cancer’ but raise questions as to the type of interventions that may best alleviate adverse symptoms such as fatigue.36
The trial by Oldervoll et al. noted above deserves further discussion in that, although it involved a similar population and had an impressive sample size, it differed significantly from ours in its center-based structure, objective outcome measures, and different implementation and training schedules. Our study, excepting the initial instructional session by PT, was home-based and encouraged a low, consistent, and gradually incremental level of daily physical exertion that was designed to integrate with participants’ usual activities. In contrast, the intervention used by Oldervoll et al. required two visits per week to a center for relatively long duration (60 minutes) sessions. The interventions, in effect, reflect different training approaches, with the intervention used by Oldervoll et al. being more akin to conventional PT and athletic training patterns. Evaluation of the relative costs and benefits of these approaches, and possible hybrid versions incorporating the most effective aspects of each, is warranted.
Both interventions enhanced physical functioning; however, ours reduced participants’ fatigue, whereas the intervention used by Oldervoll et al. did not. It is unclear whether this important difference stems from the intervention parameters outlined above or perhaps from factors such as the lower baseline mobility and higher fatigue levels of our participants. Because our outcomes were restricted to PROs, in contrast to the objective performance measures used by Oldervoll et al., we cannot ascertain whether a training effect occurred and correlated with changes in fatigue. Whether our intervention came closer to a theoretical “sweet spot,” a point at which training demands are optimally balanced with participants’ conditioning requirements and limited tolerance, is theoretically attractive but purely speculative. The fact that the drop-out rate in our intervention group (6%) was much lower than that of Oldervoll et al.’s 31%, may suggest that the tolerability of the two interventions may have differed in meaningful ways.
Limitations
This study, although adding information about the use of exercise in people with late-stage cancer, has a number of limitations. An obvious concern is the differential attention paid to the groups. Whereas we cannot completely rule out the effects of this possibility, we did make efforts to minimize its effects by limiting our contact with the subjects in the intervention group to only four short phone calls of five minutes or less versus two in their control group counterparts. It is reassuring that all the intervention group’s PROs did not improve, for example, their FACT-G scores were unaffected, and those that did so might reasonably be expected to improve with exercise. Adherence to the exercise behaviors was strongly associated with improved self-reported mobility.
Another concern might be the lack of postintervention follow-up, which makes assessment of the sustainability of the intervention’s benefits impossible. Our choice was dictated by the need to minimize the likelihood of disease progression masking the intervention’s effects, while ensuring an adequate assessment interval. Similarly, our reliance on the use of PROs also could be questioned but was dictated by our desire to minimize respondent burden on a group of patients who, for the most part, lived more than three hours from our center. The lack of objective or clinician-rated measures, however, makes it impossible to ascertain whether a training effect occurred.
Several considerations constrain the generalizeability of our results and indicate a need for further investigation. Study participants were more likely to be of Caucasian ethnicity and better educated than a more general population. It is likely that a more diverse sample would be less adherent.37,38 In fact, Quist et al. reported a low adherence rate of 8.7% in home-based training when included in a combined center- and home-based exercise intervention for patients with inoperable lung cancer.9 Also, the study’s academic cancer center site of recruitment differed from the community settings where the majority of patients receive their cancer care.39 These concerns emphasize the need for a multicenter effectiveness study involving differing treatment venues and a more demographically diverse sample. The higher number of deaths in the intervention group, although likely by chance and not statistically significant, also suggests a need for further study to critically evaluate the intervention’s safety among subgroups at higher risk for adverse outcomes.
Conclusions
A short, focused, home exercise program in association with a progressive pedometer-based walking program appears capable of improving the mobility, sleep quality, and fatigue of patients with Stage IV lung and colorectal cancer.
Acknowledgments
This work was funded by the National Institutes of Health grant KL2 RR024151-01.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Cheville AL, Beck LA, Petersen TL, Marks RS, Gamble GL. The detection and treatment of cancer-related functional problems in an outpatient setting. Support Care Cancer. 2009;17:61–67. doi: 10.1007/s00520-008-0461-x. [DOI] [PubMed] [Google Scholar]
- 2.Guadagnoli E, Mor V. Daily living needs of cancer outpatients. J Community Health. 1991;16:37–47. doi: 10.1007/BF01340467. [DOI] [PubMed] [Google Scholar]
- 3.Keogh JW, Macleod RD. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage. 2012;43:96–110. doi: 10.1016/j.jpainsymman.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Denlinger CS, Engstrom PF. Colorectal cancer survivorship: movement matters. Cancer Prev Res (Phila) 2011;4:502–511. doi: 10.1158/1940-6207.CAPR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2011;72:139–153. doi: 10.1016/j.lungcan.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Adamsen L, Stage M, Laursen J, Rorth M, Quist M. Exercise and relaxation intervention for patients with advanced lung cancer: a qualitative feasibility study. Scand J Med Sci Sports. 2011 doi: 10.1111/j.1600-0838.2011.01323.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Headley JA, Ownby KK, John LD. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004;31:977–983. doi: 10.1188/04.ONF.977-983. [DOI] [PubMed] [Google Scholar]
- 8.Oldervoll LM, Loge JH, Lydersen S, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16:1649–1657. doi: 10.1634/theoncologist.2011-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quist M, Rorth M, Langer S, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. 2012;75:203–208. doi: 10.1016/j.lungcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol. 2006;24:635–642. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 11.Clark MM, Rummans TA, Atherton PJ, et al. Randomized Controlled Trial of Maintaining Quality of Life During Radiation Therapy for Advanced Cancer. Cancer. 2012 [Epub ahead of print] [Google Scholar]
- 12.Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2010;1:CD007130. doi: 10.1002/14651858.CD007130.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jette AM, Haley SM, Tao W, et al. Prospective evaluation of the AM-PAC-CAT in outpatient rehabilitation settings. Phys Ther. 2007;87:385–398. doi: 10.2522/ptj.20060121. [DOI] [PubMed] [Google Scholar]
- 14.Tao W, Haley SM, Coster WJ, Ni P, Jette AM. An exploratory analysis of functional staging using an item response theory approach. Arch Phys Med Rehabil. 2008;89:1046–1053. doi: 10.1016/j.apmr.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss CO, Hoenig HM, Fried LP. Compensatory strategies used by older adults facing mobility disability. Arch Phys Med Rehabil. 2007;88:1217–1220. doi: 10.1016/j.apmr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Borg G. The Borg CR10 scale. In: Borg G, editor. Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998. pp. 39–43. [Google Scholar]
- 17.Coster WJ, Haley SM, Andres PL, et al. Refining the conceptual basis for rehabilitation outcome measurement: personal care and instrumental activities domain. Med Care. 2004;42(Suppl 1):I62–I72. doi: 10.1097/01.mlr.0000103521.84103.21. [DOI] [PubMed] [Google Scholar]
- 18.Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(Suppl 1):I49–I61. doi: 10.1097/01.mlr.0000103520.43902.6c. [DOI] [PubMed] [Google Scholar]
- 19.Siebens H, Andres PL, Pengsheng N, Coster WJ, Haley SM. Measuring physical function in patients with complex medical and postsurgical conditions: a computer adaptive approach. Am J Phys Med Rehabil. 2005;84:741–748. doi: 10.1097/01.phm.0000186274.08468.35. [DOI] [PubMed] [Google Scholar]
- 20.Haley SM, Siebens H, Coster WJ, et al. Computerized adaptive testing for follow-up after discharge from inpatient rehabilitation: I. Activity outcomes. Arch Phys Med Rehabil. 2006;87:1033–1042. doi: 10.1016/j.apmr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Jette AM, Haley SM, Ni P, Moed R. Adaptive short forms for outpatient rehabilitation outcome assessment. Am J Phys Med Rehabil. 2008;87:842–852. doi: 10.1097/PHM.0b013e318186b7ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 23.Victorson D, Barocas J, Song J, Cella D. Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) and its subscales: a reliability generalization. Qual Life Res. 2008;17:1137–1146. doi: 10.1007/s11136-008-9398-2. [DOI] [PubMed] [Google Scholar]
- 24.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 25.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan DR, O’Mara AM, Kelaghan JW, Minasian LM. Quality-of-life assessment in the symptom management trials of the National Cancer Institute-supported Community Clinical Oncology Program. J Clin Oncol. 2005;23:591–598. doi: 10.1200/JCO.2005.12.181. [DOI] [PubMed] [Google Scholar]
- 27.Cheville AL, Troxel AB, Basford JR, Kornblith AB. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J Clin Oncol. 2008;26:2621–2629. doi: 10.1200/JCO.2007.12.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheville AL, Yost K, Larson D, et al. The item response theory-based Ambulatory Post-Acute Care Computer Adaptive Test is responsive to functional decline in patients with late stage lung cancer. Arch Phys Med Rehabil. 2012 doi: 10.1016/j.apmr.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldervoll LM, Loge JH, Paltiel H, et al. Are palliative cancer patients willing and able to participate in a physical exercise program? Palliat Support Care. 2005;3:281–287. doi: 10.1017/s1478951505050443. [DOI] [PubMed] [Google Scholar]
- 30.Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manage. 2006;31:421–430. doi: 10.1016/j.jpainsymman.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Paltiel H, Solvoll E, Loge JH, Kaasa S, Oldervoll L. “The healthy me appears”: palliative cancer patients’ experiences of participation in a physical group exercise program. Palliat Support Care. 2009;7:459–467. doi: 10.1017/S1478951509990460. [DOI] [PubMed] [Google Scholar]
- 32.Temel JS, Greer JA, Goldberg S, et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:595–601. doi: 10.1097/JTO.0b013e31819d18e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keays KS, Harris SR, Lucyshyn JM, MacIntyre DL. Effects of Pilates exercises on shoulder range of motion, pain, mood, and upper-extremity function in women living with breast cancer: a pilot study. Phys Ther. 2008;88:494–510. doi: 10.2522/ptj.20070099. [DOI] [PubMed] [Google Scholar]
- 34.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 35.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 36.Beaton R, Pagdin-Friesen W, Robertson C, et al. Effects of exercise intervention on persons with metastatic cancer: a systematic review. Physiother Can. 2009;61:141–153. doi: 10.3138/physio.61.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan RH, Gordon NF, Chong A, Alter DA. Influence of socioeconomic status on lifestyle behavior modifications among survivors of acute myocardial infarction. Am J Cardiol. 2008;102:1583–1588. doi: 10.1016/j.amjcard.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5:105–116. doi: 10.1080/15412550801941190. [DOI] [PubMed] [Google Scholar]
- 39.National Cancer Institute (NCI) NCI community cancer centers program pilot: 2007–2010. Available from http://ncccp.cancer.gov/Media/FactSheet.htm. Accessed June 2, 2011.


