Abstract
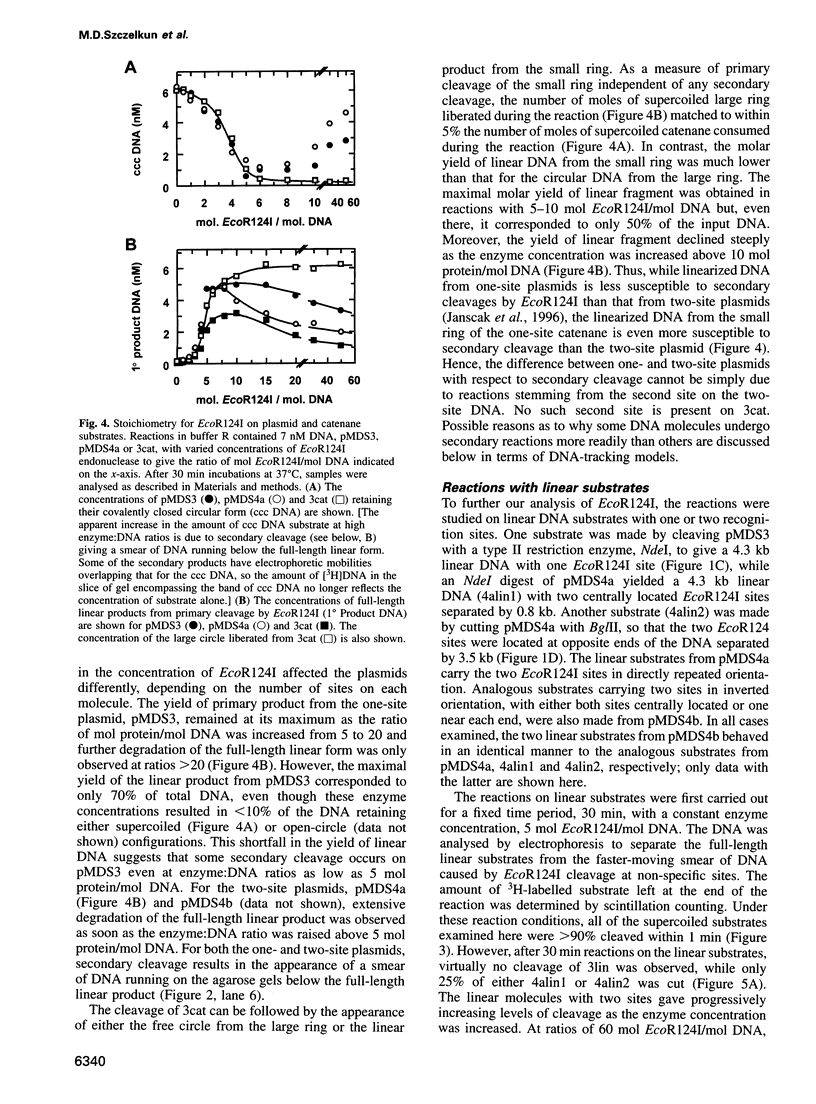
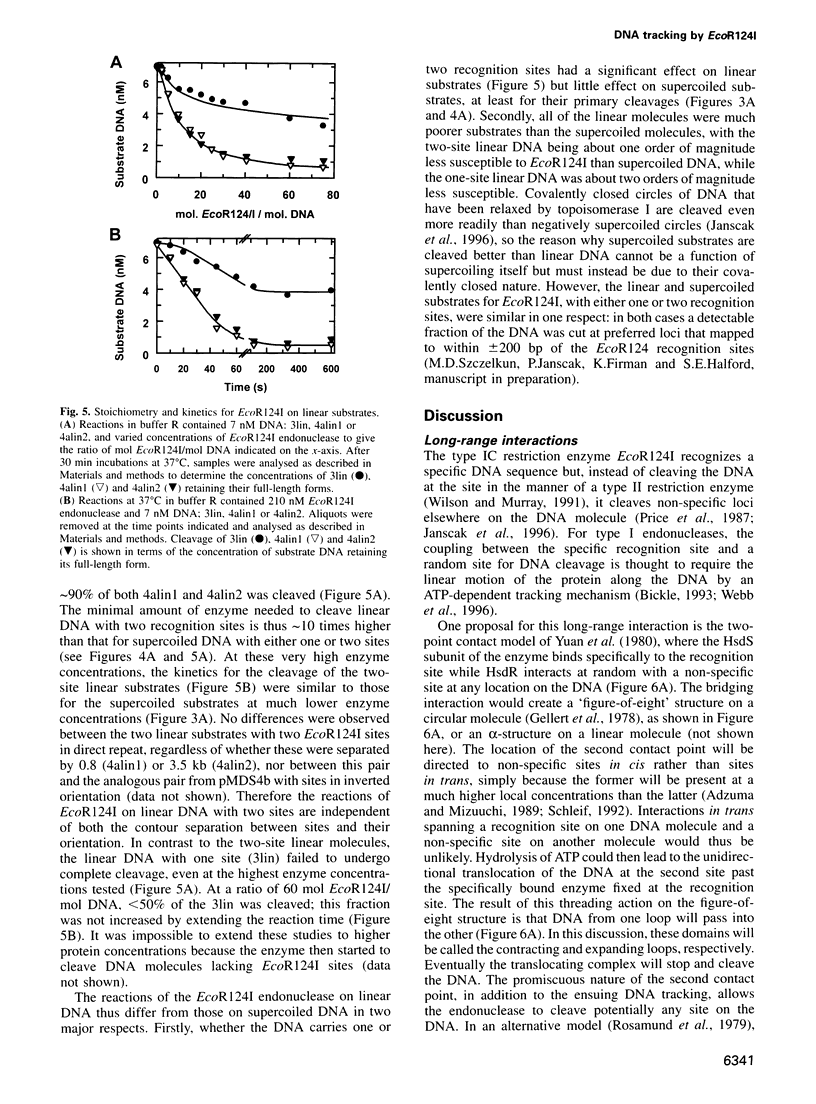
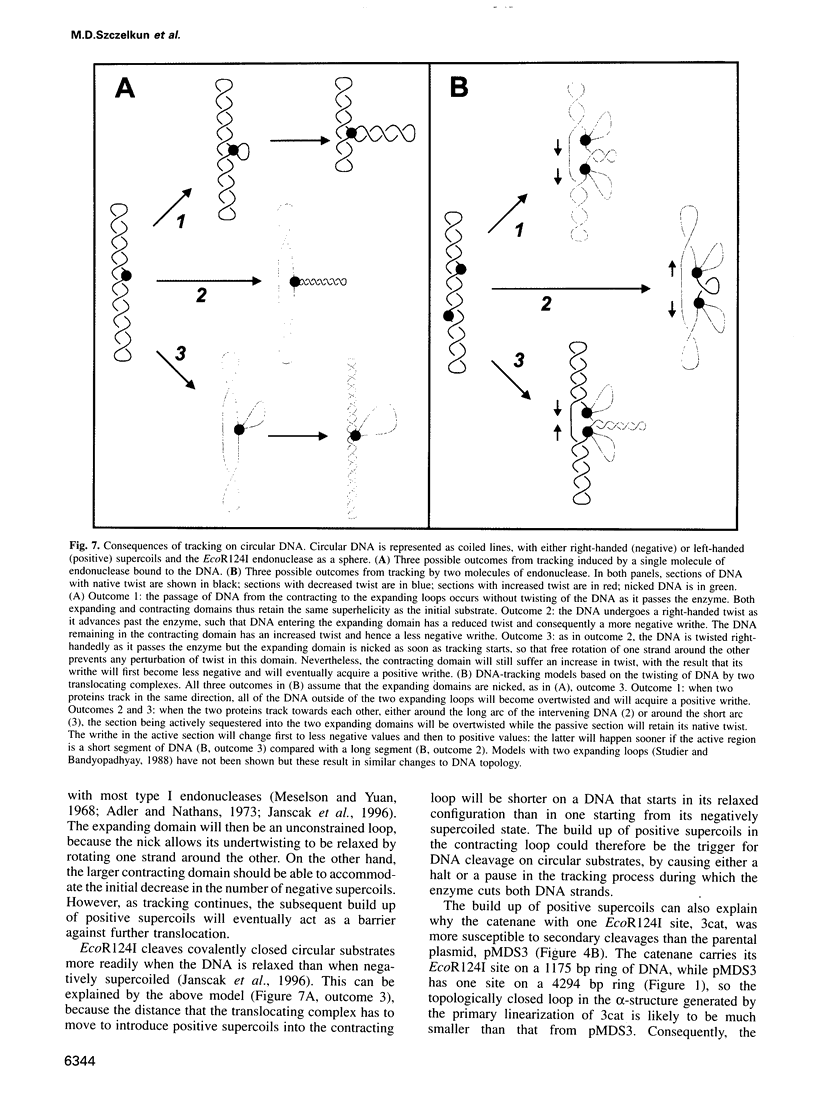
Type I restriction endonucleases such as EcoR124I cleave DNA at undefined loci, distant from their recognition sequences, by a mechanism that involves the enzyme tracking along the DNA between recognition and cleavage sites. This mechanism was examined on plasmids that carried recognition sites for EcoR124I and recombination sites for resolvase, the latter to create DNA catenanes. Supercoiled substrates with either one or two restriction sites were linearized by EcoR124I at similar rates, although the two-site molecule underwent further cleavage more readily than the one-site DNA. The catenane from the plasmid with one EcoR124I site, carrying the site on the smaller of the two rings, was cleaved by EcoR124I exclusively in the small ring, and this underwent multiple cleavage akin to the two-site plasmid. Linear substrates derived from the plasmids were cleaved by EcoR124I at very slow rates. The communication between recognition and cleavage sites therefore cannot stem from random looping. Instead, it must follow the DNA contour between the sites. On a circular DNA, the translocation of non-specific DNA past the specifically bound protein should increase negative supercoiling in one domain and decrease it in the other. The ensuing topological barrier may be the trigger for DNA cleavage.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abadjieva A., Patel J., Webb M., Zinkevich V., Firman K. A deletion mutant of the type IC restriction endonuclease EcoR1241 expressing a novel DNA specificity. Nucleic Acids Res. 1993 Sep 25;21(19):4435–4443. doi: 10.1093/nar/21.19.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackroyd A. J., Avila P., Parker C. N., Halford S. E. Site-specific recombination by mutants of Tn21 resolvase with DNA recognition functions from Tn3 resolvase. J Mol Biol. 1990 Dec 5;216(3):633–643. doi: 10.1016/0022-2836(90)90388-3. [DOI] [PubMed] [Google Scholar]
- Adams D. E., West S. C. Unwinding of closed circular DNA by the Escherichia coli RuvA and RuvB recombination/repair proteins. J Mol Biol. 1995 Mar 31;247(3):404–417. doi: 10.1006/jmbi.1995.0149. [DOI] [PubMed] [Google Scholar]
- Adler S. P., Nathans D. Studies of SV 40 DNA. V. Conversion of circular to linear SV 40 DNA by restriction endonuclease from Escherichia coli B. Biochim Biophys Acta. 1973 Mar 19;299(2):177–188. doi: 10.1016/0005-2787(73)90340-7. [DOI] [PubMed] [Google Scholar]
- Adzuma K., Mizuuchi K. Interaction of proteins located at a distance along DNA: mechanism of target immunity in the Mu DNA strand-transfer reaction. Cell. 1989 Apr 7;57(1):41–47. doi: 10.1016/0092-8674(89)90170-0. [DOI] [PubMed] [Google Scholar]
- Au K. G., Welsh K., Modrich P. Initiation of methyl-directed mismatch repair. J Biol Chem. 1992 Jun 15;267(17):12142–12148. [PubMed] [Google Scholar]
- Benjamin H. W., Cozzarelli N. R. Geometric arrangements of Tn3 resolvase sites. J Biol Chem. 1990 Apr 15;265(11):6441–6447. [PubMed] [Google Scholar]
- Bickle T. A., Brack C., Yuan R. ATP-induced conformational changes in the restriction endonuclease from Escherichia coli K-12. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3099–3103. doi: 10.1073/pnas.75.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Role of DNA topology in Mu transposition: mechanism of sensing the relative orientation of two DNA segments. Cell. 1986 Jun 20;45(6):793–800. doi: 10.1016/0092-8674(86)90554-4. [DOI] [PubMed] [Google Scholar]
- Dreier J., Bickle T. A. ATPase activity of the type IC restriction-modification system EcoR124II. J Mol Biol. 1996 Apr 19;257(5):960–969. doi: 10.1006/jmbi.1996.0215. [DOI] [PubMed] [Google Scholar]
- Dryden D. T., Sturrock S. S., Winter M. Structural modelling of a type I DNA methyltransferase. Nat Struct Biol. 1995 Aug;2(8):632–635. doi: 10.1038/nsb0895-632. [DOI] [PubMed] [Google Scholar]
- Dunaway M., Dröge P. Transactivation of the Xenopus rRNA gene promoter by its enhancer. Nature. 1989 Oct 19;341(6243):657–659. doi: 10.1038/341657a0. [DOI] [PubMed] [Google Scholar]
- Endlich B., Linn S. The DNA restriction endonuclease of Escherichia coli B. I. Studies of the DNA translocation and the ATPase activities. J Biol Chem. 1985 May 10;260(9):5720–5728. [PubMed] [Google Scholar]
- Eskin B., Linn S. The deoxyribonucleic acid modification and restriction enzymes of Escherichia coli B. II. Purification, subunit structure, and catalytic properties of the restriction endonuclease. J Biol Chem. 1972 Oct 10;247(19):6183–6191. [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Ohmori H., Tomizawa J. DNA gyrase and DNA supercoiling. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):35–40. doi: 10.1101/sqb.1979.043.01.007. [DOI] [PubMed] [Google Scholar]
- Janscak P., Abadjieva A., Firman K. The type I restriction endonuclease R.EcoR124I: over-production and biochemical properties. J Mol Biol. 1996 Apr 19;257(5):977–991. doi: 10.1006/jmbi.1996.0217. [DOI] [PubMed] [Google Scholar]
- Kanaar R., van de Putte P., Cozzarelli N. R. Gin-mediated recombination of catenated and knotted DNA substrates: implications for the mechanism of interaction between cis-acting sites. Cell. 1989 Jul 14;58(1):147–159. doi: 10.1016/0092-8674(89)90411-x. [DOI] [PubMed] [Google Scholar]
- Kneale G. G. A symmetrical model for the domain structure of type I DNA methyltransferases. J Mol Biol. 1994 Oct 14;243(1):1–5. doi: 10.1006/jmbi.1994.1624. [DOI] [PubMed] [Google Scholar]
- Kong X. P., Onrust R., O'Donnell M., Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992 May 1;69(3):425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- Levene S. D., Donahue C., Boles T. C., Cozzarelli N. R. Analysis of the structure of dimeric DNA catenanes by electron microscopy. Biophys J. 1995 Sep;69(3):1036–1045. doi: 10.1016/S0006-3495(95)79978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel A., Bickle T. A., Krüger D. H., Schroeder C. Type III restriction enzymes need two inversely oriented recognition sites for DNA cleavage. Nature. 1992 Jan 30;355(6359):467–469. doi: 10.1038/355467a0. [DOI] [PubMed] [Google Scholar]
- Meisel A., Mackeldanz P., Bickle T. A., Krüger D. H., Schroeder C. Type III restriction endonucleases translocate DNA in a reaction driven by recognition site-specific ATP hydrolysis. EMBO J. 1995 Jun 15;14(12):2958–2966. doi: 10.1002/j.1460-2075.1995.tb07296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister J., MacWilliams M., Hübner P., Jütte H., Skrzypek E., Piekarowicz A., Bickle T. A. Macroevolution by transposition: drastic modification of DNA recognition by a type I restriction enzyme following Tn5 transposition. EMBO J. 1993 Dec;12(12):4585–4591. doi: 10.1002/j.1460-2075.1993.tb06147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Batten P. L., Murray K. Restriction of bacteriophage lambda by Escherichia coli K. J Mol Biol. 1973 Dec 15;81(3):395–407. doi: 10.1016/0022-2836(73)90149-6. [DOI] [PubMed] [Google Scholar]
- Nobbs T. J., Halford S. E. DNA cleavage at two recognition sites by the SfiI restriction endonuclease: salt dependence of cis and trans interactions between distant DNA sites. J Mol Biol. 1995 Sep 29;252(4):399–411. doi: 10.1006/jmbi.1995.0506. [DOI] [PubMed] [Google Scholar]
- Ostrander E. A., Benedetti P., Wang J. C. Template supercoiling by a chimera of yeast GAL4 protein and phage T7 RNA polymerase. Science. 1990 Sep 14;249(4974):1261–1265. doi: 10.1126/science.2399463. [DOI] [PubMed] [Google Scholar]
- Parker C. N., Halford S. E. Dynamics of long-range interactions on DNA: the speed of synapsis during site-specific recombination by resolvase. Cell. 1991 Aug 23;66(4):781–791. doi: 10.1016/0092-8674(91)90121-e. [DOI] [PubMed] [Google Scholar]
- Price C., Shepherd J. C., Bickle T. A. DNA recognition by a new family of type I restriction enzymes: a unique relationship between two different DNA specificities. EMBO J. 1987 May;6(5):1493–1497. doi: 10.1002/j.1460-2075.1987.tb02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond J., Endlich B., Linn S. Electron microscopic studies of the mechanism of action of the restriction endonuclease of Escherichia coli B. J Mol Biol. 1979 Apr 25;129(4):619–635. doi: 10.1016/0022-2836(79)90472-8. [DOI] [PubMed] [Google Scholar]
- Stark W. M., Parker C. N., Halford S. E., Boocock M. R. Stereoselectivity of DNA catenane fusion by resolvase. Nature. 1994 Mar 3;368(6466):76–78. doi: 10.1038/368076a0. [DOI] [PubMed] [Google Scholar]
- Stasiak A., Tsaneva I. R., West S. C., Benson C. J., Yu X., Egelman E. H. The Escherichia coli RuvB branch migration protein forms double hexameric rings around DNA. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7618–7622. doi: 10.1073/pnas.91.16.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Bandyopadhyay P. K. Model for how type I restriction enzymes select cleavage sites in DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4677–4681. doi: 10.1073/pnas.85.13.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczelkun M. D., Halford S. E. Recombination by resolvase to analyse DNA communications by the SfiI restriction endonuclease. EMBO J. 1996 Mar 15;15(6):1460–1469. [PMC free article] [PubMed] [Google Scholar]
- Tinker R. L., Kassavetis G. A., Geiduschek E. P. Detecting the ability of viral, bacterial and eukaryotic replication proteins to track along DNA. EMBO J. 1994 Nov 15;13(22):5330–5337. doi: 10.1002/j.1460-2075.1994.tb06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vipond I. B., Baldwin G. S., Oram M., Erskine S. G., Wentzell L. M., Szczelkun M. D., Nobbs T. J., Halford S. E. A general assay for restriction endonucleases and other DNA-modifying enzymes with plasmid substrates. Mol Biotechnol. 1995 Dec;4(3):259–268. doi: 10.1007/BF02779019. [DOI] [PubMed] [Google Scholar]
- Wang Z., Dröge P. Differential control of transcription-induced and overall DNA supercoiling by eukaryotic topoisomerases in vitro. EMBO J. 1996 Feb 1;15(3):581–589. [PMC free article] [PubMed] [Google Scholar]
- Webb J. L., King G., Ternent D., Titheradge A. J., Murray N. E. Restriction by EcoKI is enhanced by co-operative interactions between target sequences and is dependent on DEAD box motifs. EMBO J. 1996 Apr 15;15(8):2003–2009. [PMC free article] [PubMed] [Google Scholar]
- Wedel A., Weiss D. S., Popham D., Dröge P., Kustu S. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science. 1990 Apr 27;248(4954):486–490. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- Wilson G. G., Murray N. E. Restriction and modification systems. Annu Rev Genet. 1991;25:585–627. doi: 10.1146/annurev.ge.25.120191.003101. [DOI] [PubMed] [Google Scholar]
- Yuan R., Hamilton D. L., Burckhardt J. DNA translocation by the restriction enzyme from E. coli K. Cell. 1980 May;20(1):237–244. doi: 10.1016/0092-8674(80)90251-2. [DOI] [PubMed] [Google Scholar]





