Abstract
Gastric (GC) and gastroesophageal junction (GEJ) cancers are two global health problems with a relatively high mortality, particularly in the advanced stage. Inhibition of angiogenesis is now contemplated as a classic treatment preference for myriad tumor types encompassing renal cell carcinoma, non-small cell lung cancer, colorectal cancer, glioblastoma, and ovarian cancer, among others. Bevacizumab and ramucirumab have been widely investigated in GC and GEJ cancer, with some controversy about their therapeutic role. Ramucirumab is a monoclonal antibody for vascular endothelial growth factor receptor-2, with demonstrated activity both as a monotherapy and as a part of combination strategy in the management of advanced GC/GEJ cancer. In this review article, we present a critical evaluation of the preclinical and clinical data underlying the use of this drug in this indication. Moreover, we provide a spotlight on the future perspectives in systemic therapy for advanced GC/GEJ cancer.
Keywords: ramucirumab, gastric cancer, gastroesophageal cancer
Video abstract
Introduction
Gastric cancer (GC), in spite of the markedly declining incidence in the past decades, is still regarded as a global health problem, ranking as the fifth most common type of cancer, after malignancies of the lung, breast, colon, and prostate, and the third leading cause of cancer mortality globally, with around 952,000 new cases and 723,000 estimated deaths in 2012.1 Noteworthy, the decline in the incidence of GC was met by an opposing relative rise in the incidence of cancers of the gastroesophageal junction (GEJ) and gastric cardia.2
Given the heterogeneity of GC, several classifications and subclassifications of GC have been postulated based on tumor histology, degree of glandular differentiation, location, and molecular foundation. Histologically, the cardinal variants include three distinct subtypes, namely, proximal nondiffuse GC, distal nondiffuse GC, and diffuse GC.3
Proper staging of GC can be accomplished through an endoscopic ultrasound performed concomitantly with esophagoduodenoscopy. This confers high diagnostic accuracy for various GC stages. A preoperative laparoscopy can also be performed to improve staging diagnosis.4
An authentic preoperative staging of nodal involvement in GC is a must, given the fact that the extent of lymph node involvement is the most important prognostic factor according to the American Joint Committee on Cancer (AJCC) cancer staging manual.5 Nevertheless, nodal sampling is technically rather challenging and the need for modern sentinel lymph node mapping technologies has driven many tests in this terrain.6
Multidetector computer tomography is currently used as the standard procedure for detecting distant metastases.7 However, according to many consensus guidelines, positron emission tomography may be used additionally in GC staging, principally for describing distant spread or lymphatic deposits.8
Current state-of-the-art treatment of GC
Significant progress has been demonstrated in surgical and perioperative treatment modalities. Surgical resection carries the best odds for cure, with 5-year survival of 50%–70%, if paired with an early onset of diagnosis. Nonetheless, the prognosis of GC remains poor. The probability of tumor recurrence exceeds in 50% of patients with initially localized disease, especially in Western countries.9,10
Moreover, most newly diagnosed patients present with advanced and unresectable disease, and around 50% of cases present with synchronous metastasis with a very unfortunate corresponding relative 5-year survival rate of 4%–10%.11,12
Of note, reports from Eastern countries have constantly revealed superior survival in GC patients, perhaps expressing geographical differences as regard epidemiology, pharmacogenomics, and molecular profiling.13,14
Cytotoxic chemotherapy represents the predominant treatment choice in the locally advanced and metastatic cohort of patients; notwithstanding, the median overall survival (OS) for advanced disease still continues to be below 1 year; while for best supportive care, it was 3–5 months.15,16 There is no universal consensus about the standard combination for first-line chemotherapy. However, it is well established that combination chemotherapy (cisplatin/fluoropyrimidine) improves outcomes compared to fluoropyrimidine monotherapy.17
In recent years, the addition of trastuzumab to standard chemotherapy in patients with human epidermal growth factor receptor-2 (HER-2)-positive tumors has dramatically changed the management algorithm in this subset of patients. This has been established after achieving statistically significant increase in OS in the Trastuzumab for Gastric Cancer (ToGA) study (hazard ratio [HR] 0.65, 95% confidence interval [CI] 0.51–0.83), corresponding to median survival of 16 months versus 11.8 months. ToGA study was a randomized controlled trial that compared cisplatin and a fluoropyrimidine combination with the same combination in addition to trastuzumab.18
According to the National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology (ESMO), and other international consensus guidelines, treatment choices for HER-2-negative patients include two- and three-drug regimens in the first-line treatment setting, including 5-fluorouracil and cisplatin, in addition to irinotecan- or docetaxel-based regimens.7,19 By the same token, the evolving prescription of second-line therapies has led to an improved survival in selected patients.20,21
The recent data on the driving mechanisms of tumor progression and the tumor profiling based on molecular basis have led to the recognition of several alternative therapeutic targets. Distinctive spotlight was shed on vascular endothelial growth factor receptor (VEGFR) as a new target. Remarkably enough, VEGFR-2 is overexpressed in GC tissues compared with normal mucosa. VEGFR overexpression was also prominently observed in the case of lymph nodal metastases.22
Efficacy studies of recent trials disclosed progression-free survival (PFS) and OS benefit with the use of the monoclonal antibody (ramucirumab) or the tyrosine kinase inhibitor (apatinib) against the VEGFR-2, as second-line or even third-line therapies.23,24
VEGF pathway and cancer
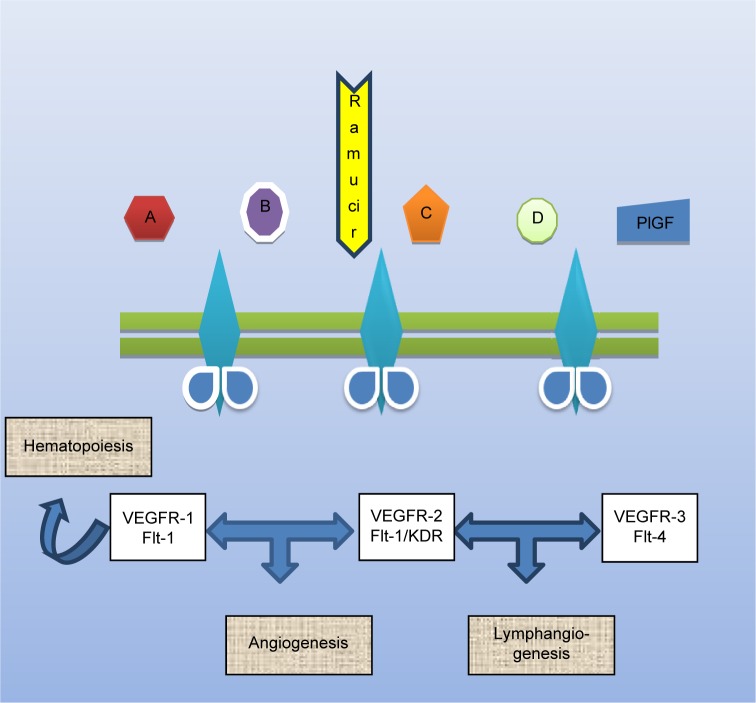
In normal cells, there is a state of harmony between pro-and anti-angiogenic factors. Sustained angiogenesis is a fundamental process for the tumor growth and development and engages the interaction between tumor cells and stromal endothelial cells through multiple growth factors and membranous receptors that finally potentiate pro-angiogenic intracellular signaling pathways, hence providing nutritional supply to dividing cells through the formation of new blood vessels (neovascularization), with subsequent cancer progression and metastasis (Figure 1).25
Figure 1.
Basic mechanism of action of ramucirumab.
Note: A–D refer to different ligands for VEGFRs.
Abbreviations: VEGFR, vascular endothelial growth factor receptor; PIGF, placental growth factor; KDR: kinase insert domain receptor.
Consequently, anti-angiogenesis has become a common therapeutic intervention for many clinical trials that proved improved survival by inhibiting angiogenesis, primarily the vascular endothelial growth factor (VEGF) axis, either by ligand inhibition,26 receptor tyrosine kinase inhibition, or inhibition of intracellular signaling kinases27 or combinations of these strategies. Anti-VEGF therapies have been associated with a survival benefit when these drugs were tested across different malignancies, including renal cell, non-small cell lung cancer, hepatocellular, and colorectal carcinomas.28 Historically, angiogenesis has been studied as a fundamental contributor to diverse physiological processes, such as embryogenesis, normal tissue turnover, and wound healing.29
The mechanism of tumor angiogenesis was first depicted as early as in 1977 as a highly complex multifaceted program. The course of this process starts from the degradation of the basement membrane of a peritumoral capillary, recruitment of stromal cells and endothelial progenitor cells, development of a solid cord, remodeling of the extracellular matrix into an empty capillary with subsequent pro-angiogenic cytokine signaling, and activation of oncogenic signaling cascades.30
VEGF was isolated for the first time in 1989 as a diffusible heparin-binding polypeptide, which targets vascular endothelial cells.31 The VEGF group of molecules includes VEGFA, VEGFB, VEGFC, VEGFD, VEGFE, and placental growth factor. These cytokines were consequently proved to be correlated with the mediation of tumor angiogenesis by interacting with different transmembrane tyrosine kinase receptors, such as VEGFR-1 (fms-like tyrosine kinase 1/Flt-1), VEGFR-2 (Flk-1/KDR), VEGFR-3 (Flt-4), along with two nonenzymatic coreceptors neuropilin-1 and -2 (NRP-1/2). NRP-1 is principally expressed in arterial endothelial cells, whereas NRP-2 exists in venous and lymphatic endothelium.32
VEGFR-1 shows high binding affinity for VEGFA, albeit it also shows weak phosphorylation activity, with a potentially negative modulator role on VEGF signaling. VEGFR-3 is primarily involved in lymphangiogenesis, while VEGFR-2 is by far nominated as the main receptor advocating angiogenesis.33
Logically, various malignancies exhibit upregulation of VEGFR-2 expression in the tumor vasculature.34 The pro-angiogenic downstream effects of VEGFR-2 signaling ultimately endorse the evolvement and maintenance of vastly haphazard and highly permeable neovascular network that promotes tumor growth and metastases.35
Engagement of VEGFs to VEGFR-2 launches receptor dimerization and booms intracellular autophosphorylation of various tyrosine residues with consequently activated pathways.36 Two of the most significant activated pathways are the mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol 3′ kinase (PI3K) pathway, ultimately resulting in enhanced cell survival through AKT/PKB, cell migration, and vascular permeability through the expression of endothelial nitric oxide synthase.37
This compelling value of angiogenesis in the mechanisms of tumor growth, proliferation, and metastasis led to the rapid development and hence the widespread clinical use of angiogenic inhibitors.38 It is also noteworthy to say that credits given to the anti-angiogenic inhibitors are not only attributed to the direct antitumoral effects mediated by the direct blockade of the process of new vessel formation, but also to the indirect antiproliferative effects acquired from the normalization of the haphazard tumor vasculature, which in turn encourages intra-tumor delivery of cytotoxic drugs.39
Pharmacology of ramucirumab
Ramucirumab is a fully humanized monoclonal antibody with a high binding affinity for the extracellular domain of VEGFR-2.40 Preclinical studies showed that targeting of this VEGF family receptor was associated with inhibition of VEGF-mediated signaling, proliferation and migration of human endothelial cells, and antitumor activity in animal models.41–44 Owing to species-specific differences in human VEGFR-2 and murine VEGFR-2 (Fk-1), the development of anti-VEGFR-2 antibodies required the production of immunoglobulins specific to both the human and murine forms of the receptor to specifically block ligand binding.
Pharmacodynamics and pharmacokinetics
A number of Phase I studies have evaluated different pharmacodynamic and pharmacokinetic aspects of ramucirumab; accordingly, they informed the selection of appropriate doses for Phase II and III studies.
In the initial Phase I study of ramucirumab monotherapy in GC, a total of 37 patients were treated with doses ranging from 2 to 16 mg/kg infused on a weekly basis. After one patient developed dose-limiting hypertension and deep venous thrombosis at 16 mg/kg, the next lower dose (13 mg/kg) was considered the maximum tolerated dose. A nonlinear effect of the ramucirumab dose was seen on the clearance rate, suggesting saturation of the clearance mechanism, which was likely to be largely receptor-mediated. However, minimal serum drug accumulation was evident over the course of the study.40
Following the earlier experience of weekly dosing of ramucirumab monotherapy, another Phase I study evaluated biweekly versus triweekly infusion of ramucirumab. A total of 25 patients were treated with ramucirumab: 13 with 6, 8, or 10 mg/kg Q2W and 12 with 15 or 20 mg/kg Q3W. Ramucirumab was well tolerated with no observed dose-limiting toxicities. Pharmacokinetic analyses showed low clearance and half-life of approximately 110–160 hours. Analysis of serum biomarkers also showed significant inter-patient variability with trends toward increased VEGFA and a transient decrease in soluble VEGFR-2. This study was the proof behind the recommended doses of 8 mg/kg every 2 weeks and 10 mg/kg every 3 weeks in further studies.45
In another Phase Ib study of ramucirumab/paclitaxel combination (ramucirumab 8 mg/kg on days 1 and 15 and paclitaxel 80 mg/m2 on days 1, 8, and 15 of a 28-day cycle), six patients were enrolled and all the patients experienced ≥1 treatment-emergent adverse event; five patients experienced grade ≥3 treatment-emergent adverse events. There were two deaths caused by disease progression. The best overall responses were stable disease (n=5) and partial response (n=1). Following a single dose of 8 mg/kg ramucirumab, infused intravenously (IV), clearance was ~0.017 L/hour, half-life (t1/2) was 138–225 hours, and steady-state volume of distribution (Vss) was ~3 L.46
Thus, the available Phase I data for ramucirumab both in monotherapy and in combination with paclitaxel settings suggested tolerability and encouraged further advancement of the drug in Phase II and III settings.
Immunogenicity
An additional characteristic phenomenon that has been observed with ramucirumab treatment is its immunogenicity. Anti-ramucirumab antibodies were detected by enzyme-linked immunosorbent assay in 33 of 443 (7.4%) of patients treated with ramucirumab in clinical trials for whom post-baseline serum samples were available, with neutralizing antibodies detected in one patient only.47 Moreover, anti-ramucirumab antibodies were not associated with infusion-related reactions in the majority of patients who developed antibodies against ramucirumab.
Efficacy, safety, and tolerability studies
Efficacy
The efficacy of ramucirumab in the second-line treatment of advanced gastric/gastroesophageal carcinomas has been proved by two randomized controlled studies (Table 1). The first one is the ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD) study, which was conducted to evaluate whether ramucirumab may improve survival in patients with advanced GC.24 Patients with advanced gastric/GEJ adenocarcinoma after progressing on first-line chemotherapy (n=355) were randomized (2:1) to receive either ramucirumab 8 mg/kg (n=238) or a placebo (n=117), IV once every 2 weeks. In patients in the ramucirumab group, median OS was 5.2 months, while in patients in the placebo group it was 3.8 months (P=0.047).
Table 1.
Summary of RCT addressing the role of ramucirumab in the treatment of advanced GC/GEJ
| Study | Study type | Assessment tools | Study end points | Number of patients (safety population)
|
Indication | OS (months) | PFS (months) | ORR (%) | Incidence of serious AEs |
|---|---|---|---|---|---|---|---|---|---|
| Treatment regimen | |||||||||
| Fuchs et al24 | Phase III RCT |
RECISTversion 1.1 | Primary: OS Secondary: PFS, 12-week PFS, ORR, duration of response, quality of life, safety, and ramucirumab immunogenicity |
Arm A: ramucirumab 8 mg/kg plus best supportive care (238 pts) Arm B: placebo plus best supportive care (117 pts) |
Advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy | 5.2 vs 3.8 (P=0.047) | 2.1 vs 1.3 (P<0.0001) | 3% in either arm (P=0.76) | 109 (46.2%) vs 51 (44.4%) |
| Wilke et al49 | Phase III RCT |
RECISTversion 1.1 | Primary: OS Secondary: PFS, ORR, DCR, patient-reported outcomes, immunogenicity, and safety |
Arm A: ramucirumab 8 mg/kg intravenously on days 1 and 15 plus paclitaxel 80 mg/m2 intravenously on days 1, 8, and 15 of a 28-day cycle (330 pts) Arm B: placebo intravenously on days 1 and 15 plus paclitaxel 80 mg/m2 intravenously on days 1, 8, and 15 of a 28-day cycle (335 pts) |
Advanced gastric or GEJ adenocarcinoma, with progression after first-line chemotherapy (platinum plus fluoropyrimidine with or without an anthracycline) | 9.6 vs 7.4 (P=0.017) | 4.4 vs 2.9 (P<0.0001) | 28% vs 16% (P=0.0001) | 159 (48.6%) vs 144 (43.8%) |
| Yoon et al48 | Phase II RCT |
RECISTversion 1.1 | Primary: PFS Secondary: OS, RR, and DCR |
Arm A: ramucirumab plus mFOLFOX6 (82 pts) Arm B: placebo plus mFOLFOX6 (80 pts) |
Front-line therapy for advanced gastric or esophageal adenocarcinoma | 11.7 vs 11.5 (HR =1.08) | 6.4 vs 6.7 (P=0.89) | 45.2% vs 46.4% | 48 (58.5%) vs 32 (40%) |
Abbreviations: AEs, adverse events; DCR, disease control rate; GC, gastric cancer; GEJ, gastroesophageal junction; HR, hazard ratio; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; pts, patients; RCT, randomized controlled trial; RECIST, Response Evaluation Criteria In Solid Tumors; REGARD, REbif vs Glatiramer Acetate in Relapsing MS Disease; RR, response rate.
The second study is the ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW) trial in which patients (n=665) with advanced gastric/GEJ adenocarcinoma in the second-line setting were randomized to paclitaxel alone (80 mg/m2 on days 1, 8, 15) or ramucirumab plus paclitaxel (8 mg/kg IV every 2 weeks) in 4-week cycles indefinitely. For patients receiving ramucirumab plus paclitaxel, median OS was 9.6 months, while it was 7.4 months for those receiving paclitaxel monotherapy (P=0.0169). Median PFS was 4.4 months in the combined treatment group and 2.9 months in the monotherapy group (P<0.0001).49 Thus, the REGARD and the RAINBOW studies have shown that ramucirumab (both alone and in combination with paclitaxel) is an effective new standard for second-line treatment of advanced gastric/GEJ adenocarcinoma.
In accordance with the two studies mentioned earlier, the US Food and Drug Administration (FDA) has approved ramucirumab both as a monotherapy and in combination with paclitaxel as a second-line treatment of advanced gastric/gastroesophageal adenocarcinoma. However, in view of the very high cost of the drug, cost-effectiveness analyses versus other cheaper second-line alternatives such as irinotecan or docetaxel should be conducted.
Safety and tolerability
Generally, the reported side effect profile in ramucirumab-controlled clinical trials in solid tumors follows the traditional class effects of other anti-angiogenic agents including hypertension, proteinuria, stomatitis, gastrointestinal perforation, and fatigue.50–54 The largest experience with safety and tolerability of ramucirumab monotherapy in gastric/gastroesophageal carcinomas was described by the REGARD trial.24 In this study, approximately 10% of patients discontinued ramucirumab treatment due to adverse events compared with 6.0% receiving placebo. Grade 3 or higher adverse events with rates that were higher in the ramucirumab versus placebo arm included hypertension, abdominal pain, fatigue, and hyponatremia. VEGF class risks of special interest to ramucirumab were modestly increased compared to the control group and were generally in line with those seen with other VEGF inhibitors.55–57
Combination chemotherapy with ramucirumab from Phase II trials showed generally no significant unexpected toxicity. While dosing regimens varied between studies with weekly or every 2- to 3-week infusions, toxicity rates seemed independent of dose or frequency of administration. Further Phase III data with combination paclitaxel, docetaxel, or FOLFIRI (in gastric, lung, or colon cancer, respectively) have not revealed any unexpected toxicities.48,49 In the RAINBOW study, grade ≥3 toxicities were higher in the combination arm (ramucirumab/paclitaxel), including neutropenia, leukopenia, hypertension, anemia, fatigue, abdominal pain, and asthenia.49
Comparison of the outcomes of bevacizumab and ramucirumab in the setting of advanced GC
Bevacizumab is a monoclonal antibody that binds VEGFA with subsequent restriction of its interaction with VEGFR-1 and VEGFR-2, thus exerting an anti-angiogenic impact. Bevacizumab was investigated in the setting of unresectable locally advanced or metastatic GC or GEJ adenocarcinomas in combination with cisplatin and capecitabine as a first-line therapy in a global randomized, placebo-controlled Phase III trial (the Avastin for Advanced Gastric Cancer trial [AVAGAST]).58
Unfortunately, unsatisfactory primary end point results were obtained, namely, nonstatistically significant OS benefit (12.1 vs 10.1 months; HR =0.87, 95% CI, 0.73–1.03, P=0.1002), coupled with statistically significant improved PFS (6.7 months vs 5.3 months; HR =0.80; P=0.0037), and response rate (46% vs 37.4%, P=0.0315). Further data extracted from the complementary Phase III AVATAR study contributed to better interpretation of the AVAGAST results in the right context.59
The inconsistent efficacy outcomes of anti-angiogenesis between bevacizumab in the AVAGAST trial and ramucirumab in the RAINBOW trial was so remarkable that it triggered many explanatory postulations. First of all, ramucirumab in the RAINBOW trial was investigated in the second-line setting, where angiogenesis is acknowledged for its pivotal role in cancer cell survival and proliferation as compared to less advanced disease. Not to mention, in the AVAGAST trial, bevacizumab was prescribed as 2.5 mg/kg/week, a dose inferior to the 5 mg/kg/week dose administered to the subjects in previous Phase II studies.60,61 Second, the impact of the chemotherapeutic agents opted in the design of each study cannot be undermined, especially pointing to the prominently established intrinsic anti-angiogenic activity of paclitaxel, which was solely utilized for the patients in the RAINBOW trial. This anti-angiogenic impact exerted by paclitaxel may be attributed to the restriction of either cell migration or tubule synthesis, along with impedance of the proliferation of the triggered endothelial lining cells.62 As a consequence, the optimacy of platinum analog as the chemotherapeutic backbone, to which anti-angiogenic therapy should be combined, has been recently called into question.63
Future perspectives
Personalized anti-angiogenic treatment of GC
Despite the encouraging results for ramucirumab in second-line treatment of GC, questions still exist on how to improve and better select ramucirumab treatment as well as other anti-angiogenic therapies in GC. As it is quite remarkable to note that the majority of studies involving anti-angiogenic therapies in GC (except ramucirumab) were negative.64–67
This may be explained in the contest that GC is a heterogeneous disease, both biology-wise and genetics-wise. The inadequacy of the AJCC TNM staging algorithm in the prognostic assessment of GC necessitated the application of comprehensive genomic approaches to deepen the understanding of crucial cellular and molecular mechanisms of GC.68
Consequently, various prognostic and predictive biomarkers were explored in many studies, yet their utilization remains uncertain. Independent validation of possible prognostic and predictive biomarkers is a must before they can be routinely employed in clinical practice. It is also important to undertake retrospective studies evaluating the status of the most promising markers in GC samples integrated in specific clinical trials and correlating them with patient outcomes to test this postulation.69–72 Detailed discussion of the candidate biomarkers may be outside the scope of this manuscript.
Ongoing studies of ramucirumab in gastric/gastroesophageal carcinoma
A number of ongoing Phase II and III studies are evaluating different ramucirumab-based combinations in gastric/gastroesophageal carcinoma. The results of these studies are expected within the next 3 years (Table 2).
Table 2.
Ongoing studies of ramucirumab in advanced gastric and gastroesophageal cancers
| ClinicalTrials.gov identifier | Study status | Hypothesis | Study type | Estimated enrollment | Estimated completion date | Treatment regimen | Current primary outcome measures | Current secondary outcome measures |
|---|---|---|---|---|---|---|---|---|
| NCT01983878 | Ongoing but not recruiting participants | A study of ramucirumab in treating Japanese participants with metastatic gastric or gastroesophageal junction cancer, following disease progression on first-line platinum-containing or -containing combination therapy in Japanese patients | Phase II interventional, single arm, open-label trial | 33 Japanese participants | September 2015 | Ramucirumab 8 mg/kg administered IV once every 2 weeks | PFS rate at 12 weeks | PFS (estimated up to 12 months) ORR DCR OS Number of participants with anti-ramucirumab antibodies |
| NCT02314117 | Currently recruiting participants | To evaluate the effectiveness of ramucirumab in combination with capecitabine and cisplatin compared to capecitabine and cisplatin alone as first-line therapy in patients with metastatic gastric or gastroesophageal junction adenocarcinoma (RAINFALL) |
Randomized, double- blind, placebo-controlled phase III study | 616 | April 2018 | 8 mg/kg ramucirumab given IV on days 1 and 8 in combination with 80 mg/m2 cisplatin given IV on day 1 of each 21-day cycle (for up to 6 cycles) and 1,000 mg/m2 capecitabine given orally twice a day on days 1 through 14. Participants who are unable to take capecitabine will be given 800 mg/m2/day 5-fluorouracil (5-FU) IV on days 1–5 of each 21-day cycle |
PFS | OS ORR DCR TTP DoR Time to deterioration in ECOG PS QoL Number of participants with anti-ramucirumab antibodies |
| NCT02082210 | Currently recruiting participants | Part A: dose-ranging study Part B: safety/efficacy study to find a recommended schedule and dose range for LY2875358 when given with ramucirumab that may be safely given to participants with advanced and/or metastatic cancer of any of the following types: gastric adenocarcinoma, gastroesophageal junction adenocarcinoma, hepatocellular cancer, renal cell carcinoma, or non-small cell lung cancer |
Phase 1b/II doseescalating and safety open-label study | 70 | December 2015 | Experimental: LY2875358 + ramucirumab (Part A) Part A: dose escalation of LY2875358 administered IV on days 1 and 15 every 28-day cycle in combination with a fixed dose of ramucirumab administered IV on days 1 and 15 of every 28-day cycle Experimental: LY2875358 + ramucirumab (Part B) Part B: recommended LY2875358 dose from Part A to be administered IV on days 1 and 15 of every 28-day cycle in combination with a fixed dose of ramucirumab administered IV on days 1 and 15 of every 28-day cycle |
Part A: number of participants who experienced DLTs Part B: ORR |
DCR PFS Number of participants with anti-ramucirumab and anti-LY2875358 antibodies |
| NCT02317991 | Not yet open for participant recruitment | To determine whether nab-paclitaxel and ramucirumab are effective when used in combination for treating patients with metastatic gastroesophageal cancer who have either progressed or not responded to prior therapy | Phase II, single-arm, open-label trial | 65 | December 2017 | All patients will receive 125 mg/m2 of nab-paclitaxel V on days 1, 8, and 15 of a 28-day cycle (weekly for 3 weeks, with 1 week of rest) Patients will receive ramucirumab 8 mg/kg IV in combination with nab-paclitaxel on days 1 and 15 of the 28-day cycle |
PFS | ORR OS TTP |
Abbreviations: 5-FU, fluorouracil 5-FU; DCR, disease control rate; DLTs, dose-limiting toxicities; DoR, duration of response; ECOG, Eastern Cooperative Oncology Group; IV, intravenously; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PS, performance status; QoL, quality of life; TTP, time to progression.
Conclusion
GC is a global health problem with a relatively high mortality, particularly in the advanced stage. Standard approach to the management of GC incorporates initial staging workup with appropriate laboratory, imaging, and endoscopic investigations. After assigning the patient to the appropriate anatomical stage, treatment recommendations will rely upon whether the disease is localized, locally advanced, or metastatic. For nonmetastatic disease, radical surgery is the cornerstone of treatment. This may be accompanied by perioperative chemo-and/or radiotherapy according to the extent of the disease. For metastatic disease, systemic chemotherapy is the backbone of treatment. According to the biological characteristics of the tumor (HER-2-positive or HER-2-negative), this may be accompanied by anti-HER-2 therapies.
Pathological angiogenesis has been considered one of the hallmarks of cancer and one of the fundamental pathological processes of almost all solid tumors. VEGF pathway has long been considered one of the principal pathways of both physiological and pathological angiogenesis. Accordingly, targeting VEGF pathway components has been considered an attractive therapeutic intervention for most solid tumors. This may be conducted either by targeting the VEGF itself or its receptors. VEGFR-2 has been found to be overexpressed by many solid tumors, and thus an enthusiastic research program has been launched to establish anti-VEGFR 2 treatments in these tumors. Ramucirumab, a monoclonal antibody against VEGFR-2, has been shown to have a significant antitumor activity against GC in preclinical and clinical studies. These efforts have been culminated in the publication of two randomized controlled studies of ramucirumab monotherapy and ramucirumab/paclitaxel combination in second-line treatment of advanced GC (REGARD and RAINBOW studies, respectively). These two studies have shown a statistically significant benefit for ramucirumab-based treatment versus control in the second-line treatment of advanced gastric/GEJ adenocarcinoma. Accordingly, both ramucirumab monotherapy and ramucirumab/paclitaxel combination have been approved by FDA in the second-line setting of advanced gastric/GEJ adenocarcinoma.
The toxicity profile of ramucirumab in these studies has been consistent with class effects from other VEGF pathway-targeted agents. This includes risks of hypertension, gastrointestinal perforation, proteinuria, and neutropenia.
There is a considerable room for improving and personalizing ramucirumab treatment in advanced GC. This includes advancing the use of ramucirumab into first-line setting, utilizing newer ramucirumab-based combinations that may have extra advantages in terms of efficacy or toxicity, and using molecular biomarkers for improved selection of patients for ramucirumab treatment.
Finally, fine-tuning of the management of advanced gastric/gastroesophageal carcinoma is a challenging job. Appropriate incorporation of preclinical and clinical models into ongoing clinical trials may lead to improved results and impressive outcomes.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [webpage on the internet] Lyon: International Agency for Research on Cancer; 2013. [Accessed March 19, 2015]. Available from: http://globocan.iarc.fr/Default.aspx. [Google Scholar]
- 2.Forman D, Burley V. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–649. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Rahman O. Targeting vascular endothelial growth factor (VEGF) pathway in gastric cancer: preclinical and clinical aspects. Crit Rev Oncol/Hematol. 2015;93(1):18–27. doi: 10.1016/j.critrevonc.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Mihaljevic AL, Friess H, Schuhmacher C, et al. Clinical trials in gastric cancer and the future. J Surg Oncol. 2013;107(3):289–297. doi: 10.1002/Jso.23120. [DOI] [PubMed] [Google Scholar]
- 5.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31(29):3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network . NCCN guidelines for gastric cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2015. [Accessed March 19, 2015]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 8.Hopkins S, Yang GY. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol. 2011;2(1):39–44. doi: 10.3978/j.issn.2078-6891.2010.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 10.Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 13.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 15.Cervantes A, Roda D, Tarazona N, Roselló S, Pérez-Fidalgo JA. Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev. 2013;39(1):60–67. doi: 10.1016/j.ctrv.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Oba K, Paoletti X, Bang YJ, et al. GASTRIC Group Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer. 2013;49(7):1565–1577. doi: 10.1016/j.ejca.012.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;(3):CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 19.ESMO guidelines working group European Society of Medical Oncology (ESMO) guidelines for Gastric Cancer. 2015. [Accessed March 2015]. Available from: http://www.esmo.org/Guidelines-Practice/Clinical-Practice-Guidelines/Gastrointestinal-Cancers/Gastric-Cancer.
- 20.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Kim HJ, Kim SY, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol. 2013;24:2850–2854. doi: 10.1093/annonc/mdt351. [DOI] [PubMed] [Google Scholar]
- 22.Han FH, Li HM, Zheng DH, He YL, Zhan WH. The effect of the expression of vascular endothelial growth factor (VEGF)-C and VEGF receptor-3 on the clinical outcome in patients with gastric carcinoma. Eur J Surg Oncol. 2010;36:1172–1179. doi: 10.1016/j.ejso.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Qin S, Xu J, et al. Apatinib for chemotherapy refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel arm, phase II trial. J Clin Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastrooesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Rahman O. Targeting vascular endothelial growth factor (VEGF) pathway in iodine-refractory differentiated thyroid carcinoma (DTC): from bench to bedside. Crit Rev Oncol/Hematol. 2014;94(1):45–54. doi: 10.1016/j.critrevonc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Ellis L, Hicklin D. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 28.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgos H. Angiogenic and growth factors in human amnio-chorion and placenta. Eur J Clin Invest. 1983;13:289–296. doi: 10.1111/j.1365-2362.1983.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 30.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Rahman O. Vascular endothelial growth factor (VEGF) pathway and neuroendocrine neoplasms (NENs): prognostic and therapeutic considerations. Tumor Biol. 2014;35(11):10615–10625. doi: 10.1007/s13277-014-2612-7. [DOI] [PubMed] [Google Scholar]
- 34.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer. Eur J Clin Invest. 2007;37(11):878–886. doi: 10.1111/j.1365-2362.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 35.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 36.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12(17):5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 38.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 39.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 40.Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu D, Shen J, Vil MD, et al. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem. 2003;278:43496–43507. doi: 10.1074/jbc.M307742200. [DOI] [PubMed] [Google Scholar]
- 42.Miao HQ, Hu K, Jimenez X, et al. Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochem Biophys Res Commun. 2006;345:438–445. doi: 10.1016/j.bbrc.2006.04.119. [DOI] [PubMed] [Google Scholar]
- 43.Jung YD, Mansfield PF, Akagi M, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133–1140. doi: 10.1016/s0959-8049(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Z, Hattori K, Zhang H, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17:604–611. doi: 10.1038/sj.leu.2402831. [DOI] [PubMed] [Google Scholar]
- 45.Chiorean EG, Hurwitz HI, Cohen RB, et al. Phase I study of every 2-or 3-week dosing of ramucirumab, a human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2 in patients with advanced solid tumors. Ann Oncol. 2015;26(6):1230–1237. doi: 10.1093/annonc/mdv144. [DOI] [PubMed] [Google Scholar]
- 46.Ueda S, Satoh T, Gotoh M, Gao L, Doi T. A phase Ib study of safety and pharmacokinetics of ramucirumab in combination with paclitaxel in patients with advanced gastric adenocarcinomas. Oncologist. 2015;20(5):493–494. doi: 10.1634/theoncologist.2014-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eli Lilly and Company Ramucirumab: Prescribing Information. 2014. Mar 19, 2015. Available form: http://www.accessdatafdagov/drugsatfda_docs/label/2014/125477lblpdf.
- 48.Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): randomized, double-blind, multicenter phase 2 trial. J Clin Oncol. 2014;32:5s. Suppl; abstr 4004. [Google Scholar]
- 49.Wilke H, Muro K, Van Cutsem EV, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Rahman O, Fouad M. Risk of cardiovascular toxicities in patients with solid tumors treated with sunitinib, axitinib, cediranib or regorafenib: an updated systematic review and comparative meta-analysis. Crit Rev Oncol/Hematol. 2014;92(3):194–207. doi: 10.1016/j.critrevonc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Qi WX, Sun YJ, Tang LN, Shen Z, Yao Y. Risk of gastrointestinal perforation in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Crit Rev Oncol/Hematol. 2014;89(3):394–403. doi: 10.1016/j.critrevonc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Rahman O, Fouad M. Risk of cardiovascular toxicities in patients with solid tumors treated with sorafenib: an updated systematic review and meta-analysis. Future Oncol. 2014;10(12):1981–1992. doi: 10.2217/fon.14.42. [DOI] [PubMed] [Google Scholar]
- 53.Santoni M, Conti A, Massari F, et al. Treatment-related fatigue with sorafenib, sunitinib and pazopanib in patients with advanced solid tumors: an up-to-date review and meta-analysis of clinical trials. Int J Cancer. 2015;136(1):1–10. doi: 10.1002/ijc.28715. [DOI] [PubMed] [Google Scholar]
- 54.Abdel-Rahman O, Fouad M. Risk of mucocutaneous toxicities in patients with solid tumors treated with sunitinib: a critical review and meta-analysis. Expert Rev Anticancer Ther. 2015;15(1):129–141. doi: 10.1586/14737140.2015.985660. [DOI] [PubMed] [Google Scholar]
- 55.Full Prescribing Information: Stivarga. [Accessed April 10, 2013]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203085lbl.pdf.
- 56.Full Prescribing Information: Avastin. [Accessed April 10, 2013]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125085s267lbl.pdf.
- 57.Full Prescribing Information: Zaltrap. [Accessed April 10, 2013]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125418s000lbl.pdf.
- 58.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 59.Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study) Gastric Cancer. 2015;18(1):168–176. doi: 10.1007/s10120-014-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24(33):5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 61.Uronis HE, Bendell JC, Altomare I, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab in the treatment of metastatic esophagogastric adenocarcinomas. Oncologist. 2013;18(3):271–272. doi: 10.1634/theoncologist.2012-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasquier E, Honore S, Pourroy B, et al. Antiangiogenic concentrations of paclitaxel induce an increase in microtubule dynamics in endothelial cells but not in cancer cells. Cancer Res. 2005;65(6):2433–2440. doi: 10.1158/0008-5472.CAN-04-2624. [DOI] [PubMed] [Google Scholar]
- 63.De Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 64.Moehler M, Mueller A, Hartmann JT, et al. An open-label, multicentre biomarker-oriented AIO phase II trial of sunitinib for patients with chemo-refractory advanced gastric cancer. Eur J Cancer. 2011;47:1511–1520. doi: 10.1016/j.ejca.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Bang YJ, Kang YK, Kang WK, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449–1458. doi: 10.1007/s10637-010-9438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun W, Powell M, O’Dwyer PJ, Catalano P, Ansari RH, Benson AB., 3rd Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947–2951. doi: 10.1200/JCO.2009.27.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satoh T, Yamada Y, Muro K, et al. Phase I study of cediranib in combination with cisplatin plus fluoropyrimidine (S-1 or capecitabine) in Japanese patients with previously untreated advanced gastric cancer. Cancer Chemother Pharmacol. 2012;69:439–446. doi: 10.1007/s00280-011-1723-8. [DOI] [PubMed] [Google Scholar]
- 68.Potts SJ, Huff SE, Lange H, et al. Tissue pattern recognition error rates and tumor heterogeneity in gastric cancer. Appl Immunohistochem Mol Morphol. 2013;21(1):21–30. doi: 10.1097/PAI.0b013e31825552a3. [DOI] [PubMed] [Google Scholar]
- 69.Durães C, Almeida GM, Seruca R, et al. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014;464(3):367–378. doi: 10.1007/s00428-013-1533-y. [DOI] [PubMed] [Google Scholar]
- 70.Abdel-Rahman O. Targeting the hepatocyte growth factor/mesenchymal epithelial transition pathway in gastric cancer: biological rationale and clinical applications. Expert Rev Anticancer Ther. 2015;15(2):235–245. doi: 10.1586/14737140.2014.974564. [DOI] [PubMed] [Google Scholar]
- 71.Abdel-Rahman O. Insulin-like growth factor pathway aberrations and gastric cancer; evaluation of prognostic significance and assessment of therapeutic potentials. Med Oncol. 2015;32(1):431. doi: 10.1007/s12032-014-0431-8. [DOI] [PubMed] [Google Scholar]
- 72.Abdel-Rahman O. Hedgehog pathway aberrations and gastric cancer; evaluation of prognostic impact and exploration of therapeutic potentials. Tumor Biol. 2015;36(3):1367–1374. doi: 10.1007/s13277-015-3216-6. [DOI] [PubMed] [Google Scholar]