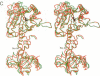Abstract
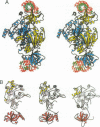
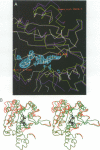
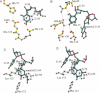
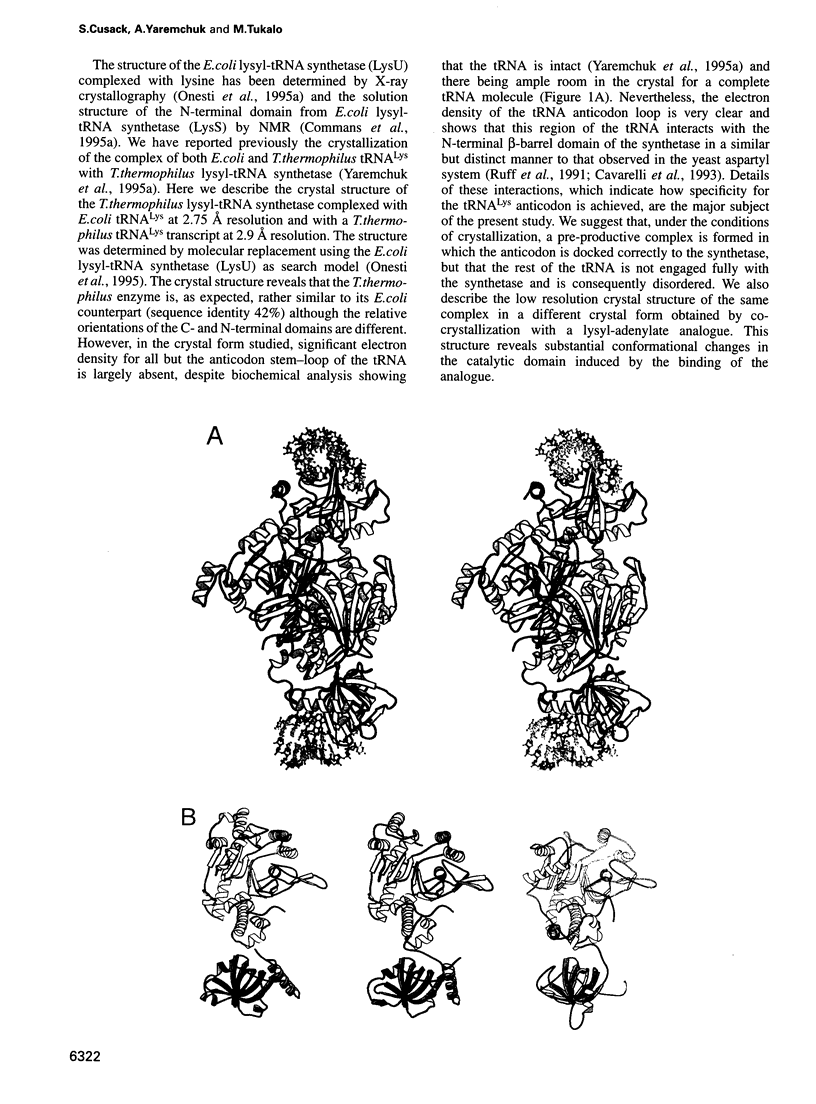
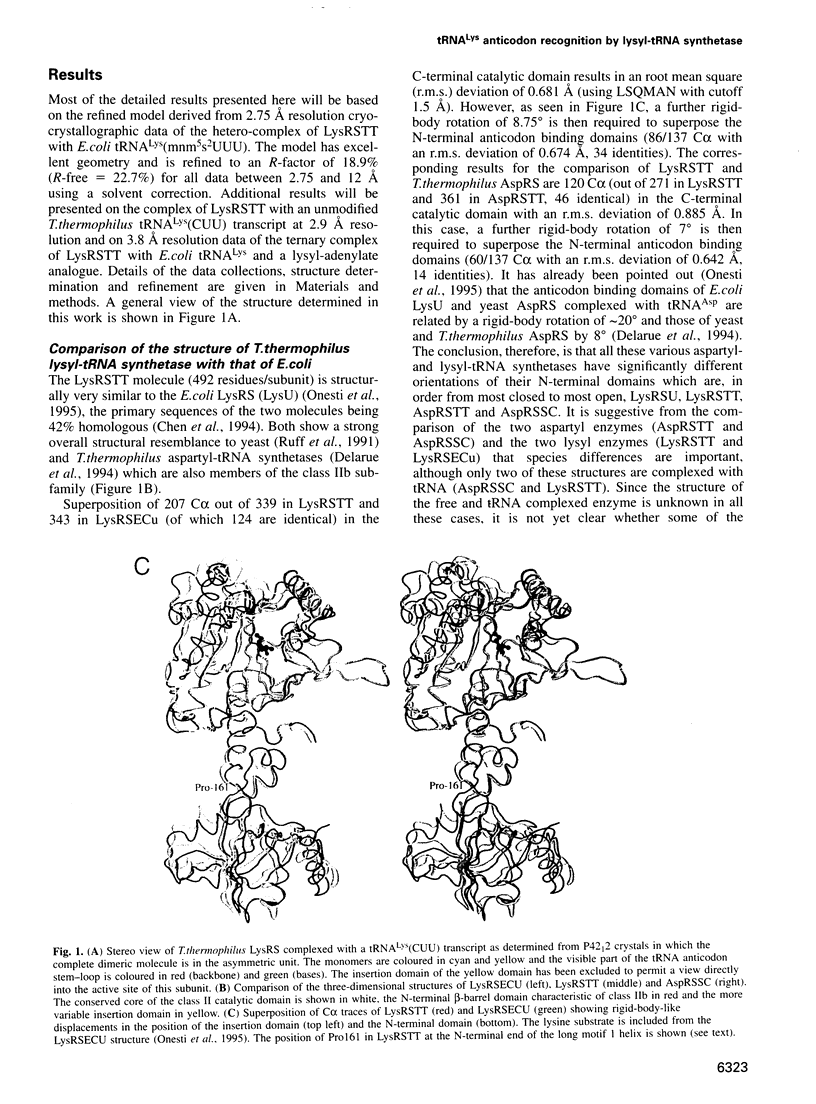
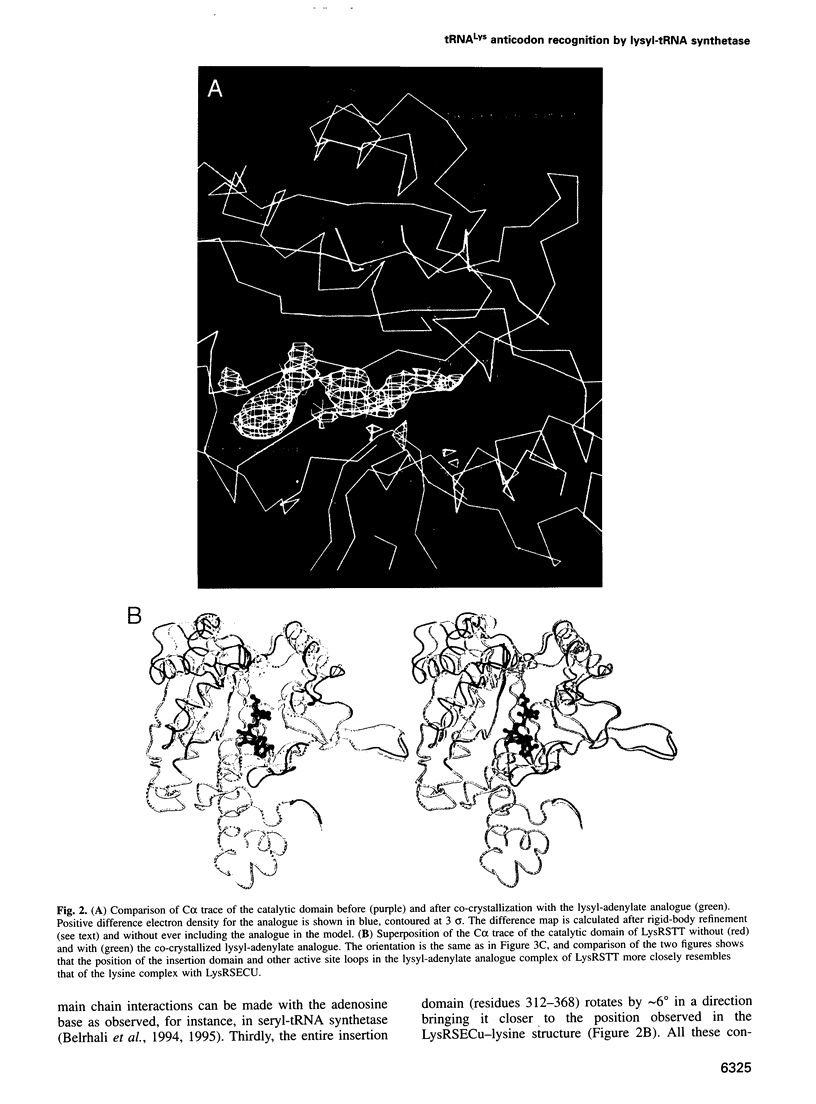
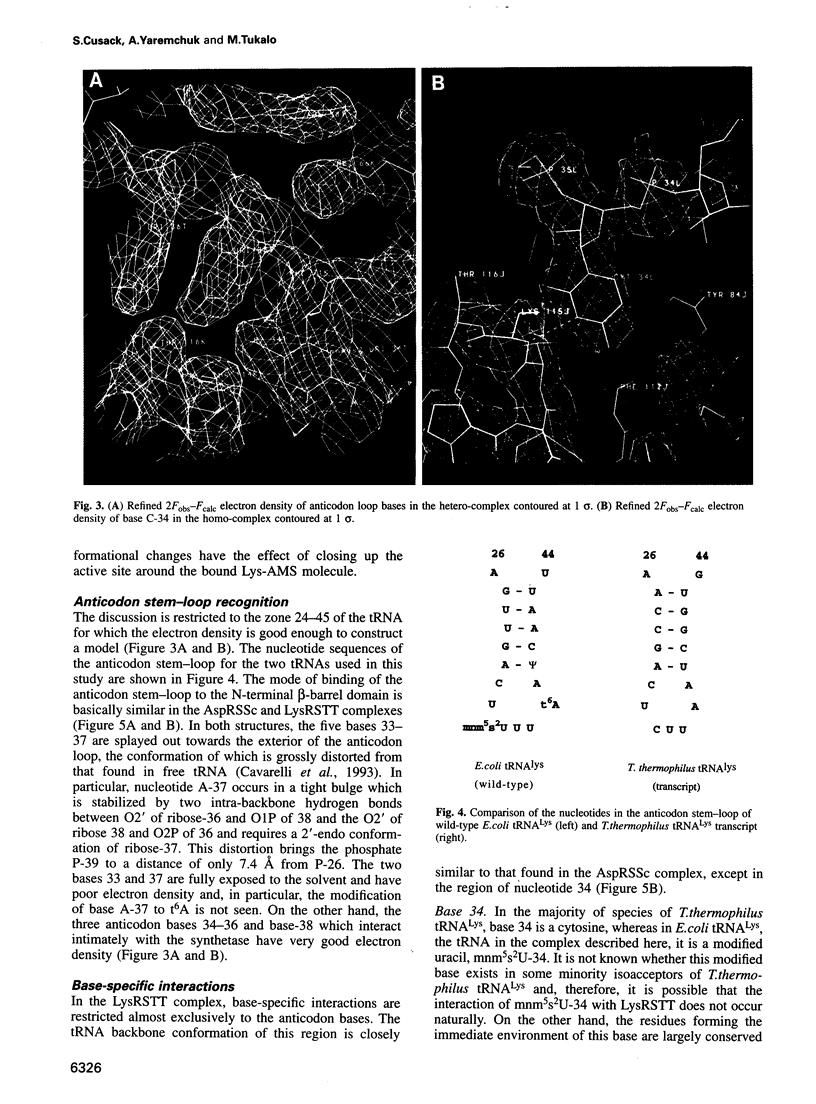
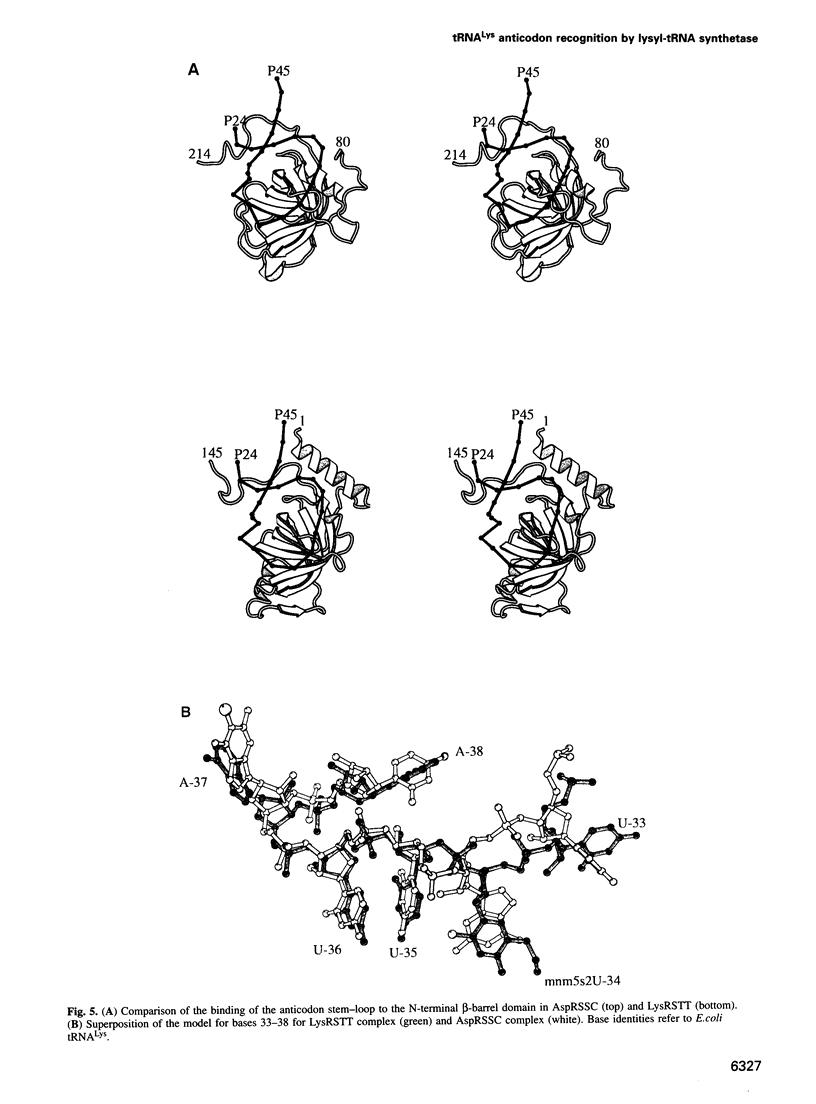
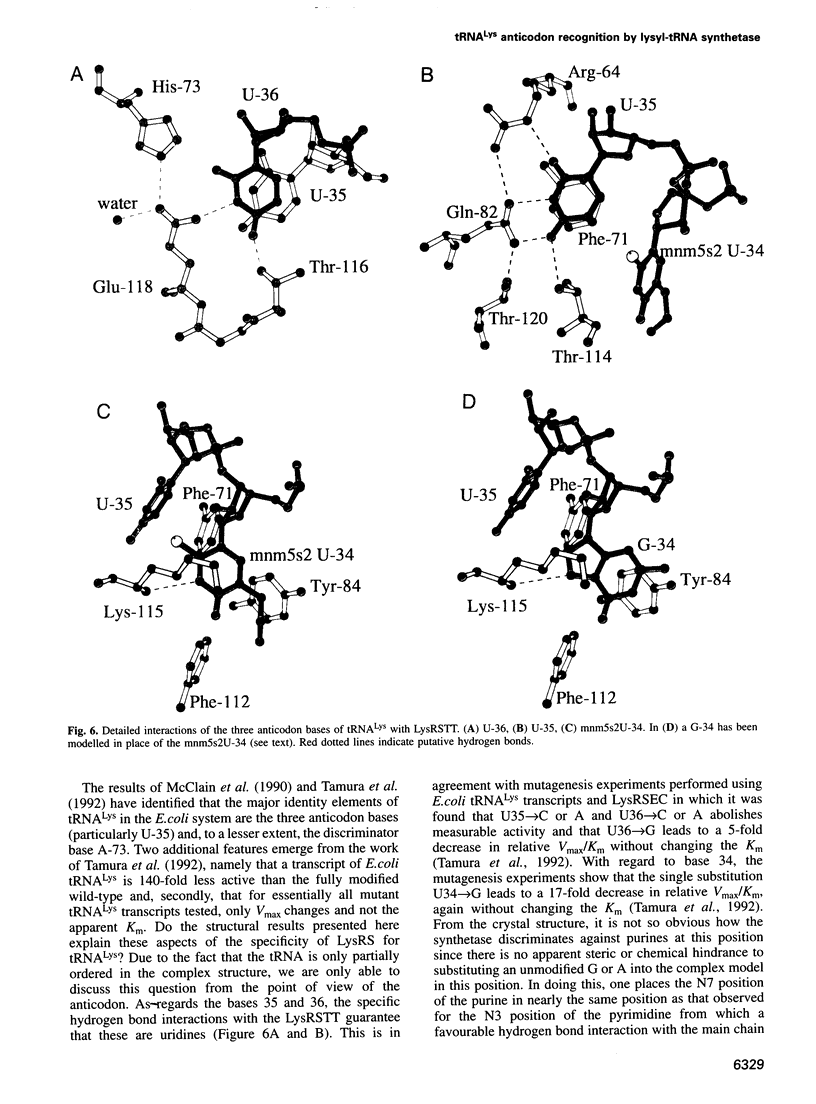
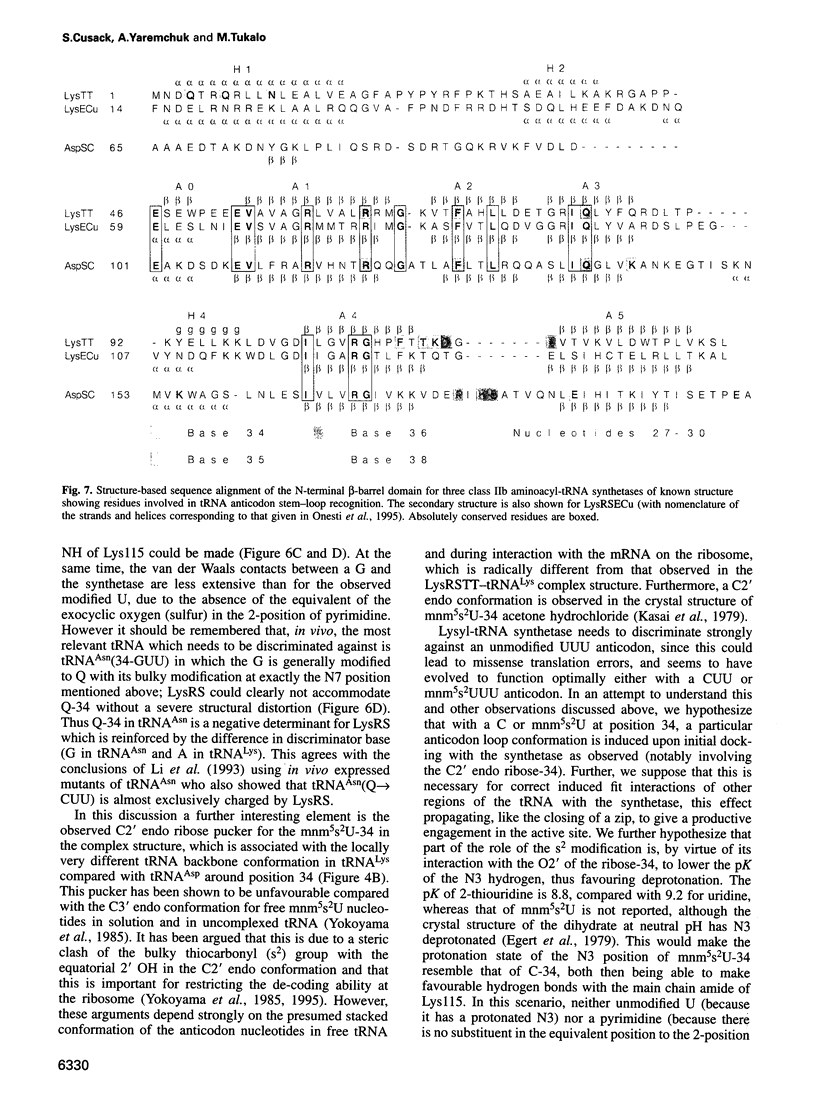
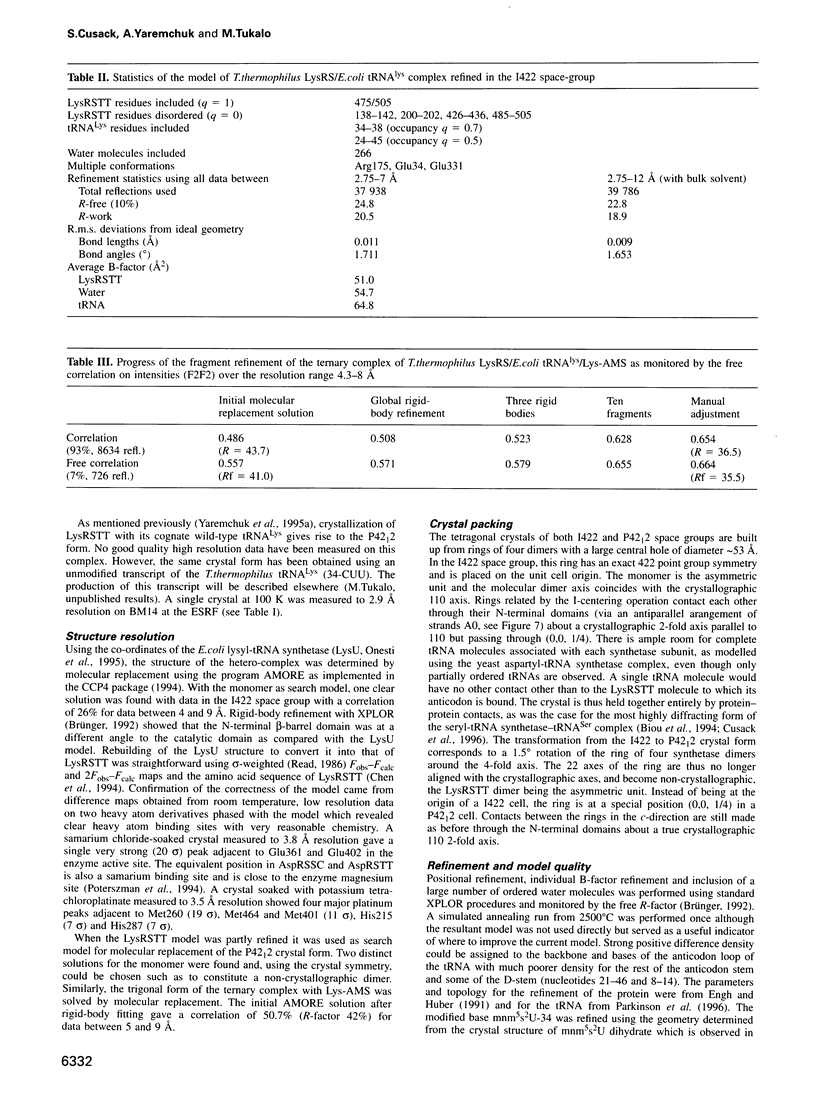
The crystal structures of Thermus thermophilus lysyl-tRNA synthetase, a class IIb aminoacyl-tRNA synthetase, complexed with Escherchia coli tRNA(Lys)(mnm5 s2UUU) at 2.75 A resolution and with a T. thermophilus tRNA(Lys)(CUU) transcript at 2.9 A resolution are described. In both complexes only the tRNA anticodon stem-loop is well ordered. The mode of binding of the anticodon stem-loop to the N-terminal beta-barrel domain is similar to that previously found for the homologous class IIb aspartyl-tRNA synthetase-tRNA(Asp) complex except in the region of the wobble base 34 where either mnm5 s2U or C can be accommodated. The specific recognition of the other anticodon bases, U-35 and U-36, which are both major identity elements in the lysine system, is also described. Additional crystallographic data on a ternary complex with a lysyl-adenylate analogue show that binding of the intermediate induces significant conformational changes in the vicinity of the active site of the enzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belrhali H., Yaremchuk A., Tukalo M., Berthet-Colominas C., Rasmussen B., Bösecke P., Diat O., Cusack S. The structural basis for seryl-adenylate and Ap4A synthesis by seryl-tRNA synthetase. Structure. 1995 Apr 15;3(4):341–352. doi: 10.1016/s0969-2126(01)00166-6. [DOI] [PubMed] [Google Scholar]
- Belrhali H., Yaremchuk A., Tukalo M., Larsen K., Berthet-Colominas C., Leberman R., Beijer B., Sproat B., Als-Nielsen J., Grübel G. Crystal structures at 2.5 angstrom resolution of seryl-tRNA synthetase complexed with two analogs of seryl adenylate. Science. 1994 Mar 11;263(5152):1432–1436. doi: 10.1126/science.8128224. [DOI] [PubMed] [Google Scholar]
- Biou V., Yaremchuk A., Tukalo M., Cusack S. The 2.9 A crystal structure of T. thermophilus seryl-tRNA synthetase complexed with tRNA(Ser). Science. 1994 Mar 11;263(5152):1404–1410. doi: 10.1126/science.8128220. [DOI] [PubMed] [Google Scholar]
- Cavarelli J., Eriani G., Rees B., Ruff M., Boeglin M., Mitschler A., Martin F., Gangloff J., Thierry J. C., Moras D. The active site of yeast aspartyl-tRNA synthetase: structural and functional aspects of the aminoacylation reaction. EMBO J. 1994 Jan 15;13(2):327–337. doi: 10.1002/j.1460-2075.1994.tb06265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavarelli J., Rees B., Ruff M., Thierry J. C., Moras D. Yeast tRNA(Asp) recognition by its cognate class II aminoacyl-tRNA synthetase. Nature. 1993 Mar 11;362(6416):181–184. doi: 10.1038/362181a0. [DOI] [PubMed] [Google Scholar]
- Chen J., Brevet A., Lapadat-Tapolsky M., Blanquet S., Plateau P. Properties of the lysyl-tRNA synthetase gene and product from the extreme thermophile Thermus thermophilus. J Bacteriol. 1994 May;176(9):2699–2705. doi: 10.1128/jb.176.9.2699-2705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commans S., Blanquet S., Plateau P. A single substitution in the motif 1 of Escherichia coli lysyl-tRNA synthetase induces cooperativity toward amino acid binding. Biochemistry. 1995 Jun 27;34(25):8180–8189. doi: 10.1021/bi00025a025. [DOI] [PubMed] [Google Scholar]
- Commans S., Plateau P., Blanquet S., Dardel F. Solution structure of the anticodon-binding domain of Escherichia coli lysyl-tRNA synthetase and studies of its interaction with tRNA(Lys). J Mol Biol. 1995 Oct 13;253(1):100–113. doi: 10.1006/jmbi.1995.0539. [DOI] [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Cusack S. Eleven down and nine to go. Nat Struct Biol. 1995 Oct;2(10):824–831. doi: 10.1038/nsb1095-824. [DOI] [PubMed] [Google Scholar]
- Cusack S., Härtlein M., Leberman R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991 Jul 11;19(13):3489–3498. doi: 10.1093/nar/19.13.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack S., Yaremchuk A., Tukalo M. The crystal structure of the ternary complex of T.thermophilus seryl-tRNA synthetase with tRNA(Ser) and a seryl-adenylate analogue reveals a conformational switch in the active site. EMBO J. 1996 Jun 3;15(11):2834–2842. [PMC free article] [PubMed] [Google Scholar]
- Delarue M., Poterszman A., Nikonov S., Garber M., Moras D., Thierry J. C. Crystal structure of a prokaryotic aspartyl tRNA-synthetase. EMBO J. 1994 Jul 15;13(14):3219–3229. doi: 10.1002/j.1460-2075.1994.tb06623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Eriani G., Dirheimer G., Gangloff J. Aspartyl-tRNA synthetase from Escherichia coli: cloning and characterisation of the gene, homologies of its translated amino acid sequence with asparaginyl- and lysyl-tRNA synthetases. Nucleic Acids Res. 1990 Dec 11;18(23):7109–7118. doi: 10.1093/nar/18.23.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francklyn C., Harris D., Moras D. Crystallization of histidyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1994 Aug 12;241(2):275–277. doi: 10.1006/jmbi.1994.1498. [DOI] [PubMed] [Google Scholar]
- Hillen W., Egert E., Lindner H. J., Gassen H. G. Restriction or amplification of wobble recognition: the structure of 2-thio-5-methylaminomethyluridine and the interaction of odd uridines with the anticodon loop backbone. FEBS Lett. 1978 Oct 15;94(2):361–364. doi: 10.1016/0014-5793(78)80977-6. [DOI] [PubMed] [Google Scholar]
- Isel C., Lanchy J. M., Le Grice S. F., Ehresmann C., Ehresmann B., Marquet R. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the post-transcriptional modifications of primer tRNA3Lys. EMBO J. 1996 Feb 15;15(4):917–924. [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., Nojima H., Nukiwa N., Ishizuka M., Nakajima T., Yasuhara T., Tanaka T., Oshima T. High guanine plus cytosine content in the third letter of codons of an extreme thermophile. DNA sequence of the isopropylmalate dehydrogenase of Thermus thermophilus. J Biol Chem. 1984 Mar 10;259(5):2956–2960. [PubMed] [Google Scholar]
- Kasai H., Nishimura S., Vorbrüggen H., Iitaka Y. Crystal and molecular structure of the acetonide of 5-methylaminomethyl-2-thiouridine: a minor constituent of Escherichia coli tRNAs. FEBS Lett. 1979 Jul 15;103(2):270–273. doi: 10.1016/0014-5793(79)81343-5. [DOI] [PubMed] [Google Scholar]
- Li S., Pelka H., Schulman L. H. The anticodon and discriminator base are important for aminoacylation of Escherichia coli tRNA(Asn). J Biol Chem. 1993 Aug 25;268(24):18335–18339. [PubMed] [Google Scholar]
- McClain W. H., Foss K., Jenkins R. A., Schneider J. Nucleotides that determine Escherichia coli tRNA(Arg) and tRNA(Lys) acceptor identities revealed by analyses of mutant opal and amber suppressor tRNAs. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9260–9264. doi: 10.1073/pnas.87.23.9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzin A. G. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 1993 Mar;12(3):861–867. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onesti S., Miller A. D., Brick P. The crystal structure of the lysyl-tRNA synthetase (LysU) from Escherichia coli. Structure. 1995 Feb 15;3(2):163–176. doi: 10.1016/s0969-2126(01)00147-2. [DOI] [PubMed] [Google Scholar]
- Parkinson G., Vojtechovsky J., Clowney L., Brünger A. T., Berman H. M. New parameters for the refinement of nucleic acid-containing structures. Acta Crystallogr D Biol Crystallogr. 1996 Jan 1;52(Pt 1):57–64. doi: 10.1107/S0907444995011115. [DOI] [PubMed] [Google Scholar]
- Poterszman A., Delarue M., Thierry J. C., Moras D. Synthesis and recognition of aspartyl-adenylate by Thermus thermophilus aspartyl-tRNA synthetase. J Mol Biol. 1994 Nov 25;244(2):158–167. doi: 10.1006/jmbi.1994.1716. [DOI] [PubMed] [Google Scholar]
- Pütz J., Puglisi J. D., Florentz C., Giegé R. Identity elements for specific aminoacylation of yeast tRNA(Asp) by cognate aspartyl-tRNA synthetase. Science. 1991 Jun 21;252(5013):1696–1699. doi: 10.1126/science.2047878. [DOI] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Saks M. E., Sampson J. R., Abelson J. N. The transfer RNA identity problem: a search for rules. Science. 1994 Jan 14;263(5144):191–197. doi: 10.1126/science.7506844. [DOI] [PubMed] [Google Scholar]
- Sylvers L. A., Rogers K. C., Shimizu M., Ohtsuka E., Söll D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry. 1993 Apr 20;32(15):3836–3841. doi: 10.1021/bi00066a002. [DOI] [PubMed] [Google Scholar]
- Tamura K., Himeno H., Asahara H., Hasegawa T., Shimizu M. In vitro study of E.coli tRNA(Arg) and tRNA(Lys) identity elements. Nucleic Acids Res. 1992 May 11;20(9):2335–2339. doi: 10.1093/nar/20.9.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer A. J. Specific incorporation of selenium into lysine- and glutamate- accepting tRNAs from Escherichia coli. J Biol Chem. 1983 Jul 25;258(14):8637–8641. [PubMed] [Google Scholar]
- Yaremchuk A. D., Cusack S., Aberg A., Gudzera O., Kryklivyi I., Tukalo M. Crystallization of Thermus thermophilus histidyl-tRNA synthetase and its complex with tRNAHis. Proteins. 1995 Aug;22(4):426–428. doi: 10.1002/prot.340220413. [DOI] [PubMed] [Google Scholar]
- Yaremchuk A. D., Krikliviy I. A., Cusack S., Tukalo M. A. Cocrystallization of lysyl-tRNA synthetase from Thermus thermophilus with its cognate tRNAlys and with Escherichia coli tRNAlys. Proteins. 1995 Mar;21(3):261–264. doi: 10.1002/prot.340210309. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]