Abstract
The normal heart beat intervals are neither strictly stationary nor completely random, and continuously shift from one period to another. Decoding the ECG identifies this “hidden” information that imparts inherent complexity to the heart-beating interval time series. Loss of this complexity in cardiovascular disease is manifested as a reduction in heart rate variability (HRV) and this reduction correlates with an increase in both morbidity and mortality. Because HRV measurements are noninvasive and easy to perform, they have emerged as an important tool in cardiology. However, the identities of specific mechanisms that underline the changes in HRV that occur in cardiovascular diseases remain largely unknown. Changes in HRV have mainly been interpreted on a neural basis, ie due to changes in autonomic impulses to the heart: sympathetic activity decreases both the average heart beat interval and HRV, and parasympathetic activity increases both. It has now become clear, however, that the heart rate and HRV are also determined by intrinsic properties of the pacemaker cells that comprise the sinoatrial node, and the responses of these properties to autonomic receptor stimulation. Here we review how changes in the properties of coupled-clock mechanisms intrinsic to pacemaker cells that comprise the sinoatrial node and their impaired response to autonomic receptor stimulation are implicated in the changes of HRV observed in heart diseases.
Keywords: Cardiac denervation, Coupled-clock pacemaker system, Fractal-like behavior of the heart rate
Introduction
The normal heart beat intervals are neither strictly stationary nor completely random (ie, chaotic), and continuously shift from one period to another. This chaotic heart rhythm is due to nonlinear oscillators interacting together in a complex dynamic.1 Decoding the ECG identifies this “hidden” information that imparts inherent complexity to the heart beating interval time series by revealing the existence of fractal-like dynamic behaviors that operate over multiple time scales. Loss of this complexity in cardiovascular disease is manifested as a reduction in heart rate variability (HRV), and this reduction correlates with an increase in both morbidity and mortality (review in2). Because HRV measurements are noninvasive and easy to perform, they have emerged as an important tool in cardiology. However, the identities of specific mechanisms that underline the changes in HRV that occur in cardiovascular diseases remain largely unknown.
Changes in HRV have mainly been interpreted on a neural basis, due to modification of autonomic impulses to the heart: sympathetic activity decreases both the average heart beat interval and HRV, and parasympathetic activity increases both (Fig. 1A). Therefore, an imbalance of flux between the two arms of the autonomic system in the presence of cardiovascular disease has been thought to be the basis of changes in both the heart beat interval and HRV. The sinoatrial node (SAN) is the tissue area within the heart from which the cardiac impulse originates. Because parasympathetic and sympathetic nerves discharge neurotransmitters that bind to β-adrenergic or cholinergic receptors of pacemaker cells within the SAN tissue and modulate the heart rate and rhythm, the readout of HRV is a direct output of pacemaker cell function. Specifically, experimental evidence3–6 has indicated that the graded changes in the rate of action potential firing by the SAN are nonlinear functions of the graded autonomic receptor stimulation, indicating that intrinsic properties of pacemaker cells within the sinoatrial node are not only determinants of heart rate, but also are important determinants of HRV (for extensive review see7). Cardiac control by autonomic neural impulses is much more complex than only sympathetic and parasympathetic nerve stimulations. For example, in the recent years the complex role of intrinsic cardiac ganglia in maintaining the adequate cardiac output has been revealed.8, 9 The average number of ganglia per porcine is in the range of 350, and the majority of which are distributed in the atria with a smaller part is located in the ventricular. Moreover, it was shown recently that this cardiac ganglia can directly affect SAN function.10 In this paper we describe the basic methods for assessment of HRV, discuss novel perspectives on the dynamic of the coupled nonlinear oscillators intrinsic to pacemaker cells residing within the SAN, and how signaling via autonomic receptors on pacemaker cells links neural impulses to the intrinsic pacemaker cell signaling pathways to alter normal automaticity. Finally, we hypothesize and provide evidence how deficits in intrinsic regulatory properties of pacemaker cells during cardiac disease can affect heart rate and HRV.
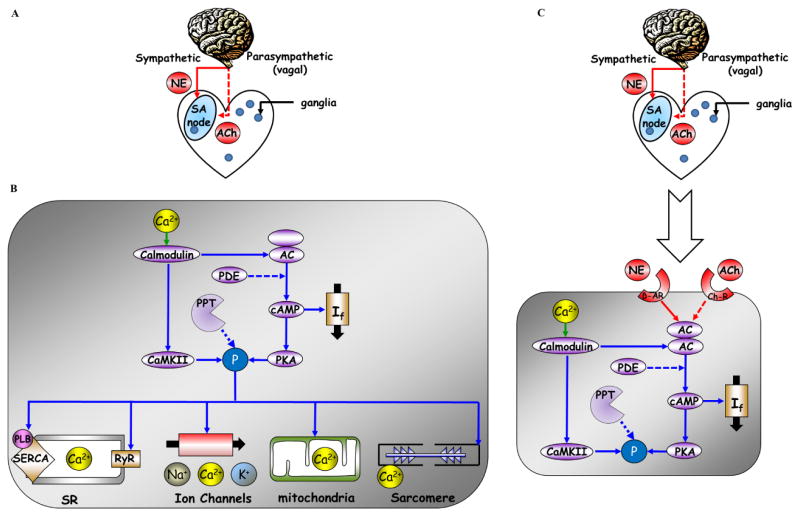
Figure 1. The crosstalk between the autonomic nervous system and the sinoatrial node system.
(A) In vivo, neural impulses via parasympathetic and sympathetic nerves influence the sinoatrial node rate and rhythm. (B) Coupled-clock molecules that drive basal automaticity of SANC: basal Ca2+-calmodulin activation of adenylyl cyclases (AC), which produce cAMP-PKA-dependent phosphorylation signaling. Ca2+-calmodulin activates, in parallel to AC, calmodulin-dependent kinase II (CaMKII) phosphorylation signaling. cAMP positively shifts the f-channel activation curve. Phosphodiesterases (PDE) degrade cAMP production, while protein phosphatase (PPT) degrades phosphorylation activity. PKA and CaMKII phosphorylate sarcoplasmic reticulum (SR) Ca2+ cycling proteins (ryanodine receptor (RyR), phospholamban (PLB), which bind to and inhibit the sarcoplasmic reticulum Ca2+-ATPase (SERCA)), surface membrane ion channel proteins, ATP production mechanisms in the mitochondria and sarcomere. (C) Neurotransmitters discharged by the sympathetic and parasympathetic nerves bind to β-adrenergic or cholinergic receptors, respectively, of pacemaker cells within the sinoatrial node. Autonomic receptor signaling couples to G-proteins and leads to modulation of many of the same coupled-clock molecules that drive basal automaticity of SANC. (modified from Yaniv et al. Cardiovascular Pharmacology: Open Access).
Methods to decode the complexity of heart rate variability
HRV analytic methods are used to quantify the statistical variability of the adjacent intervals in the ECG recordings in vivo, electrical activity in SAN tissue, or action potentials in single pacemaker cells.11 The major methods for quantifying HRV are categorized as time domain, frequency domain, geometric, and nonlinear. Time-series analysis is the simplest means of evaluating HRV to identify variation in beat-to-beat intervals over time: 1) SDNN, standard deviation of all beat intervals; 2) SDANN, standard deviation of 5-minute average beat intervals; 3) RMSSD, square root of the mean of the squares of successive beat intervals; 4) pNN50, the percentage of intervals with 50 ms or more different from the preceding interval; 5) CV, coefficient of variation of all beat intervals.
Fast Fourier transformation of the beat intervals has traditionally been employed to quantify cyclic fluctuations of the beat intervals in the frequency domain at frequencies lower than the heart rate.12 For this measurement in humans the ECG is usually recorded over periods ranging from 5 min (approximately 256 beats) to 24 hours. For a short ECG recordings (~ 5 min), only two peaks are present in the beating interval power spectrum: a high frequency (HF) peak, which in humans is present in the frequency domain between 0.15 and 0.4 Hz (between 2 and 6 heart cycles); and a low frequency (LF) peak, which in humans is present between 0.04 and 0.15 Hz (between 6 and 25 heart cycles). Two other power spectrum peaks can be defined within longer ECG recordings: ultralow-frequency (ULF), which in humans is present in the segment below 0.0033 Hz (below 300 heart cycles) and very low frequency (VLF), defined in humans as the segment between 0.0033 and 0.04 Hz (between 25 and 300 heart cycles).
Poincaré plot analysis (also called a return map) is a geometrical technique that was first applied by Woo et al.13 to beat intervals from patients with cardiac disease. In a Poincaré plot, each beat interval is visualized as a function of the preceding interval (Fig. 2). A higher scattering around the mean beat interval implies the presence of a “complex” dynamic within the time-series of beating intervals where a low scattering (eg, “cigar shape”) implies an absence of a complex dynamic.
Figure 2. Poincaré plot analysis.
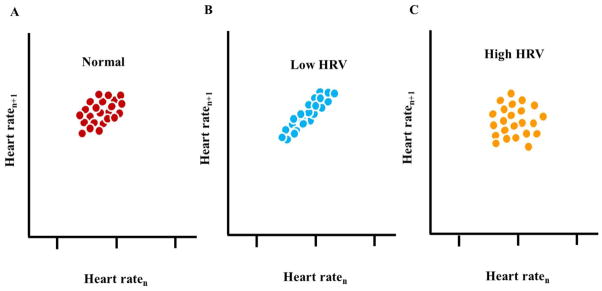
Schematic illustration of each beat interval as a function of the preceding interval. The dots illustrate experimental data.
Considering the nonlinear nature of biological systems, nonlinear analyses are required to fully characterize complex dynamics. For example, differences in the time series of heart-rate intervals lie not only in the distribution of the inter-beat variations around the mean heart rate, but also in their time ordering: different dynamic patterns can occur at different frequency scales that correspond to the actual frequency of an underlying physiological process.14 Although the nature of a specific mechanism that produces this complexity within the heart beat intervals has yet to be discovered, that this complexity has been documented to be reduced in advanced age and in the context of cardiovascular disease may imply the HRV is necessary for healthy heart function. Fractal analysis of the heart beats is a non-linear method to decode the secrets hidden in the heart-beat intervals.15 One method to identify and quantify fractal-like behavior is the power law function slope (Fig. 3). Power spectra that embody fractal-like behavior have a high density of power at low frequencies which exponentially decrease at high frequencies.16 Plotting this exponential relationship in a log-log scale transforms it to a linear function, the slope of which is defined as β. In a log-log plot of healthy mammals, the power law slope in VLF is linear with a negative slope, β~−1. Short- and long-term fractal-scaling exponent quantified by detrended fluctuation analysis (DFA) characterizes the degree of correlation among time scales embedded within the heart beat intervals (Fig. 3).17 The short-term exponent, α1, is a measure of the degree to which the beat intervals are correlated on a scale of 3–11 beats, and the long-term exponent, α2, is a measure of the degree to which the beat intervals are correlated on a scale of 12–20 beats. Note that if β or α is less than 0.5, the system dynamic is described as white noise (ie, a random signal with a constant power spectral), and if β or α equals 1.5, the system dynamic is described as Brownian noise (ie, an integral of white noise that has more energy at lower frequencies).
Figure 3. Analytic methods of fractal-like behavior.
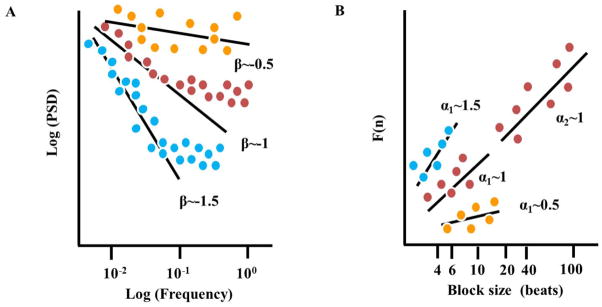
Schematic illustration of (A) the linear function derived from a log-log plot (slope β) of the power spectral analysis (PSD, calculated from Fourier analysis of heart rate intervals) vs. frequency, (B) the short and long-term linear relationship (slope α1 and α2, respectively) of the amplitude of detrended fluctuations (F(n)), calculated from heart rate intervals vs. the block size n (in beats), on a log-log scale. The dots illustrate experimental data. Increase in the slope implies decrease in signal complexity while increase in the slope implies a random signal.
Assessment of approximate entropy is another nonlinear method to assess HRV, and it is used to quantify the regularity and complexity of time-series data. Larger values of entropy correspond to greater apparent randomness or irregularly, whereas smaller values correspond to instances of recognizable (ie, Brownian Noise) within patterns in the data. That the level of approximate entropy is lower than apparent randomness, but higher than recognizable patterns, may imply that the healthy heart operates with optimum entropy efficiency.
Increased HRV can predict the risk assessment among patients with heart disease
The combination of functional or structural indices such as the left ventricular ejection fraction with indices derived from HRV analysis has been proposed as a useful and reliable method for risk assessment among patients with heart disease.18 In a random effects meta-analysis, a low SDNN was associated with an increase of a first cardiovascular event in populations without known cardiovascular disease.19 Moreover, patients with a high cardiovascular risk profile have lower time-domain HRV indices, and a reduced LF power and LF:HF ratio20. Finally, entropy generally decreases with cardiac vascular disease risk progression.21 Identification of the mechanisms for changes in HRV will enable the design of specific novel treatments for different cardiac diseases. Evidence from in vivo and ex vivo experimental studies suggests that HRV is not only dependent upon the autonomic nervous system impulse balance, but may also depend on intrinsic mechanisms of SAN tissue: 1) even in the presence of complete denervation, the fractal-like behavior of the heart in vivo is still present22, although the fractal-like slope (β) is reduced; 2) in isolated hearts (ie, when the heart is completely detached from both hormonal and neural input), there is a non-linear relationship between the HRV and heart beating rate in numerous species;23 3) fractal-like behavior is present in monolayers cultured cells with pacemaker-like activity.24
Control mechanisms of heart rate and rhythm in pacemaker cells
The SAN is an anatomically and electrophysiologically heterogeneous structure; its pacemaker cells express a unique set of ion channels and Ca2+ proteins necessary for the generation and propagation of electrical activity in the heart. Recent evidence (review in25) shows that the spontaneous beating of pacemaker cells isolated from the SAN, even in the absence of receptor stimulation by neuronal input, is driven by Ca2+ activation of calmodulin-adenylyl cyclase (AC)-dependent protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) which effect phosphorylation of proteins that drive biophysical mechanisms leading to coupling of clocks within pacemaker cells (Fig. 1B):26 the sarcoplasmic reticulum (SR) is a Ca2+ clock that spontaneously generates chaotic local diastolic Ca2+ releases (LCRs) that activate an inward Na+-Ca2+ exchanger current, which accelerates the rate of diastolic membrane depolarization. Na+-Ca2+ exchange current and the f-channel current, another member of the ensemble of sarcolemmal electrogenic molecules (“membrane clock”), concurrently drive the diastolic membrane depolarization to ignite the next action potential (AP). This spontaneous diastolic phase is a unique feature of the pacemaker cells that is also functional in other peacemaking tissue of the heart (ie atrioventricular node, bundle of His, bundle branches, and His-Purkinje system). During late diastolic depolarization L-type channels are activated and Ca2+ influx via these channels induce Ca2+ release from the SR via ryanodine channels. This released Ca2+ binds to calmodulin that activates AC, inactivates L-type channel and modulates the Na+-Ca2+ exchange current, contributing to the Ca2+ transient decay by causing Ca2+ efflux from the cell. A substantial component of Ca2+ influx via the L-type channels is pumped directly into the SR, and replenishes cell Ca2+ load to balance Ca2+ efflux via Na+-Ca2+ exchange. The occurrence of AP-triggered Ca2+ release synchronizes the depleted SR Ca2+ state, that temporarily suspends generation of spontaneous LCRs. Moreover, membrane depolarization during the AP activates K+ channels, which effect AP repolarization.
The rate at which this intrinsic coupled-clock system ticks becomes further accelerated, or reduced, respectively, by adrenergic or cholinergic autonomic receptor stimulation (Fig. 1C). Other neurotransmitters, (eg, serotonin, substance P, and opioid peptides), hormonal influences (eg, epinephrine, atrial-natriuretic peptide, brain-natriuretic peptide), and mechanical factors (eg, atrial stretch and arterial pressure) also modulate the in vivo heart rate. Adrenergic autonomic receptor stimulation of isolated pacemaker cells synchronizes intrinsic clock mechanisms via Ca2+-calmodulin activation of AC/PKA and CaMKII-dependent phosphorylation of Ca2+ and membrane proteins.27 Cholinergic receptor stimulation, in contrast, reduces Ca2+-calmodulin activation of AC/PKA and CaMKII signaling and desynchronizes intrinsic clock mechanisms.28
Variations in the coupled-clock function of pacemaker cells residing in the SAN lead to variation in the rate of action potential firing and can induce scale-invariant dynamics in the cardiac impulse, thus imparting variability in the heart rate and rhythm measured by the ECG in vivo. As evidence, in isolated rabbit SANC, beat-to-beat variations in the spontaneous AP firing rate are directly correlated with average variations in the period of local Ca2+ releases that are controlled by the coupled-clock system period.5 However, it is not known whether or how the pacemaker cells contribute to HRV in vivo (review in 29 and 30). Here we discuss evidence that impaired intrinsic properties of pacemaker cells becomes manifest in an altered heart rate and HRV in the context of heart disease, the main causes of morbidity and mortality in developed countries.31
Change in HRV and fractal-like behavior in cardiac diseases
Heart Failure
Heart failure is a highly prevalent syndrome that affects close to 6 million adults in the US alone, with 600,000 new cases diagnosed each year.32 Patients with chronic heart failure have a higher heart rate, reduced SDNN and RMSDD and reduced power within VLF and LF frequency domains.33 These HRV parameters become further reduced in patients with decompensated chronic heart failure compared to those with stable chronic heart failure.33 It is particularly noteworthy that in heart failure patients there is an increase of the amplitude of LF early stages and a reduction of the LF component at the end stage.34 Furthermore, in heart failure patients the Poincaré plot reveals fewer complex dynamics.13 Finally, it has been observed that with heart failure heart beat regularity is decreased and α1 of the DFA analysis is reduced (ie, fractal-like behavior in heart failure becomes more like Brownian noise).35
HRV analysis has been also found to be effective in predicting mortality risk in heart failure patients. An average SDNN<50 ms for more than four weeks in a row is associated with a three-fold increase in mortality risk.36,37 Moreover, a reduced VLF power is a powerful and independent risk predictor of mortality in these patients.38
Because an increase in sympathetic and a decrease in parasympathetic activity have been documented in heart failure patients,39 it has been assumed that a modification in the autonomic nervous impulse to pacemaker cells is the major reason for changes in HRV. However, changes in intrinsic mechanisms in pacemaker cells have also been documented. In single pacemaker cells (ie, the autonomic nervous system is not intact) from heart failure rabbits the intrinsic beat interval increases compared to the control. Moreover, membrane ionic channel remodeling accompanies heart failure: both If and Iks densities become reduced.40 Finally, in SAN of rabbits with heart failure, the bradycardiac response to acetylcholine is much higher, while the response to sympathertic activity is preserved.41 Zaza et al.4, however, have shown that when the β adrenergic receptor is stimulated, the spontaneous AP firing rate in isolated SANC increases and the HRV becomes reduced. That the relationship between HRV and the beating rate is non-linear indicates that changes in spontaneous beating interval in single isolated SANC are not simply surrogates of autonomic receptor stimulation. Therefore, an increase in sympathetic activity during heart failure not only increases the beating rate in pacemaker cells residing in the SAN tissue, but also likely reduces the HRV. In other terms, changes in the properties of cells that comprise the sinoatrial node are also likely implicated in the reduction of HRV observed in heart failure patients.
Note that catecholamine levels are enhanced in heart failure patients and long-term activation of adrenergic receptor on pacemaker cells augments the severity of symptoms accompanying heart failure.42 The response to this activation is blunted due to desensitization of the β receptors.43 Therefore, the modulation of intrinsic mechanisms in pacemaker cells is also blunted and both components can affect long term changes in HRV.
Myocardial ischemia
Myocardial ischemia is associated with sinus bradycardia (ie, a sinous node beating rate of less than 50 bpm). Myocardial ischemia is present in many cardiac diseases and its early detection is of substantial concern. Compared to patients without ischemia, night time α1 was significantly lower (fractal-like behavior became more like white noise) than during the daytime in patients with postoperative myocardial ischemia.44 Because ischemia impairs heart beat initiation within the sinoatrial node, due to a failure of its resident pacemaker cells,45 changes in the properties of pacemaker cells are also likely implicated in the change of HRV and fractal-like slope in the context of myocardial ischemia. Although changes in If, IKr and ICa,L in single isolated pacemaker cells are not involved in the ischemia-induced increase in beating interval, reductions in ICa,T and INCX substantially contribute to a reduced rate of diastolic depolarization that underlies an increase in the beating interval.
Myocardial infarction
In patients with a previous myocardial infarction, both the time and frequency domain measures of HRV are reduced, but the approximate entropy is significantly higher than in healthy subjects.46 Moreover, compared to healthy subjects, the slope fractal-like behavior within the heart-beat intervals is somewhat steeper in patients who have sustained a myocardial infarction.22 Time and frequency domain HRV analyses indicate that VLF power of HRV is the strongest independent predictor of ventricular tachycardia in patients that had sustained a prior myocardial infraction.47 Interestingly, although different parameters in both time and frequency domains can predict mortality of patients following acute myocardial infraction (review in48), the fractal-like short-term scaling exponent (α1) of the heart-beat interval is a better predictor of death than the traditional measures of heart rate variability.49 Because parasympathetic tone decreases after acute myocardial infarction and the balance of sympathetic to parasympathetic tone increases,50 it has been assumed that modification in the autonomic nervous system impulse is the reason for changes in HRV following an infarction. However, because the number of viable pacemaker cell also decreases following myocardial infarction, their absence can contribute to changes in HRV and increase in entropy observed in vivo. In fact, sick sinus syndrome (see below) following an acute myocardial infarction appears to persist chronically in a high number of these patients.51
Arrhythmia
The occurrence of major arrhythmic events in patients with arrhytmogenic right ventricular cardiomyopathy is associated with a reduced SDNN in the time domain and reduced LF power in the frequency domain.52 Just prior to an arrhythmogenic event the LF/HF ratio increases.53 Because ventricular arrhythmias can induce pH changes and cause reentrant tachycardia, in which the reentry circuit returns to the atrium via accessory AV connections, changes in signaling intrinsic to the sinoatrial node may cause changes in HRV. Moreover, patients with a transplanted heart (ie, the autonomic nervous system is not intact) have respiratory sinus arrhythmia heart rate fluctuations (HRV) similar to those in healthy subjects.54
Brugada Syndrome
Patients with Brugada syndrome have a mutations in the gene encoding cardiac sodium channels and this disease is the major cause of ventricular tachyarrhtmia in young patients.55 In these patients SDNN56 and LF power57 of beating interval are reduced. A lack of sympathetic drive in the presence of sustained parasympathetic, drive together with a reduction in cAMP level (due to a direct feedback from a reduction in Na that decreases the Ca2+ balance in the cell), were documented in patients with Brugada syndrome.55 More experimental data on the changes in characteristics of the SAN function in Brugada syndrome are needed to understand the mechanisms behind the reduction in HRV.
Change in HRV and fractal-like behavior of the heart-beat intervals due to diminished intrinsic coupled-clock mechanisms of pacemaker cells
Sick sinus syndrome
Sick sinus syndrome is associated with abnormal impulse initiation and propagation from the SAN. Patients affected by this disease have sinus bradycardia, sinus pauses or arrest, atrial chronotropic incompetence, and SAN exit block (review in58). Sick sinus node dysfunction occurs most commonly in older patients, but even healthy older patient show higher regularity and altered fractal scaling, consisting of a loss of complex variability.59 In the sick sinus patients, the average spread around the mean interval (quantified by Poincaré plot) is higher than in control patients. 60 Similar results were documented in dogs with sick sinus syndrome.61 Finally, the fractal-like behavior of heart-beat intervals is closer to a white noise in sick sinus patients.60
Genetic manipulation of coupled-clock proteins
Genetic manipulation of protein phosphorylation of intracellular Ca2+ and surface membrane ion channels, (ie, coupled-clock proteins) also affect heart rate and HRV.
Ankyrins are adapter proteins required for targeting channels and transporters to the cytoskeleton in diverse cells. Mutations of ankyrin-B in humans are linked to sick sinus syndrome.62 Mice with mutation in ankyrin-based pathways exhibit, in addition to bradycardia, an increased resting heart interval variability in vivo. Pacemaker cells from mice with ankyrin-B deletion mutations have reduced expression of Na+/Ca2+ exchanger, Na+/K+-ATPase and IP3 receptor compared to the wild-type mice.62 Therefore, a failure of intrinsic coupled-clock mechanisms not only causes bradycardia but also increases HRV observed in vivo.
Genetic manipulations of intracellular Ca2+ and surface membrane ion protein phosphorylation of the coupled-clock system induce change in both heart rate and rhythm. It is notable that mice in which genetic manipulation of HCN let to a reduced binding of cAMP to HCN (ie pacemaker channels) do not have bradycardia, but do develop recurrent sinus pauses. In patients (HCN4-695X mutant carriers) there is no significant difference in HRV, although heart rate variance is slightly higher in mutant carriers than in normal patients.63 Similarly mean heart rate of GIRK4-deficient mice that lack cardiac IKACh does not differ from wild-type, but LF and HF powers are reduced in vivo.64 Ryanodine receptor mutations (R4496C) induced in mice that confers a reduced threshold for ryanodine activation and polymorphic ventricular tachycardia, in response to β-adrenergic stimulation exhibit an increase in heart-beat interval and increased HRV in vivo65. Finally, loss of Cav1.3 (CACNA1D) function in mice is associated with both increased beating interval and HRV.66
Direct pharmacological inhibition of coupled-clock proteins
Pharmacological interventions that impact on intrinsic SANC mechanisms (Fig. 1), eg, intracellular Ca2+ 67, can cause the AP firing rate to become irregular. For example, ivabradine, a new selective If current blocker at the level of SAN, significantly decreases LF:HF ratio in healthy volunteers during tilt and exercise.68 In heart failure patients, ivabradine not only induces a heart rate reduction but also increases indices of heart rate variability.69 It is important to note, however, that due to the crosstalk between the membrane and Ca2+ clocks that regulates pacemaker cell function, whenever either the membrane or Ca2+ clock becomes directly perturbed, the other clock is perturbed indirectly. Therefore, due to clock crosstalk, the steady-state bradycardia associated with different HCN4 mutations, ivabradine or Ca2+ regulatory proteins is likely mediated, only in part by If inhibition, and in part, by changes in the Ca2+ clock.70
Aging
Although aging is not a disease per se, it is a complex process that is characterized by a gradual decline in organ functional reserve.71, 72 Moreover, the prevalence of heart diseases described above increases in advanced age. Reductions in HRV,59 entropy2 and fractal-like slope39 have been documented in advanced age. Although a decrease in catecholamine responsiveness, increased resting levels of sympathetic tone and decreased parasympathetic tone accompany advancing age (review in73), the age-associated decrease in HRV may, in part, be associated with changes in intrinsic intracellular coupled-clock mechanisms that accompany advanced age. In fact, aging is associated with deficient intrinsic cAMP-PKA-Ca2+ signaling of sinatrial node pacemaker cell.74 Therefore, in this case, changes that occur in the properties of the cells in the sinoatrial node likely contribute to age-associated changes in both HRV and fractal-like behavior.
Inflammation
Inflammation is a response to altered homeostasis and occur by releasing different cytokines eg tumor necrosis factor α, interleukin-1,4,6,10 (review in75) from the endocrine. Inflammatory markers, such as cytokines are correlated with increase in mortality and morbidity in patients with cardiac disease,76 and are associated with a reduction in HRV mainly SDNN, VLF power and LF power in patients with coronary artery disease,77 or with heart failure.78 Inflammation increases both sympathetic and parasympathetic activities but also the oxidative stress.75 Therefore, increase in oxidative stress can increase CaMKII activity in sinoatrial node cells,79 and thereby affects intrinsic mechanism of the sinoatrial node.
Therapeutic perspectives
Dietary fish oil
Omega-3 polyunsaturated fatty acids from fish oil decreases heart rate and increases HRV.80 Interestingly, this supplement reduces the heart rate to the same level in patients with and without vagal innervation.81 It was shown recently that If is reduced in rabbits that were fed with fish oil diet, indicating a direct effect on properties intrinsic to pacemaker cells.82 Note, that as mentioned above due to the crosstalk between the membrane and Ca2+ clocks that regulates pacemaker cell function, whenever either the membrane or Ca2+ clock becomes directly perturbed, the other clock is perturbed indirectly. Therefore, due to clock crosstalk, the steady-state bradycardia associated with reduced If current, is likely mediated, only in part by If inhibition, and in part, by changes in the Ca2+ clock.70
Cervical vagal stimulation
In recent years non-pharmacological interventions that can modulate the cardiac autonomic tone have been suggested for the treatment of heart failure (for review see83). Vagal nerve stimulation reduces arrhythmias and increases long-term survival.83 Vagal stimulation increases parasympathetic tone and VLF power and reduces LF:HF power. The left vagus nerve is associated with cardiac contractility while the right vagus nerve is associated with the sinoatrial node function. Therefore, due to vagal stimulation the properties intrinsic to pacemaker cells change. These results, however, need to be confirmed in larger studies.
Chronic exercise improves heart rate complexity in cardiac disease states
Based on the guidelines for secondary prevention through cardiac rehabilitation in patients with documented coronary disease, an increase in physical activity may reduce mortality.84 Following exercise training in coronary disease patients, significant increases in SDNN and the HF power and decreases in the LF:HF are generally observed.85 Note that a decrease in SDNN is associated with increased cardiac mortality (see above). Although exercise training decreases catecholamine levels and β-adrenergic receptor density,85 in heart transplant patients (ie, the nervous system is not intact) increases in both HRV and the HF power during acute exercise occur that are similar to increases in normal subjects.86 Similar results were also obtained with pharmacological inhibition of the nervous system.87 These data suggest that during exercise a non-autonomic mechanism takes the control of HRV.
Summary
In summary, during health and cardiac disease, the HRV is determined by intrinsic properties of sinoatrial node pacemaker cells and their modulation by the competing influences of the two branches of autonomic neural input to the heart. A reduced HRV and power within VLF and LF domains has been observed in patients with heart failure, myocardial ischemia or myocardial infraction. Therefore these patients have reduced complex beating interval dynamics. However, an increased HRV and entropy has been observed in patients with arrhythmias, sinus sick syndrome, or mutations in intracellular Ca2+ or surface membrane ion channel protein. Because all these diseases are associated with increased mortality, it seems that the heart in vivo has an optimum level of heart-beat interval complexity, and reduction in this complexity or an increase in the level of disorder are both associated with increased mortality. Finally, from the details above one can conclude that changes in the intrinsic properties of cells that comprise the sinoatrial node are also likely implicated in the changes of HRV and power-law behavior observed in heart failure, ischemia, myocardial infarction and during occurrence of arrhythmia.
Future directions
Dissection of mechanisms of the HRV at different hierarchical levels (intact heart, denervated heart, isolated sinoatrial node and iolated pacemaker cell) is required for a complete understanding of the mechanisms that regulate HRV in health and induce changes in HRV in the presence of cardiovascular disease. Moreover, an understanding the changes in the intracellular pathways that link autonomic receptors of pacemaker cells to neural impulses from the brain (eg, phosphorylation signaling, receptor densities, ionic channel proteins) will, in our opinion, be the key to identification of novel therapeutic strategies for treatment of heart disease.
Acknowledgments
The work was partially supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Non-standard abbreviations and acronyms
- AC
Adenylyl cyclase
- AP
Action potential
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- DFA
Detrended fluctuation analysis
- HF
High frequency
- HRV
Heart rate variability
- LCR
Local diastolic Ca2+ releases
- LF
Low frequency
- PKA
Protein kinase A
- SAN
Sinoatrial node
- SR
Sarcoplasmic reticulum
- VLF
Very low frequency
- ULF
Ultralow frequency
References
- 1.Lakatta EG, Yaniv Y, Maltsev VA. Minding the gaps that link intrinsic circadian clock within the heart to its intrinsic ultradian pacemaker clocks. Focus on “The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility”. Am J Physiol Cell Physiol. 2013;304(10):C941–944. doi: 10.1152/ajpcell.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa MD, Peng CK, Goldberger AL. Multiscale analysis of heart rate dynamics: entropy and time irreversibility measures. Cardiovasc Eng. 2008;8(2):88–93. doi: 10.1007/s10558-007-9049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocchetti M, Malfatto G, Lombardi F, Zaza A. Role of the input/output relation of sinoatrial myocytes in cholinergic modulation of heart rate variability. J Cardiovasc Electrophysiol. 2000;11(5):522–530. doi: 10.1111/j.1540-8167.2000.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 4.Zaza A, Lombardi F. Autonomic indexes based on the analysis of heart rate variability: a view from the sinus node. Cardiovasc Res. 2001;50(3):434–442. doi: 10.1016/s0008-6363(01)00240-1. [DOI] [PubMed] [Google Scholar]
- 5.Monfredi O, Maltseva LA, Spurgeon HA, Boyett MR, Lakatta EG, Maltsev VA. Beat-to-Beat Variation in Periodicity of Local Calcium Releases Contributes to Intrinsic Variations of Spontaneous Cycle Length in Isolated Single Sinoatrial Node Cells. PLoS One. 2013;8(6):e67247. doi: 10.1371/journal.pone.0067247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monfredi OJ, Maltseva LA, Boyett MR, Lakatta EG, Maltsev VA. Stochastic Beat-To-Beat Variation in Periodicity of Local Calcium Releases Predicts Intrinsic Cycle Length Variability in Single Sinoatrial Node Cells. Biophys J. 2011;100(3):558a. doi: 10.1371/journal.pone.0067247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaioannou VE, Verkerk AO, Amin AS, de Bakker JM. Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr Cardiol Rev. 2013;9(1):82–96. doi: 10.2174/157340313805076359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batulevicius D, Skripka V, Pauziene N, Pauza DH. Topography of the porcine epicardiac nerve plexus as revealed by histochemistry for acetylcholinesterase. Auton Neurosci. 2008;138(1–2):64–75. doi: 10.1016/j.autneu.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Kawashima T. Anatomy of the cardiac nervous system with clinical and comparative morphological implications. Anat Sci Int. 2011;86(1):30–49. doi: 10.1007/s12565-010-0096-0. [DOI] [PubMed] [Google Scholar]
- 10.Zarzoso M, Rysevaite K, Milstein ML, et al. Nerves projecting from the intrinsic cardiac ganglia of the pulmonary veins modulate sinoatrial node pacemaker function. Cardiovasc Res. 2013;99(3):566–575. doi: 10.1093/cvr/cvt081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 13.Woo MA, Stevenson WG, Moser DK, Trelease RB, Harper RM. Patterns of beat-to-beat heart rate variability in advanced heart failure. Am Heart J. 1992;123(3):704–710. doi: 10.1016/0002-8703(92)90510-3. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov P, Rosenblum MG, Peng CK, et al. Scaling and universality in heart rate variability distributions. Physica A. 1998;249:587–593. doi: 10.1016/s0378-4371(97)00522-0. [DOI] [PubMed] [Google Scholar]
- 15.Mandelbort B. The fractal geometry of nature. San Francisco, CA: Freeman; 1983. [Google Scholar]
- 16.Saul JP, Arai Y, Berger RD, Lilly LS, Colucci WS, Cohen RJ. Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am J Cardiol. 1988;61(15):1292–1299. doi: 10.1016/0002-9149(88)91172-1. [DOI] [PubMed] [Google Scholar]
- 17.Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 18.Priori SG, Aliot E, Blomstrom-Lundqvist C, et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J. 2001;22(16):1374–1450. doi: 10.1053/euhj.2001.2824. [DOI] [PubMed] [Google Scholar]
- 19.Hillebrand S, Gast KB, de Mutsert R, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. doi: 10.1093/europace/eus341. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Villegas JF, Lam-Espinosa E, Ramirez-Moreno DF, Calvo-Echeverry PC, Agredo-Rodriguez W. Heart rate variability dynamics for the prognosis of cardiovascular risk. PLoS One. 2011;6(2):e17060. doi: 10.1371/journal.pone.0017060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelinek HF, Md Imam H, Al-Aubaidy H, Khandoker AH. Association of cardiovascular risk using non-linear heart rate variability measures with the framingham risk score in a rural population. Front Physiol. 2013;4:186. doi: 10.3389/fphys.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bigger JT, Jr, Steinman RC, Rolnitzky LM, Fleiss JL, Albrecht P, Cohen RJ. Power law behavior of RR-interval variability in healthy middle-aged persons, patients with recent acute myocardial infarction, and patients with heart transplants. Circulation. 1996;93(12):2142–2151. doi: 10.1161/01.cir.93.12.2142. [DOI] [PubMed] [Google Scholar]
- 23.Monfredi O, Nirmalan M, Zhang H, Boyett MR. Heart rate variability as a measure of autonomic nerve activity is fundamentally flawed. Heart Rhythm. 2013;10(5):S181. [Google Scholar]
- 24.Mandel Y, Weissman A, Schick R, et al. Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation. 2012;125(7):883–893. doi: 10.1161/CIRCULATIONAHA.111.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res. 2010;106(4):659–673. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaniv Y, Sirenko S, Ziman BD, Spurgeon HA, Maltsev VA, Lakatta EG. New evidence for coupled clock regulation of the normal automaticity of sinoatrial nodal pacemaker cells: Bradycardic effects of ivabradine are linked to suppression of intracellular Ca cycling. J Mol Cell Cardiol. 2013;62C:80–89. doi: 10.1016/j.yjmcc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinogradova TM, Lyashkov AE, Zhu W, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98(4):505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 28.Lyashkov AE, Vinogradova TM, Zahanich I, et al. Cholinergic receptor signaling modulates spontaneous firing of sinoatrial nodal cells via integrated effects on PKA-dependent Ca(2+) cycling and I(KACh) Am J Physiol Heart Circ Physiol. 2009;297(3):H949–959. doi: 10.1152/ajpheart.01340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaniv Y, Lyashkov AE, Lakatta EG. The fractal-like complexity of heart rate variability beyond neurotransmitters and autonomic receptors: signaling intrinsic to sinoatrial node pacemaker cells. Cardiovascular Pharmacology: Open Access. 2013;2(3):111. doi: 10.4172/2329-6607.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binah O, Weissman A, Itskovitz-Eldor J, Rosen MR. Integrating beat rate variability: from single cells to hearts. Heart Rhythm. 2013;10(6):928–932. doi: 10.1016/j.hrthm.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization; 2011. [Google Scholar]
- 32.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rydlewska A, Jankowska EA, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Ponikowski P. Changes in autonomic balance in patients with decompensated chronic heart failure. Clin Auton Res. 2011;21(1):47–54. doi: 10.1007/s10286-010-0089-z. [DOI] [PubMed] [Google Scholar]
- 34.Guzzetti S, Cogliati C, Turiel M, Crema C, Lombardi F, Malliani A. Sympathetic predominance followed by functional denervation in the progression of chronic heart failure. Eur Heart J. 1995;16(8):1100–1107. doi: 10.1093/oxfordjournals.eurheartj.a061053. [DOI] [PubMed] [Google Scholar]
- 35.Platisa MM, Gal V. Dependence of heart rate variability on heart period in disease and aging. Physiol Meas. 2006;27(10):989–998. doi: 10.1088/0967-3334/27/10/005. [DOI] [PubMed] [Google Scholar]
- 36.Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110(16):2389–2394. doi: 10.1161/01.CIR.0000139841.42454.78. [DOI] [PubMed] [Google Scholar]
- 37.Bilchick KC, Fetics B, Djoukeng R, et al. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs’ Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure) Am J Cardiol. 2002;90(1):24–28. doi: 10.1016/s0002-9149(02)02380-9. [DOI] [PubMed] [Google Scholar]
- 38.Hadase M, Azuma A, Zen K, et al. Very low frequency power of heart rate variability is a powerful predictor of clinical prognosis in patients with congestive heart failure. Circ J. 2004;68(4):343–347. doi: 10.1253/circj.68.343. [DOI] [PubMed] [Google Scholar]
- 39.Peng CK, Havlin S, Hausdorff JM, Mietus JE, Stanley HE, Goldberger AL. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. J Electrocardiol. 1995;28 (Suppl):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 40.Verkerk AO, Wilders R, Coronel R, Ravesloot JH, Verheijck EE. Ionic remodeling of sinoatrial node cells by heart failure. Circulation. 2003;108(6):760–766. doi: 10.1161/01.CIR.0000083719.51661.B9. [DOI] [PubMed] [Google Scholar]
- 41.Opthof T, Coronel R, Rademaker HM, Vermeulen JT, Wilms-Schopman FJ, Janse MJ. Changes in sinus node function in a rabbit model of heart failure with ventricular arrhythmias and sudden death. Circulation. 2000;101(25):2975–2980. doi: 10.1161/01.cir.101.25.2975. [DOI] [PubMed] [Google Scholar]
- 42.Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: fine-tuning cardiac stimulation. Trends Mol Med. 2007;13(12):503–511. doi: 10.1016/j.molmed.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Santulli G. Adrenal Signaling in Heart Failure: Something More Than a Distant Ship’s Smoke on the Horizon. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.02382. [DOI] [PubMed] [Google Scholar]
- 44.Laitio TT, Huikuri HV, Makikallio TH, et al. The breakdown of fractal heart rate dynamics predicts prolonged postoperative myocardial ischemia. Anesth Analg. 2004;98(5):1239–1244. table of contents. [PubMed] [Google Scholar]
- 45.Du YM, Nathan RD. Ionic basis of ischemia-induced bradycardia in the rabbit sinoatrial node. J Mol Cell Cardiol. 2007;42(2):315–325. doi: 10.1016/j.yjmcc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Makikallio TH, Seppanen T, Niemela M, Airaksinen KE, Tulppo M, Huikuri HV. Abnormalities in beat to beat complexity of heart rate dynamics in patients with a previous myocardial infarction. J Am Coll Cardiol. 1996;28(4):1005–1011. doi: 10.1016/s0735-1097(96)00243-4. [DOI] [PubMed] [Google Scholar]
- 47.Huikuri HV, Koistinen MJ, Yli-Mayry S, et al. Impaired low-frequency oscillations of heart rate in patients with prior acute myocardial infarction and life-threatening arrhythmias. Am J Cardiol. 1995;76(1):56–60. doi: 10.1016/s0002-9149(99)80801-7. [DOI] [PubMed] [Google Scholar]
- 48.Huikuri HV, Stein PK. Clinical application of heart rate variability after acute myocardial infarction. Front Physiol. 2012;3:41. doi: 10.3389/fphys.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huikuri HV, Makikallio TH, Peng CK, Goldberger AL, Hintze U, Moller M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101(1):47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- 50.Basu S, Sinha SK, Shao Q, Ganguly PK, Dhalla NS. Neuropeptide Y modulation of sympathetic activity in myocardial infarction. J Am Coll Cardiol. 1996;27(7):1796–1803. doi: 10.1016/0735-1097(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 51.Simonsen E, Nielsen BL, Nielsen JS. Sinus node dysfunction in acute myocardial infarction. Acta Med Scand. 1980;208(6):463–469. doi: 10.1111/j.0954-6820.1980.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 52.Battipaglia I, Scalone G, Macchione A, et al. Association of heart rate variability with arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ J. 2012;76(3):618–623. doi: 10.1253/circj.cj-11-1052. [DOI] [PubMed] [Google Scholar]
- 53.Postolache G, Oliveira M, Rocha I, Girao PS, Postolache O. New insight into arrhythmia onset using HRV and BPV analysis. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2691–2694. doi: 10.1109/IEMBS.2011.6090739. [DOI] [PubMed] [Google Scholar]
- 54.Hrushesky WJ, Fader D, Schmitt O, Gilbertsen V. The respiratory sinus arrhythmia: a measure of cardiac age. Science. 1984;224(4652):1001–1004. doi: 10.1126/science.6372092. [DOI] [PubMed] [Google Scholar]
- 55.Paul M, Meyborg M, Boknik P, et al. Autonomic dysfunction in patients with Brugada syndrome: further biochemical evidence of altered signaling pathways. Pacing Clin Electrophysiol. 2011;34(9):1147–1153. doi: 10.1111/j.1540-8159.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- 56.Pierre B, Babuty D, Poret P, et al. Abnormal nocturnal heart rate variability and QT dynamics in patients with Brugada syndrome. Pacing Clin Electrophysiol. 2007;30 (Suppl 1):S188–191. doi: 10.1111/j.1540-8159.2007.00635.x. [DOI] [PubMed] [Google Scholar]
- 57.Krittayaphong R, Veerakul G, Nademanee K, Kangkagate C. Heart rate variability in patients with Brugada syndrome in Thailand. Eur Heart J. 2003;24(19):1771–1778. doi: 10.1016/j.ehj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115(14):1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 59.Pikkujamsa SM, Makikallio TH, Sourander LB, et al. Cardiac interbeat interval dynamics from childhood to senescence : comparison of conventional and new measures based on fractals and chaos theory. Circulation. 1999;100(4):393–399. doi: 10.1161/01.cir.100.4.393. [DOI] [PubMed] [Google Scholar]
- 60.Bergfeldt L, Haga Y. Power spectral and Poincare plot characteristics in sinus node dysfunction. J Appl Physiol (1985) 2003;94(6):2217–2224. doi: 10.1152/japplphysiol.01037.2002. [DOI] [PubMed] [Google Scholar]
- 61.Gladuli A, Moise NS, Hemsley SA, Otani NF. Poincare plots and tachograms reveal beat patterning in sick sinus syndrome with supraventricular tachycardia and varying AV nodal block. J Vet Cardiol. 2011;13(1):63–70. doi: 10.1016/j.jvc.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Scouarnec S, Bhasin N, Vieyres C, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105(40):15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schweizer PA, Duhme N, Thomas D, et al. cAMP sensitivity of HCN pacemaker channels determines basal heart rate but is not critical for autonomic rate control. Circ Arrhythm Electrophysiol. 2010;3(5):542–552. doi: 10.1161/CIRCEP.110.949768. [DOI] [PubMed] [Google Scholar]
- 64.Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20(1):103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 65.Neco P, Torrente AG, Mesirca P, et al. Paradoxical effect of increased diastolic Ca(2+) release and decreased sinoatrial node activity in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circulation. 2012;126(4):392–401. doi: 10.1161/CIRCULATIONAHA.111.075382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baig SM, Koschak A, Lieb A, et al. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14(1):77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 67.Yaniv Y, Maltsev VA, Escobar AL, et al. Beat-to-beat Ca(2+)-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm. J Mol Cell Cardiol. 2011;51(6):902–905. doi: 10.1016/j.yjmcc.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joannides R, Moore N, Iacob M, et al. Comparative effects of ivabradine, a selective heart rate-lowering agent, and propranolol on systemic and cardiac haemodynamics at rest and during exercise. Br J Clin Pharmacol. 2006;61(2):127–137. doi: 10.1111/j.1365-2125.2005.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borer JS, Le Heuzey JY. Characterization of the heart rate-lowering action of ivabradine, a selective I(f) current inhibitor. Am J Ther. 2008;15(5):461–473. doi: 10.1097/MJT.0b013e3181758855. [DOI] [PubMed] [Google Scholar]
- 70.Yaniv Y, Lakatta EG. Pacemaker gene mutations, bradycardia, arrhythmias and the coupled clock theory. J Cardiovasc Electrophysiol. 2013;24(12):E28–29. doi: 10.1111/jce.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73(2):413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 72.Yaniv Y, Juhaszova M, Sollott SJ. Age-related changes of myocardial ATP supply and demand mechanisms. Trends Endocrinol Metab. 2013;24(10):495–505. doi: 10.1016/j.tem.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santulli G, Iaccarino G. Pinpointing beta adrenergic receptor in ageing pathophysiology: victim or executioner? Evidence from crime scenes. Immun Ageing. 2013;10(1):10. doi: 10.1186/1742-4933-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sirenko S, Shukla S, Liu J, Lakatta EG. Age associated decrease in intrinsic action potential (AP) firing rate in sinoatrial node cells is linked to deficient intrinsic cAMP-PKA-Ca signaling. Biophys J. 2011;100(3):434a. [Google Scholar]
- 75.Papaioannou V, Pneumatikos I, Maglaveras N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: current strengths and limitations. Front Physiol. 2013;4:174. doi: 10.3389/fphys.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phillips AN, Neaton JD, Cook DG, Grimm RH, Shaper AG. Leukocyte count and risk of major coronary heart disease events. Am J Epidemiol. 1992;136(1):59–70. doi: 10.1093/oxfordjournals.aje.a116421. [DOI] [PubMed] [Google Scholar]
- 77.Hamaad A, Sosin M, Blann AD, Patel J, Lip GY, MacFadyen RJ. Markers of inflammation in acute coronary syndromes: association with increased heart rate and reductions in heart rate variability. Clin Cardiol. 2005;28(12):570–576. doi: 10.1002/clc.4960281207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol. 2001;12(3):294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 79.Erickson JR, Joiner ML, Guan X, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol. 2006;97(8):1127–1130. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 81.Harris WS, Gonzales M, Laney N, Sastre A, Borkon AM. Effects of omega-3 fatty acids on heart rate in cardiac transplant recipients. Am J Cardiol. 2006;98(10):1393–1395. doi: 10.1016/j.amjcard.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 82.Verkerk AO, den Ruijter HM, Bourier J, et al. Dietary fish oil reduces pacemaker current and heart rate in rabbit. Heart Rhythm. 2009;6(10):1485–1492. doi: 10.1016/j.hrthm.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 83.Singh JP, Kandala J, John Camm A. Non-pharmacological modulation of the autonomic tone to treat heart failure. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht436. [DOI] [PubMed] [Google Scholar]
- 84.Corra U, Piepoli MF, Carre F, et al. Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training: key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur Heart J. 2010;31(16):1967–1974. doi: 10.1093/eurheartj/ehq236. [DOI] [PubMed] [Google Scholar]
- 85.Oliveira NL, Ribeiro F, Alves AJ, Teixeira M, Miranda F, Oliveira J. Heart rate variability in myocardial infarction patients: Effects of exercise training. Rev Port Cardiol. 2013;32(9):687–700. doi: 10.1016/j.repc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 86.Bernardi L, Salvucci F, Suardi R, et al. Evidence for an intrinsic mechanism regulating heart rate variability in the transplanted and the intact heart during submaximal dynamic exercise? Cardiovasc Res. 1990;24(12):969–981. doi: 10.1093/cvr/24.12.969. [DOI] [PubMed] [Google Scholar]
- 87.Casadei B, Moon J, Johnston J, Caiazza A, Sleight P. Is respiratory sinus arrhythmia a good index of cardiac vagal tone in exercise? J Appl Physiol (1985) 1996;81(2):556–564. doi: 10.1152/jappl.1996.81.2.556. [DOI] [PubMed] [Google Scholar]