Abstract
BACKGROUND
Transcatheter left atrial appendage (LAA) ligation may represent an alternative to oral anticoagulation for stroke prevention in atrial fibrillation..
OBJECTIVES
This study sought to assess the early safety and efficacy of transcatheter ligation of the LAA for stroke prevention in atrial fibrillation..
METHODS
This was a retrospective, multicenter study of consecutive patients undergoing LAA ligation with the Lariat device at 8 U.S. sites. The primary endpoint was procedural success, defined as device success (suture deployment and <5 mm leak by post-procedure transesophageal echocardiography), and no major complication at discharge (death, myocardial infarction, stroke, Bleeding Academic Research Consortium bleeding type 3 or greater, or cardiac surgery). Post-discharge management was per operator discretion.
RESULTS
A total of 154 patients were enrolled. Median CHADS2 score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack, or thromboembolism [doubled]) was 3 (interquartile range: 2 to 4). Device success was 94%, and procedural success was 86%. A major complication occurred in 15 patients (9.7%). There were 14 major bleeds (9.1%), driven by the need for transfusion (4.5%). Significant pericardial effusion occurred in 16 patients (10.4%). Follow-up was available in 134 patients at a median of 112 days (interquartile range: 50 to 270 days): Death, myocardial infarction, or stroke occurred in 4 patients (2.9%). Among 63 patients with acute closure and transesophageal echocardiography follow-up, there were 3 thrombi (4.8%) and 13 (20%) with residual leak.
CONCLUSIONS
In this initial multicenter experience of LAA ligation with the Lariat device, the rate of acute closure was high, but procedural success was limited by bleeding. A prospective randomized trial is required to adequately define clinical efficacy, optimal post-procedure medical therapy, and the effect of operator experience on procedural safety.
Keywords: atrial fibrillation, Lariat, left atrial appendage, stroke
A trial fibrillation. is a major risk factor for stroke and systemic embolism (1). The primary source of thromboembolism in atrial fibrillation. appears to be the left atrial appendage (LAA) (2). Oral anticoagulation with vitamin K antagonists (VKAs) reduces thromboembolic risk and is recommended for stroke prevention in patients who are not at very low risk according to standardized risk scores (1). Novel oral anticoagulant agents are noninferior and in some cases superior to VKA for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. (3–6). However, both VKAs and the novel oral anticoagulant agents increase the risk of major bleeding, particularly from a gastrointestinal source, and are not suitable in a large proportion of patients because of prohibitive bleeding risk or other clinical reasons (7,8). Therefore, there exists a substantial clinical need for alternative approaches to stroke prevention in atrial fibrillation.. A randomized clinical trial demonstrated that the efficacy of transcatheter occlusion of the LAA with a nitinol-based device and subsequent discontinuation of oral anticoagulation was noninferior to VKA (9), and terminal therapy analysis of this trial further supported the contention that LAA closure is an effective alternative to systemic anticoagulation (10). However, a permanent implant has several potential limitations, including device embolism, thrombus formation, erosion, and infection (9,11–13). These issues may be mitigated by an “implant-free” approach to LAA obliteration.
The Lariat device (SentreHeart, Redwood City, California) allows for the percutaneous ligation of the LAA through the delivery of a surgical suture via a combined transseptal and subxiphoid approach (14). This device has received a section 510(k) clearance from the U.S. Food and Drug Administration for the approximation of soft tissue and has been applied to LAA ligation in approximately 2,000 patients in the United States, according to the device manufacturer. To date, the safety and efficacy of this approach have been explored in a few small, single-center studies that enrolled patients predominantly outside the United States. The objectives of this multicenter registry were to determine the clinical characteristics and post-procedure management of patients undergoing Lariat LAA ligation in current practice within the United States and to determine the safety and early efficacy of the procedure.
METHODS
PATIENT SELECTION
This was a retrospective, multicenter study from 8 sites in the United States of patients with atrial fibrillation. undergoing attempted transcatheter LAA ligation with the Lariat device for the purpose of stroke prevention. The enrolled patients constituted the entire Lariat experience at each site. An attempted LAA ligation was defined as a procedure in which pericardial access was attempted, or transseptal puncture was attempted if done before pericardial access, with the intent to ligate the LAA with the Lariat. Patients were not included if they did not undergo a planned transcatheter LAA ligation because of issues not pertaining to the procedure (e.g., LAA thrombus on pre-procedure transesophageal echocardiography [TEE], or if a patient was screened for the procedure but was not a candidate on the basis of findings of cardiac computed tomography [CT].) The institutional review board of the coordinating center approved the protocol (Scripps Clinic, La Jolla, California), and each participating hospital research ethics board provided permission to collect data.
DATA COLLECTION
Baseline demographics, clinical and procedural characteristics, in-hospital events, concomitant medications, follow-up duration, and out-of-hospital events were collected by use of case report forms. CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack, or thromboembolism [doubled]), CHA2DS2VASC (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism [doubled], vascular disease, age 65 to 74 years, sex category), and HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) scores (15,16) were independently calculated by the coordinating center from the appropriate data fields. All events were site-reported.
STUDY ENDPOINTS AND DEFINITIONS
Device success was defined as suture deployment and a residual shunt <5 mm by post-procedural TEE. The primary endpoint was procedural success, defined as device success, and no major complication at hospital discharge (death, myocardial infarction [MI], stroke, major bleeding, or emergency surgery). Other endpoints included significant pericardial effusion, defined as an effusion requiring further intervention, such as pericardiocentesis or vasopressors for hemodynamic support; major bleeding; and major adverse cardiovascular events, defined as death, MI, or stroke. Major bleeding was defined as Bleeding Academic Research Consortium type 3 or greater (17).
TRANSCATHETER LAA LIGATION
The Lariat procedure has previously been described in detail (14). In brief, patients underwent a screening contrast CT scan to confirm that the LAA anatomy was amenable to Lariat ligation. Pericardial access was performed with a micropuncture or 17-G epidural needle, and a 13.5F soft-tipped sheath was introduced into the pericardial space over a 0.035-inch guidewire. Transseptal puncture was then performed via the femoral vein by a standard technique. Unfractionated heparin was administered to achieve a goal-activated clotting time of 250 to 300 seconds. A magnet-tipped 0.025-inch guidewire was advanced into the anterior aspect of the LAA. A magnet-tipped 0.035-inch wire was advanced into the pericardium through the pericardial sheath to form a connection with the magnet-tipped wire in the LAA, over which the Lariat snare was advanced and closed at the mouth of the LAA using TEE and fluoroscopic guidance. The preloaded suture was then released from the snare and tightened with the suture-tensioning device, and the snare was removed and the suture cut using a suture cutter. The pericardial sheath was exchanged for a drain, which was generally left in place for at least 4 to 6 hours, although the duration of drainage was at the discretion of the operator. Post-procedure medical therapy (i.e., analgesic, anti-inflammatory and anticoagulation therapy) was prescribed and clinical and imaging follow-up (generally, 1 to 3 months post-procedure) were performed according to operator preference.
STATISTICAL ANALYSIS
Categorical variables are reported as counts and percentages and continuous variables as mean ± SD or median and interquartile range (IQR). Analyses were performed with SPSS (Statistical Package for the Social Sciences, version 12.0, SPSS Inc., Chicago, Illinois).
RESULTS
PATIENT POPULATION
A total of 154 patients were enrolled at 8 sites (Appendix). A median of 19 patients (range 5 to 35 patients) were enrolled at each site. The average age was 72.1 ± 9.4 years; 38% of patients were female, 36% had diabetes mellitus, and 14% had a prior hemorrhagic stroke. The median CHADS2 score was 3 (IQR: 2 to 4), the median CHA2DS2VASC score was 4 (IQR: 3 to 5), and the median HAS-BLED score was 3 (IQR: 2 to 4) (Table 1). Before the procedure, 92 patients (60%) were being treated with an oral anticoagulant, 43 patients (28%) were on antiplatelet therapy alone, and 19 patients (12%) were on no anticoagulant or antiplatelet agent.
TABLE 1.
Baseline Demographic and Clinical Characteristics of the Study Population (n = 154)
| Age (yrs) | 72.1 ± 9.4 |
| Age >75 yrs | 70 (45) |
| Male | 96 (62) |
| Hypertension | 125 (81) |
| Diabetes mellitus | 56 (36) |
| History of heart failure | 53 (34) |
| Peripheral arterial disease | 21 (14) |
| Prior CVA/TIA | 58 (38) |
| Prior hemorrhagic CVA | 21 (14) |
| Prior major bleed or propensity for bleeding | 96 (62) |
| Labile INR measurements | 31 (20) |
| Concomitant chronic NSAID use | 22 (14) |
| Liver disease | 9 (6) |
| Renal disease | 14 (9) |
| Significant alcohol consumption | 16 (10) |
| CHADS2 score | 3 (2–4) |
| CHA2DS2VASC score | 4 (3–5) |
| HAS-BLED score | 3 (2–4) |
| CHADS2 score | 2.8 ± 1.4 |
| CHA2DS2VASC score | 4.1 ± 1.6 |
| HAS-BLED score | 3.2 ± 1.2 |
Values are mean ± SD, n (%), or median (interquartile range).
CHADS2 = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism (doubled); CHA2DS2VASC = congestive heart failure, hypertension, age ≥75 years [doubled], diabetes mellitus, prior stroke or TIA or thromboembolism [doubled], vascular disease, age 65 to 74 years, sex category; CVA = cerebrovascular accident; HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly; INR = international normalized ratio; IQR = interquartile range; NSAID = nonsteroidal anti-inflammatory drug; TIA = transient ischemic attack.
PROCEDURAL CHARACTERISTICS
The average procedure duration was 76.6 ± 2.6 min. In 9 cases, the suture could not be delivered for the following reasons: pericardial adhesions preventing either pericardial sheath placement (2 cases) or limiting advancement of the epicardial wire or snare (3 cases); inability to advance the Lariat snare over the LAA due to challenging anatomy (2 cases); and emergency surgery after right ventricular perforation (2 cases). Among the 145 cases in which the suture was delivered, TEE demonstrated complete LAA closure at the end of the procedure in 133 (92%) and a residual leak <5 mm in 11 cases (7%) and ≥5 mm in 1 case. Device success (delivery of suture and residual leak <5 mm) was therefore achieved in 144 cases (94%).
PROCEDURAL COMPLICATIONS AND IN-HOSPITAL OUTCOMES
There were a total of 15 patients (10%) with at least 1 major periprocedural complication (death, MI, stroke, major bleed, or emergent cardiac surgery). Major bleeding occurred in 14 patients (9%), driven by the need for transfusion (Table 2). Emergency surgery was required in a total of 3 patients (2%), 2 for right ventricular perforation during pericardial access with subsequent cardiac tamponade and 1 for repair of LAA perforation. One patient died in the hospital 19 days post-procedure of respiratory failure, sepsis, and subsequent nosocomial pneumonia. There were no in-hospital strokes or MIs. Overall, procedural success was achieved in 132 patients (86%) (Table 3).
TABLE 2.
Major Bleeding Events During Hospitalization in the Study Population (n = 154)*
| Major bleed | 14 (9.1) |
| Any transfusion with overt bleeding | 7 (4.5) |
| Overt bleed, hemoglobin drop 3 to <5 g/dl | 5 (3.2) |
| Overt bleed, hemoglobin drop ≥5 g/dl | 3 (1.9) |
| Cardiac tamponade | 7 (4.5) |
| Bleeding requiring surgical control | 2 (1.3) |
| Bleeding requiring vasoactive agents | 4 (2.6) |
| Fatal bleeding | 0 |
Values are n (%). Bleeding Academic Research Consortium type 3A or greater.
More than 1 bleeding event may have occurred in a single patient.
TABLE 3.
Reasons for Procedural Failure of Left Atrial Appendage Ligation With the Lariat Device (N = 22)
| Lariat unable to be deployed | 9 (48) |
| Pericardial adhesions | 5 |
| LAA anatomy | 2 |
| Aborted procedure after RV perforation | 2 |
|
| |
| Residual Leak ≥ 5 mm | 1 (6) |
|
| |
| Major complication before discharge | 15 (71) |
Values are n or n (%). In 2 patients with procedural failure, there was both a major complication and the lariat was not deployed. In 1 patient, there was both a residual leak ≥ 5 mm and a major complication.
LAA = left atrial appendage; RV = right ventricular.
Significant pericardial effusion occurred in 16 patients (10%) and pleural effusion in 4 patients (3%). The cause of pericardial effusion was thought to be secondary to LAA perforation/laceration in 4 of these cases (25%), a result of pericardial access in 4 cases (25%), and from an unclear cause in the remainder.
MEDICAL MANAGEMENT AT TIME OF DISCHARGE
Anticoagulant and/or antiplatelet therapy at discharge was heterogeneous (Table 4). The most frequent treatment at discharge was aspirin monotherapy. No antiplatelet or oral anticoagulant agent was prescribed in 29 patients (19%).
TABLE 4.
Medical Therapy at Discharge After Transcatheter Left Atrial Appendage Ligation (n = 154)
| Aspirin monotherapy | 47 (31) | |
|
| ||
| Dual antiplatelet therapy | 37 (24) | |
|
| ||
| Oral anticoagulation | 36 (23) | |
| Warfarin | 24 (16) | |
| Rivaroxaban | 7 (5) | |
| Dabigatran | 5 (3) | |
|
| ||
| No antiplatelet or oral anticoagulation | 29 (19) | |
|
| ||
| Clopidogrel monotherapy | 11 (7) | |
| Aggrenox | 1 (0.6) | |
Values are n (%).
OUT-OF-HOSPITAL OUTCOMES
Follow-up was available in 134 patients (87%) at a median period of 112 days (IQR: 50 to 270 days) after discharge. The composite of out-of-hospital death, MI, or stroke occurred in 4 patients (2.9%). There were 3 deaths, 1 of a noncardiovascular cause and 2 of cardiovascular causes (sudden death at 22 days post-discharge and infarcted bowel/stroke at 49 days post-discharge). Stroke occurred in 2 patients (including 1 who also died), pericardial effusion occurred in 3 patients, and late pleural effusions were noted in 3 patients.
RESIDUAL LEAK OVER FOLLOW-UP
TEE follow-up was performed in 63 patients in whom the Lariat deployment was successful. Immediately post-procedure, there was complete LAA closure in 58 patients (92%) and a leak <5 mm in the remainder (8%). At follow-up, there was complete closure in 50 patients (79%), a leak <5 mm in 9 patients (14%), and a leak ≥ 5 mm in 4 patients (6%).
THROMBUS FORMATION
Left atrial thrombus originating near the LAA stump occurred in 3 patients (5%) with TEE follow-up at 46, 82, and 104 days post-procedure. These patients had been discharged post-procedure on aspirin and clopidogrel, aspirin monotherapy, and no antiplatelet or anticoagulant agent, respectively. One additional thrombus was incidentally noted by CT 17 days post-procedure in a patient who had been noncompliant with dabigatran therapy. All patients were treated successfully with oral anticoagulation without clinical sequelae.
DISCUSSION
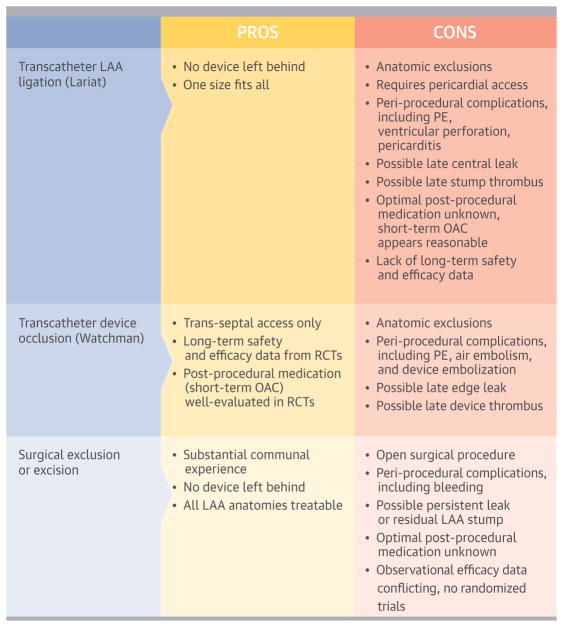
In this multicenter U.S. registry of patients undergoing transcatheter LAA ligation with the Lariat device, treated patients were at significant thromboembolic risk according to standard risk scores and frequently had a clinical history consistent with intolerance of anticoagulant agents. Device success was high (94%), but procedural success was limited by the occurrence of major bleeding. Surveillance TEE, when performed, identified occasional LAA stump thrombus (3%) and late leakage into the appendage (20%). Our findings provide insight into the results of this procedure in clinical practice and have important implications regarding patient selection, procedural technique, and post-procedural management (Central Illustration).
CENTRAL ILLUSTRATION . LAA Closure.
Review of pros and cons with varied methods for closure of the left atrial appendage (LAA) for stroke prevention. LAA = left atrial appendage; OAC = oral anticoagulation; PE = pericardial effusion; RCT = randomized clinical trial.
To date, information regarding the acute safety and efficacy of transcatheter LAA suture ligation has been limited to small, single-center studies. The technical results of the procedure were similar in the current multicenter study compared with these previous reports. In a single-center experience, the Lariat procedure was technically successful in 85 of 92 patients in whom it was attempted (92%), similar to the 94% success in our study and another, smaller experience (14,18). The most common reason for an aborted procedure in our study was unanticipated pericardial adhesions, observed in approximately 3% of patients, which were not detected before the procedure by CT or by echocardiographic imaging. This rate of unanticipated adhesions was also consistent with a previous report (14).
The rates of procedural complications and anatomic closure that we observed differ from prior reports. Major procedural complications occurred in 10% of cases, most of which were major bleeding events not related to transseptal access and driven by the occurrence of blood transfusions and serious pericardial effusions. Compared with the initial Lariat experience reported by Bartus et al. (14), our patient population was substantially older (45% versus 12% were ≥75 years of age) and had a higher prevalence of comorbidities such as heart failure (34% versus 12%) and diabetes (36% versus 10%), which may have contributed in part to a greater rate of complications. Pericardial effusion is also the most common procedural complication associated with device occlusion of the LAA. Pericardial effusion occurred in 5% of patients randomly assigned to the Watchman device (Boston Scientific, Natick, Massachusetts) in the PROTECT-AF trial (Percutaneous Left Atrial Appendage Closure for Stroke Prophylaxis in Patients With Atrial Fibrillation) (9), although the incidence has decreased to 2.2% with increased operator experience (19). Unique to the Lariat procedure, pericardial effusions may result from right ventricular perforation or irritation during pericardial access, from tearing the thin LAA wall during advancement of the magnet-tipped wire within the LAA during manipulation of the Lariat snare over the LAA, or during suture tightening. Acute management of a serious pericardial effusion during the Lariat procedure may be relatively straightforward, because a large sheath (albeit without an aspiration port) is initially placed within the pericardium, which allows for evacuation of blood if necessary. However, beyond initial stabilization, the clinical management of pericardial bleeding may require surgical repair. Additionally, post-procedure pericardial effusions can develop that may require drainage. Whether the incidence of serious pericardial effusion can be reduced with increasing operator experience and technical refinements, as observed in the CAP (Continuing Access to PROTECT-AF) registry (19), remains to be determined. Post-discharge outcomes were less favorable than previously reported, because the composite of death, MI, or stroke occurred in 4 patients (2.9%) and a late pericardial effusion occurred in 3 patients (2.2%). The causes of these are likely multifactorial but again may reflect a higher severity of underlying comorbidities in our collective patient population compared with the initial Lariat experiences (14).
Although we observed a very high acute closure rate, our results demonstrate that late residual leak can occur after the Lariat procedure. In a prior single-center observational study of 85 patients with successful ligation, there was only 1 leak (<2 mm) in the 65 patients who underwent surveillance TEE (14). In contradistinction, we observed a late leak in 20% of the 60 patients who had a surveillance TEE, approximately one-third of which were ≥5 mm in diameter. Potential reasons for this greater rate of late leak may include patient selection or operator experience, although this is speculative. Peri-device leaks were frequent after Watchman implantation but do not appear to be associated with thromboembolic events (20). Incomplete surgical LAA ligation has been associated with subsequent thrombus formation and clinical events (21). The clinical consequences of residual leaks after the Lariat procedure, if any, are unknown, but leaks can be treated successfully with percutaneous approaches (22,23), although the safety and efficacy of such leak closure are unproven.
This study provides insights into the real-world application of the Lariat technology in the United States. Patients who underwent percutaneous LAA ligation were at high risk for thromboembolic events. Approximately two-thirds of the patients had a prior major bleeding event or a propensity for major bleeding, and 14% had a prior intracranial bleeding event, which placed them at high risk for recurrent bleeding on oral anticoagulation. This patient population was not well represented in the clinical trial that demonstrated the superiority of apixaban over aspirin in patients who were not suitable for or unwilling to take warfarin anticoagulation (7). Given the incidence of procedural complications we observed and the lack of robust, long-term efficacy data, it appears reasonable that if percutaneous LAA ligation is to be performed, it should be reserved for individuals at substantial thromboembolic and bleeding risk who are not candidates for prospective U.S. Food and Drug Administration studies of LAA occlusion. Furthermore, whether a percutaneous approach to surgical ligation with the Lariat procedure provides any advantage over minimally invasive surgical ligation (24) deserves exploration.
Medical therapy at discharge after the Lariat procedure was heterogeneous. In addition to our experience, several cases of LAA thrombus after the Lariat procedure have been reported (25,26). Although our study has insufficient power to identify predictors of post-procedure thrombus formation, it would appear reasonable to administer at least a short course of post-procedural oral anticoagulation until follow-up imaging is performed, given our findings of occasional thrombus formation in patients treated with no therapy and with anti-platelet therapy. Prospective studies are required to define the optimal medical regimen post-procedure. According to our multicenter experience, it appears that post-procedural TEE is not routinely performed in current real-world practice. However, given our findings, routine follow-up imaging appears advisable.
STUDY LIMITATIONS
Our study has several limitations. It is retrospective, and all events were site-reported. Patient management and clinical and imaging follow-up were not uniform; however, one objective of this study was to describe how percutaneous LAA ligation is currently being used within the United States. Pericardial effusion can be challenging to define after the Lariat procedure, but we limited our definition to effusions that required intervention because of hemodynamic compromise or had other clinical sequelae. Data regarding post-procedural chest pain, pericardial drainage, and pericarditis were not routinely collected. It is unknown what size residual leak is of clinical importance. Although the definition of a significant leak used in this study is consistent with that of the PROTECT-AF trial, in which warfarin was continued over follow-up if there was peri-device flow ≥5 mm, we may have underestimated the incidence of clinically important leaks with the Lariat device. There was a broad range in the number of patients enrolled at each site. Increasing operator experience could be associated with improved outcomes. However, given the study size, we did not have statistical power to identify interactions, if any, between outcomes and site, operator experience, or post-procedure medical therapy.
CONCLUSIONS
In this first multicenter report of clinical outcomes after transcatheter ligation of the LAA with the Lariat device, device success was high, but procedural success was limited by major bleeding, which occurred in 9% of cases. Occasional thrombus and late leak were observed on imaging follow-up. A large prospective trial is required to adequately define safety, clinical efficacy, and post-procedure management. Until such data are available, consideration for the procedure should be limited to patients who are at high risk for thromboembolic events and bleeding and are not candidates for currently enrolling protocols of LAA occlusion.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The Lariat device is designed to facilitate percutaneous catheter-based ligation of the left atrial appendage to prevent stroke in patients with nonvalvular atrial fibrillation. using combined transseptal and transpericardial approaches. The most frequent acute complications of the procedure are hemopericardium and other major bleeding.
TRANSLATIONAL OUTLOOK
Proper assessment of the relative safety and efficacy of the Lariat device as an alternative to long-term anticoagulant therapy in patients with atrial fibrillation. will require carefully designed randomized trials that address both thromboembolism and bleeding outcomes.
Acknowledgments
This study was funded by an investigator-initiated grant from SentreHeart, Redwood City, California. Dr. Price has received consulting honoraria from Boston Scientific, Janssen Pharmaceuticals, Daiichi Sankyo, and St. Jude Medical; served as a proctor for Boston Scientific, St. Jude Medical, SentreHeart, and W.L. Gore; and received research support from SentreHeart, Inc. Dr. Gibson has received consulting honoraria from SentreHeart. Dr. Yakubov has received consulting honoraria from SentreHeart and Boston Scientific. Dr. Di Biase has received consulting honoraria from Biosense Webster, Hansen Medical, and St. Jude Medical; and speaker honoraria from Biotronik and Atricure. Dr. Natale has received consulting honoraria from Biosense Webster, Biotronik, Janssen Pharmaceuticals, St. Jude Medical, Boston Scientific, and Medtronic. Dr. Burkhardt has served as a proctor for SentreHeart, Inc. Dr. Pershad has received consulting honoraria from Boston Scientific, Asahi Intecc, Edwards Lifesciences, and Medtronic. Dr. Byrne has received consulting fees and honoraria from Medtronic, Inc. Dr. Gidney has received speaking and/or advisory fees from Biosense Webster, Medtronic, Sanofi-Aventis, and Boehringer Ingelheim. Dr. Aragon has received consulting and speakers’ honoraria from Boston Scientific. Dr. Valderrábano has received speaking honoraria from SentreHeart and Boston Scientific; and consulting honoraria with Boston Scientific, SentreHeart, St. Jude Medical, Medtronic, and Hansen Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CT
computed tomography
- IQR
interquartile range
- LAA
left atrial appendage
- RV
right ventricular
- TEE
transesophageal echocardiography
- VKA
vitamin K antagonist
APPENDIX
Participating Sites and Enrollment: Scripps Clinic, La Jolla, CA (36); Houston Methodist Hospital, Houston, TX (35); St David’s Hospital, Austin, TX (24); Cottage Hospital, Santa Barbara, CA (19); Banner Hospital, Phoenix, AZ (19); Riverside Methodist Hospital, Columbus, OH (9); Prairie Heart Cardiology, Springfield, IL (7); Northwestern University, Chicago, II (5).
References
- 1.Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223–42. doi: 10.1016/j.jacc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–9. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. for the ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, et al. the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, et al. for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano RP, Ruff CT, Braunwald E, et al. for the ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 7.Connolly SJ, Eikelboom J, Joyner C, et al. for the AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 8.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation : a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Holmes DR, Reddy VY, Turi ZG, et al. for the PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation.: a randomised non-inferiority trial. Lancet. 2009;374:534–42. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation.: 2. 3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) Trial. Circulation. 2013;127:720–9. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 11.Plicht B, Konorza TF, Kahlert P, et al. Risk factors for thrombus formation on the Amplatzer Cardiac Plug after left atrial appendage occlusion. J Am Coll Cardiol Intv. 2013;6:606–13. doi: 10.1016/j.jcin.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Sepahpour A, Ng MK, Storey P, McGuire MA. Death from pulmonary artery erosion complicating implantation of percutaneous left atrial appendage occlusion device. Heart Rhythm. 2013;10:1810–1. doi: 10.1016/j.hrthm.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 13.Gasparini M, Ceriotti C, Bragato R. Huge left atrial thrombus after left atrial appendage occlusion with a Watchman device. Eur Heart J. 2012;33:1998. doi: 10.1093/eurheartj/ehs025. [DOI] [PubMed] [Google Scholar]
- 14.Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62:108–18. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation. using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation. : the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–80. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 18.Stone D, Byrne T, Pershad A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation. at high risk for stroke and anticoagulation. Catheter Cardiovasc Interv. 2013 Jun 13; doi: 10.1002/ccd.25065. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417–24. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 20.Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–9. doi: 10.1016/j.jacc.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924–9. doi: 10.1016/j.jacc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 22.Mosley WJ, 2nd, Smith MR, Price MJ. Percutaneous management of late leak after Lariat transcatheter ligation of the left atrial appendage in patients with atrial fibrillation at high risk for stroke. Catheter Cardiovasc Interv. 2014;83:664–9. doi: 10.1002/ccd.25251. [DOI] [PubMed] [Google Scholar]
- 23.Yeow WL, Matsumoto T, Kar S. Successful closure of residual leak following LARIAT procedure in a patient with high risk of stroke and hemorrhage. Catheter Cardiovasc Interv. 2013 Oct 2; doi: 10.1002/ccd.25219. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–9. doi: 10.1016/j.jtcvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 25.Briceno DF, Fernando RR, Laing ST. Left atrial appendage thrombus post LARIAT closure device. Heart Rhythm. 2013 Nov 1; doi: 10.1016/j.hrthm.2013.10.053. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Baker MS, Paul Mounsey J, Gehi AK, Chung EH. Left atrial thrombus after appendage ligation with LARIAT. Heart Rhythm. 2014;11:1489. doi: 10.1016/j.hrthm.2013.10.024. [DOI] [PubMed] [Google Scholar]