Abstract
Background
The Keap1-Nrf2 signaling pathway regulates host cell defense responses against oxidative stress and maintains the cellular redox balance. Aims&Methods: We investigated the function/molecular mechanisms by which Keap1-Nrf2 complex may influence liver ischemia/reperfusion injury (IRI) in a mouse model of hepatic cold storage (20h at 4 C) followed by orthotopic liver transplantation (OLT).
Results
The Keap1 hepatocyte-specific knock-out (HKO) in the donor liver ameliorated post-transplant IRI, evidenced by improved hepatocellular function and OLT outcomes (Keap1HKO Keap1HKO; 100% survival), as compared with controls (WT WT; 50% survival; p<0.01). In contrast, donor liver Nrf2 deficiency exacerbated IRI in transplant recipients (Nrf2KO Nrf2KO; 40% survival). Ablation of Keap1 signaling reduced macrophage/neutrophil trafficking, pro-inflammatory cytokine programs, and hepatocellular necrosis/apoptosis, while simultaneously promoting anti-apoptotic functions in OLTs. At the molecular level, Keap1HKO increased Nrf2 levels, stimulated Akt phosphorylation, and enhanced expression of anti-oxidant Trx1, HIF-1 , and HO-1. Pretreatment of liver donors with PI3K inhibitor (LY294002) disrupted Akt/HIF-1 signaling and recreated hepatocellular damage in otherwise IR-resistant Keap1HKO transplants. In parallel in vitro studies, hydrogen peroxide-stressed Keap1-deficient hepatocytes were characterized by enhanced expression of Nrf2, Trx1, and Akt phosphorylation, in association with decreased release of lactate dehydrogenase (LDH) in cell culture supernatants.
Conclusions
Keap1-Nrf2 complex prevents oxidative injury in IR-stressed OLTs through Keap1 signaling, which negatively regulates Nrf2 pathway. Activation of Nrf2 induces Trx1 and promotes PI3K/Akt, crucial for HIF-1 activity. HIF-1 -mediated overexpression of HO-1/CyclinD1 facilitates cytoprotection by limiting hepatic inflammatory responses, and hepatocellular necrosis/apoptosis in PI3K-dependent manner.
Introduction
Ischemia/reperfusion injury (IRI) remains the major challenge in clinical liver transplantation, hepatic resection, trauma, and shock. This innate immune-dominated cascade includes reactive oxygen species (ROS) generation, which initiate tissue injury, and local inflammatory responses leading to endothelial and Kupffer cell activation, cytokine/chemokine release, and cell apoptosis [1]. It becomes recognized that oxidative stress-induced IR-damage involves multiple cell signaling pathways that result in liver failure or hepatoprotection and homeostasis [2]. Our group has pioneered the concept of cytoprotection by overexpression of heme-oxygenase-1 (HO-1) in IR-stressed organ transplants [3, 4].
Keap1 (Kelch-like ECH-associated protein 1) has been shown to interact with Nrf2 (nuclear factor erythroid 2-related factor 2), a master regulator of intracellular redox homeostasis [5]. Under normal conditions, Nrf2 is anchored in the cytoplasm through binding to Keap1, and facilitates ubiquitination/proteolysis of Nrf2 [6]. Inactivation of Keap1 leads to stabilization of Nrf2, which in turn translocates into the nuclei to activate cytoprotective target genes through binding to the anti-oxidant response element (ARE) [7]. Modification of Keap1 may also damage structural integrity of Keap1–Cul3 E3 ligase complex, decrease the ubiquitination activity and increase Nrf2 accumulation [8]. Nrf2-driven regulation of anti-oxidant and anti-inflammatory functions is important in cytoprotection. Indeed, genetic disruption of Nrf2 augments the severity of ischemic/nephrotoxic acute kidney injury in mice [9]. In contrast, activation of Nrf2 has been reported to protect against cerebral [10], retinal [11], cardiac [12] and intestinal [13] IR-tissue damage. Interestingly, human livers from older donors have lower levels of Nrf2, perhaps exposing them to increased IRI, and hence influencing the clinical outcomes [14]. While Nrf2 promotes cell growth/survival under oxidative stress conditions, its deletion reduces both constitutive and inducible expression of cytoprotective genes, and aggravates cellular damage. Moreover, disruption of Nrf2 signaling impairs angiogenic endothelial cell capacity and anti-oxidant gene expression, leading to cardiac hypertrophy, myocardial fibrosis and apoptosis in response to hemodynamic stress [15]. The diverse Nrf2-mediated cell survival and protection phenotypes may progress through Keap1-Nrf2-ARE pathway [16]. Disruption of Keap1 signaling in the liver enhances Nrf2 activity and increases expression of ROS-detoxifying cytoprotective genes [17]. Moreover, dysfunction of Keap1 gene activated Nrf2 and promoted cancer cell growth [18-20], whereas the loss of Keap1 activity led to constitutive activation of Nrf2 and anti-oxidant genes [21]. Thus, Keap1 is one of the key molecules to negatively regulate Nrf2 during oxidative stress.
Here, we report on novel regulatory mechanisms by which Keap1-Nrf2 complex prevents inflammation and exerts cytoprotection in a clinically-relevant mouse model of prolonged hepatic cold ischemia and orthotopic liver transplantation (OLT). Thus, Keap1-dependent Nrf2 activation enhanced anti-oxidant Trx1 and stimulated PI3K/Akt system, which in turn facilitated HIF-1α signaling to promote hepatoprotection in PI3K-dependent manner.
Materials and Methods
Animals
Male Keap1 hepatocyte-specific knock-out (Alb-Cre::Keap1 flox/−; Keap1HKO) and Nrf2 knock-out (Nrf2−/−; Nrf2KO) mice (BL/6; weighted 21-27g) were used (breeding pairs provided by Dr. T. Kensler, The Johns Hopkins University, Baltimore, MD). Wild-type (WT; C57BL/6) mice at 6-8 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in UCLA animal facility under specific pathogen-free conditions, received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985).
Mouse liver cold ischemia and transplantation model
We have developed a mouse model of ex-vivo hepatic cold ischemia followed by OLT [22]. Donor livers stored in UW solution at 4°C for 20h were transplanted in the following experimental groups: WT WT; Keap-1 HKO Keap-1 HKO; and Nrf2KO Nrf2KO. Animals were sacrificed at 1h, 6h, and 24h post-OLT or followed for survival at day 14. Separate groups of WT “sham” controls underwent the same procedures but without ischemia/OLT. In some experiments, donor mice were treated i.p. with PI3K inhibitor (LY294002; Calbiochem; 0.5mg/kg) or vehicle [10% dimethyl sulfoxide (DMSO) and 90% PBS] at 1h prior to liver procurement.
Hepatocellular function assay
Serum alanine aminotransferase (sALT) levels, an indicator of hepatocellular injury, were measured by IDEXX Laboratories (Westbrook, ME).
Histology and immunohistochemistry
Liver sections (5 m) were stained with hematoxylin and eosin (H&E). The severity of IRI was graded using Suzuki's criteria on a scale from 0-4 [23]. Liver macrophages and neutrophils were detected using primary rat anti-mouse CD68 (AbD Serotec, Raleigh, NC) and Ly6G (BD Biosciences, San Jose, CA) mAb, respectively. The secondary, biotinylated goat anti-rat IgG (Vector, Burlingame, CA) was incubated with immunoperoxidase (ABC Kit, Vector). Positive cells were counted blindly in 10 HPF/section.
Caspase-3 activity and TUNEL assays
Caspase-3 activity was analyzed by an assay kit (Calbiochem, La Jolla, CA), as described [24]. The Klenow-FragEL DNA Fragmentation Detection Kit (EMD Chemicals, Gibbstown, NJ) was used to detect DNA fragmentation characteristic of apoptosis in formalin-fixed paraffin-embedded liver sections [24]. Results were scored semi-quantitatively by averaging the number of TUNEL+ apoptotic cells/microscopic field at 200 magnification. Ten fields were evaluated per tissue sample.
Quantitative RT-PCR analysis
Quantitative RT-PCR was performed using the DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA). In a final reaction volume of 25 l, the following were added: 1 SuperMix (Platinum SYBR Green qPCR Kit; Invitrogen, San Diego, CA) cDNA and 10 M of each primer. Amplification conditions were: 50°C (2min), 95°C (5min), followed by 40 cycles of 95°C (15sec) and 60°C (30sec). Primers used to amplify specific gene fragments are shown (Supplementary Table).
Western blot analysis
Proteins (30 g/sample) from cell cultures/liver samples were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). Monoclonal rabbit anti-mouse Keap1, Trx1, p-Akt, Bcl-2, Bcl-xl, cleaved caspase-3, and -actin (Cell Signaling Technology, Danvers, MA), polyclonal rabbit anti-mouse Nrf2, HIF-1 (Santa Cruz Biotechnology, Santa Cruz, CA) and HO-1 (Stressgen Biotech, Victoria, BC, Canada) Abs were used. The relative quantities of proteins were determined by densitometer, and expressed in absorbance units (AU).
Mouse hepatocyte cultures
Primary hepatocytes from WT, Keap1 HKO or Nrf2 KO mice were isolated, as described [25]. Livers were perfused with warm (37 C) saline, followed by a collagenase-buffer (collagenase type IV, Sigma, St Louis, MO), and William's E culture medium (WEM) containing 10% FBS, 2μg/ml gentamycin, 15mM HEPES, 0.1μM dexamethasone, 4μg/ml insulin (Sigma) and 4mM glutamax (Invitrogen). Cells were purified by Percoll gradient centrifugation. Viable hepatocytes, suspended in WEM + 10% FBS, were added to 24-well dishes (1.5×105 cells/well). Cells were allowed to attach for 4h at 37°C and 5% CO2; medium was then changed to WEM without FBS, and cultures continued for another 24h.
In vitro experiments
Primary Keap1 HKO hepatocytes (5×105 cells/well) were pretreated with PI3K inhibitor (LY294002, 10 M; Calbiochem) or DMSO (6.5 l/ml) for 1h. In some experiments, WT hepatocytes were transfected with Keap1 or Nrf2 siRNA (100nM; Santa Cruz Biotechnology) using lipofectamine 2000 reagent (Invitrogen) and incubated for 24h. Cells were then treated with HIF inhibitor (YC-1, 100 M; Calbiochem) for 1h, and supplemented with H2O2 (200 M) for additional 12h. Cell viability, assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Life Technologies) assay, was expressed as percentage of total number of cells. Cell death was screened by lactate dehydrogenase (Stanbio Laboratory) release, and expressed as LDH activity (U/L), according to the manufacturer's instructions.
Statistical analysis
Data are expressed as mean SD. Statistical comparisons between groups were analyzed by Student's t-test. All differences were considered statistically significant at the p-value of <0.05.
Results
Hepatocyte deletion of the Keap1 gene confers resistance against IRI in OLTs
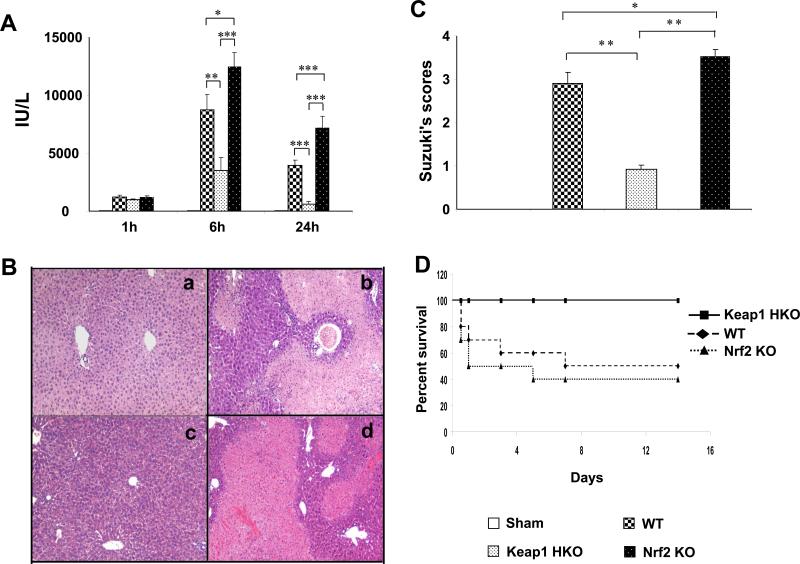
Donor livers were stored for 20h at 4 C prior to transplantation in WT WT; Keap-1 HKO Keap-1 HKO; and Nrf2KO Nrf2KO groups. The hepatocellular function in OLT recipients was assessed by sALT levels (IU/L) (Fig. 1A). Ablation of Keap1 signaling decreased sALT levels at 6h and 24h post-transplant, as compared with Keap1 proficient (WT) controls (6h: 3540±1059 and 8736±1339, respectively; p<0.01; 24h: 625±216 and 3956±432, respectively; p<0.0005). In contrast, Nrf2 deficiency exacerbated the hepatocellular damage, as evidenced by increased sALT levels at 6h (12467±1224; p<0.05) and 24h (7189±1009; p<0.0005) of reperfusion, as compared with controls.
Figure 1.
Hepatocyte-specific Keap1 deficiency (HKO) ameliorates hepatic IRI in OLTs. Donor livers were stored in UW solution (4 C) for 20h prior to the transplant. Experimental groups: (a; □) sham; (b;  ) WT WT; (c;
) WT WT; (c;  ) Keap1HKO Keap1HKO; (d;
) Keap1HKO Keap1HKO; (d;  ) Nrf2KO Nrf2KO. (A) sALT levels. Mean SD; n=4 mice/group. *p<0.05; **p<0.01; ***p<0.0005. (B) Representative OLT histology (H&E; magnification x100); (C) Suzuki's histological grading of IRI (24h). Mean SD; n=4-6 mice/group. *p<0.05, **p<0.001. (D) OLT survival: (■) Keap1HKO Keap1HKO (100%); (◆) WT WT (50%); (▲) Nrf2KO Nrf2KO (40%; p<0.01). N=10 mice/group.
) Nrf2KO Nrf2KO. (A) sALT levels. Mean SD; n=4 mice/group. *p<0.05; **p<0.01; ***p<0.0005. (B) Representative OLT histology (H&E; magnification x100); (C) Suzuki's histological grading of IRI (24h). Mean SD; n=4-6 mice/group. *p<0.05, **p<0.001. (D) OLT survival: (■) Keap1HKO Keap1HKO (100%); (◆) WT WT (50%); (▲) Nrf2KO Nrf2KO (40%; p<0.01). N=10 mice/group.
These functional data correlated with Suzuki's histological grading of IR-mediated liver damage at 24h post-transplant (Fig. 1B, C). Unlike WT controls, which showed moderate to severe sinusoidal congestion, cytoplasmic vacuolization, and hepatocellular necrosis (Panel b; score=2.9 0.26), Keap1HKO liver transplants revealed minimal pathological changes (Panel c; score=0.93 0.09; p<0.001). In contrast, Nrf2KO liver transplants were characterized by widespread edema, profound sinusoidal congestion/cytoplasmic vacuolization, and extensive (30-50%) necrosis (Panel d; score=3.52 0.17; p<0.001).
Having shown that hepatocyte Keap1-deficiency ameliorates, whereas Nrf2 deficiency enhances hepatocellular damage, we analyzed animal survival at day 14 in IR-stressed OLT groups (Fig. 1D). Unlike disruption of hepatocyte Keap1 signaling in donor livers, which led to 100% animal survival (10/10), Nrf2 deficiency diminished the survival to 40% (4/10), comparable with 50% survival (5/10) seen in unmodified WT controls (p<0.01).
Keap1HKO reduces macrophage/neutrophil trafficking and regulates inflammatory program in IR-stressed OLTs
To determine whether hepatocyte Keap1 deficiency may have influenced macrophage/neutrophil trafficking, we stained IR-stressed OLTs for CD68 and Ly6G infiltrating cells at 24h of reperfusion (Fig. 2). Indeed, disruption of Keap1 signaling decreased accumulation of CD68+ macrophages (Fig. 2A, Panel b; 6.5 3.2), as compared with WT controls (Panel a: 28.1 4.6, p<0.0001). In contrast, liver transplants devoid of Nrf2 signaling showed increased macrophage sequestration (Panel c: 37.4 6.4, p<0.005). Consistent with our immunostaining data (Fig. 2B), reduced mRNA levels coding for TNF- , IL-1 and CXCL-10 were recorded in Keap1HKO but not Nrf2KO OLTs (Fig. 2C). Furthermore, Keap1 deficiency decreased Ly6G+ neutrophil accumulation in OLTs (Fig. 2D, Panel b: 8.6 3.1), as compared with WT controls (Panel a: 37.3 4.4, p<0.0001); Nrf2KO liver transplants showed increased Ly6G+ infiltrate (Panel c: 48.6 8.1, p<0.001). Consistent with the immunostaining data (Fig. 2D, E), MPO assay (Fig. 2F), reflecting hepatic neutrophil activity (U/g), was decreased in Keap1-deficient OLTs (6h: 1.67 0.3, p<0.05; 24h: 1.17 0.28, p<0.05), as compared with WT controls (6h: 4.34 0.31; 24h: 3.37 0.29). Enhanced MPO activity found in Nrf2KO transplants (6h: 5.1 0.26, p<0.01; 24h: 4.1 0.52, p<0.01) was accompanied by increased CXCL-1 mRNA, a major neutrophil chemoattractant (Fig. 2G).
Figure 2.
Hepatocyte-specific Keap1 deficiency (HKO) reduces macrophage and neutrophil trafficking/activation in IR-stressed OLTs: (a;  ) WT WT; (b;
) WT WT; (b;  ) Keap1HKO Keap1HKO; (c;
) Keap1HKO Keap1HKO; (c;  ) Nrf2KO Nrf2KO. (A/B) Immunohistochemical staining for CD68+ cells. Representative of 4 mice/group; magnification ×400; *p<0.005, **p<0.0001. (C) Quantitative RT-PCR-assisted cytokine/chemokine gene expression; Mean±SD; n=3-4/group; *p<0.05, **p<0.005. (D/E) Immunohistochemical staining for LY6G+ cells. Representative of 4 mice/group; magnification ×400; *p<0.001, **p<0.0001. (F) Neutrophil MPO activity (U/gm). Mean±SD; n=3-4/group; *p<0.05, **p<0.01. (G) Quantitative RT-PCR-assisted detection of CXCL-1. Mean±SD; n=3-4/group; *p<0.05, **p<0.01.
) Nrf2KO Nrf2KO. (A/B) Immunohistochemical staining for CD68+ cells. Representative of 4 mice/group; magnification ×400; *p<0.005, **p<0.0001. (C) Quantitative RT-PCR-assisted cytokine/chemokine gene expression; Mean±SD; n=3-4/group; *p<0.05, **p<0.005. (D/E) Immunohistochemical staining for LY6G+ cells. Representative of 4 mice/group; magnification ×400; *p<0.001, **p<0.0001. (F) Neutrophil MPO activity (U/gm). Mean±SD; n=3-4/group; *p<0.05, **p<0.01. (G) Quantitative RT-PCR-assisted detection of CXCL-1. Mean±SD; n=3-4/group; *p<0.05, **p<0.01.
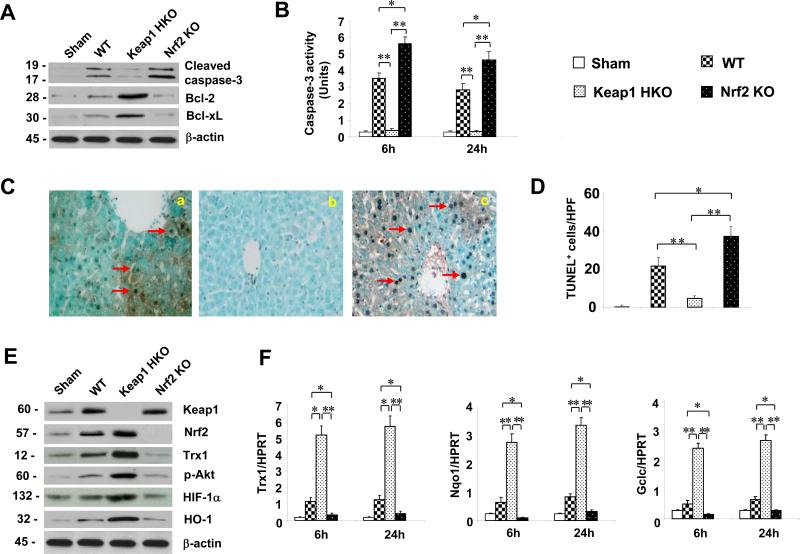
Keap1HKO promotes anti-apoptotic functions and reduces apoptosis in IR-stressed OLTs
By 24h of reperfusion, Keap1 hepatocyte deficiency downregulated Western-assisted expression (AU) of cleaved caspase-3 (Fig. 3A, 0.3-0.4), yet upregulated Bcl-2, and Bcl-xL (1.8-2.0 and 1.6-1.7, respectively) in OLTs. In contrast, Nrf2 deficiency diminished Bcl-2 and Bcl-xL (0.1-0.3 and 0.1-0.2) but increased cleaved caspase-3 (1.5-1.7) expression. These results were confirmed by decreased caspase-3 activity (U) in Keap1HKO transplants (Fig. 3B: 6h: 0.37 0.29, p<0.005; 24h: 0.29 0.09, p<0.0005, respectively), compared with WT (6h: 3.54 0.34; 24h: 2.84 0.39). We then employed TUNEL assay to analyze liver cell apoptosis (Fig. 3C, D). OLTs devoid of hepatocyte-specific Keap1 signaling showed decreased (p<0.001) frequency of TUNEL+ cells (Panel b: 4.6 1.1), as compared with WT (Panel a: 21.6 4.4) or Nrf2- deficient (Panel c: 37.2 5.1) OLTs. Moreover, Keap1 knockdown decreased total percentage of dead cells (apoptotic+necrotic cells=4.4%) in OLTs, as compared with WT controls (32.6%, p<0.0001; Suppl. Fig. 2B). Nrf2 deficiency, however, further increased liver transplant frequency of dead cells (41.8%, p<0.0001).
Figure 3.
Hepatocyte-specific Keap1 deficiency (HKO) promotes anti-apoptotic functions, reduces apoptosis and activates Nrf2-mediated Trx1/Akt/HIF-1 in IR-stressed OLTs. (A) Western analysis of cleaved caspase-3 and Bcl-2/Bcl-xl. Representative of three experiments. (B). Caspase-3 activity. Mean±SD; n=3-4/group; *p<0.005; **p<0.0005. (C/D) TUNEL staining: (a;  ) WT WT; (b;
) WT WT; (b;  ) Keap1HKO Keap1HKO; (c;
) Keap1HKO Keap1HKO; (c;  ) Nrf2KO Nrf2KO. Representative of 4 mice/group; magnification ×200 *p<0.05; **p<0.001. (E) Western analysis of Keap1, Nrf2, Trx1, p-Akt, HIF-1 , and HO-1 in OLTs. β-actin served as an internal control. Representative of three experiments. (F) Quantitative RT-PCR-assisted detection of mRNA coding for Trx1, Nqo1 and Gclc. Data were normalized to HPRT gene expression. Mean±SD; n=3-4/group; *p<0.05, **p<0.01.
) Nrf2KO Nrf2KO. Representative of 4 mice/group; magnification ×200 *p<0.05; **p<0.001. (E) Western analysis of Keap1, Nrf2, Trx1, p-Akt, HIF-1 , and HO-1 in OLTs. β-actin served as an internal control. Representative of three experiments. (F) Quantitative RT-PCR-assisted detection of mRNA coding for Trx1, Nqo1 and Gclc. Data were normalized to HPRT gene expression. Mean±SD; n=3-4/group; *p<0.05, **p<0.01.
Keap1HKO activates Nrf2-mediated Trx1 and Akt/HIF-1 signaling in IR-stressed OLTs
We next investigated the role of Keap1 in the regulation of Nrf2 and other anti-oxidant molecules. By 24h of reperfusion, disruption of hepatocyte Keap1 augmented Western-assisted expression (AU) of Nrf2 and anti-oxidant Trx1 (Fig. 3E, 2.8-3.0 and 3.0-3.2), compared with Nrf2-deficient (0.2-0.4) or WT (0.5-0.7) OLTs. In parallel, Keap1HKO enhanced hepatic p-Akt, and HIF-1 /HO-1 expression (2.9-3.1, 2.8-2.9, and 2.4-2.5, respectively). Furthermore, Keap1 but not Nrf2 knock-out increased mRNA levels coding for anti-oxidant Trx1, Nqo1 and Gclc at both 6h and 24h post-OLT, as compared with WT controls (Fig. 3F).
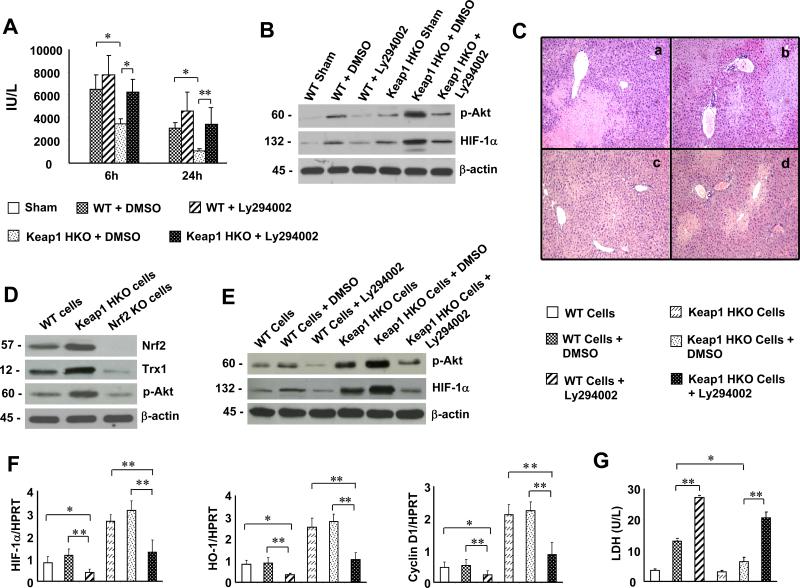
Inhibition of PI3K disrupts Akt/HIF-1 signaling and recreates IRI in Keap1HKO OLTs
As Keap1HKO triggered activation of Nrf2-mediated Trx1 and Akt/HIF-1 (Fig. 3E, F), we then investigated cross-regulation between PI3K/Akt and HIF-1 signaling in our model. Keap1HKO donor mice were treated with PI3K inhibitor (LY294002) prior to liver procurement. At 6h and 24h post-OLT, sALT levels (IU/L) were increased in Keap1HKO transplant group given adjunctive PI3K inhibitor (Fig. 4A, 6h: 6244±1098, p<0.05; 24h: 3428±1430, p<0.01), compared with Keap1HKO controls given DMSO (6h: 3448±437, 24h: 1068±206). OLTs from Keap1HKO donors treated with DMSO showed minimal liver sinusoidal congestion without edema, vacuolization or necrosis (Fig. 4C, Panel c; score= 1.2 0.63, p<0.0005). In contrast, those given PI3K inhibitor in WT or Keap1HKO groups revealed significant edema, sinusoidal congestion, cytoplasmic vacuolization, and necrosis (30-50%; Fig. 4C, Panel c; score=3.2 0.42; Panel d; score=2.5 0.71). Although Keap1HKO increased Western blot-assisted expression (AU) of p-Akt (Fig. 4B, 1.9-2.1) and HIF-1 (1.8-2.0), adjunctive PI3K inhibition decreased p-Akt (0.5-0.7) and HIF-1 expression (0.6-0.8) in IR-stressed OLTs.
Figure 4.
Inhibition of PI3K disrupts Akt/HIF-1 signaling and recreates liver IRI in Keap1HKO OLTs. Groups of WT or Keap1HKO liver donor mice were pre-treated with Ly294002 or DMSO (-1 h). (A) sALT levels (IU/L): (□) sham; ( ) WT+DMSO; (
) WT+DMSO; ( ) WT+ Lly294002; (
) WT+ Lly294002; ( ) Keap1 HKO+DMSO; (
) Keap1 HKO+DMSO; ( ) Keap1 HKO+Ly294002. Mean±SD; n=4 mice/group; *p<0.05, **p<0.01. (B) Western analysis of p-Akt and HIF-1 in OLTs. β-actin served as an internal control. Representative of three experiments. (C) Representative H&E staining of OLTs (n=4) at 24h: Panel (a) WT+DMSO; (b) WT+Ly294002; (c) Keap1HKO+DMSO; (d) Keap1HKO+Ly294002 (magnification x100). Keap1-dependent Nrf2 activation promoted Trx1/Akt/HIF-1 signaling in mouse hepatocytes in vitro. (D) Western blot expression of Nrf2, Trx1 and p-Akt in primary H2O2-stressed hepatocyte (WT, Keap1HKO or Nrf2KO) cultures. Representative of three experiments. (E) Primary H2O2-stressed hepatocytes were pretreated with PI3K inhibitor (LY294002) or DMSO; p-Akt/HIF-1 expression was analyzed by Western blots. Representative of three experiments. (F) Quantitative RT-PCR-assisted detection of HIF-1 , HO-1, and Cyclin D1 in LY294002/DMSO-pretreated H2O2-stressed hepatocyte cultures. (G) LDH release (U/L) in LY294002/DMSO-treated hepatocytes. (F-G): (□) WT cells; (
) Keap1 HKO+Ly294002. Mean±SD; n=4 mice/group; *p<0.05, **p<0.01. (B) Western analysis of p-Akt and HIF-1 in OLTs. β-actin served as an internal control. Representative of three experiments. (C) Representative H&E staining of OLTs (n=4) at 24h: Panel (a) WT+DMSO; (b) WT+Ly294002; (c) Keap1HKO+DMSO; (d) Keap1HKO+Ly294002 (magnification x100). Keap1-dependent Nrf2 activation promoted Trx1/Akt/HIF-1 signaling in mouse hepatocytes in vitro. (D) Western blot expression of Nrf2, Trx1 and p-Akt in primary H2O2-stressed hepatocyte (WT, Keap1HKO or Nrf2KO) cultures. Representative of three experiments. (E) Primary H2O2-stressed hepatocytes were pretreated with PI3K inhibitor (LY294002) or DMSO; p-Akt/HIF-1 expression was analyzed by Western blots. Representative of three experiments. (F) Quantitative RT-PCR-assisted detection of HIF-1 , HO-1, and Cyclin D1 in LY294002/DMSO-pretreated H2O2-stressed hepatocyte cultures. (G) LDH release (U/L) in LY294002/DMSO-treated hepatocytes. (F-G): (□) WT cells; ( ) WT cells+DMSO; (
) WT cells+DMSO; ( ) WT cells+Ly294002; (
) WT cells+Ly294002; ( ) Keap1 HKO cells; (
) Keap1 HKO cells; ( ) Keap1 HKO cells+DMSO; (
) Keap1 HKO cells+DMSO; ( ) Keap1 HKO cells+Ly294002. Mean±SD; n=3-4/group; *p<0.005, **p<0.0005.
) Keap1 HKO cells+Ly294002. Mean±SD; n=3-4/group; *p<0.005, **p<0.0005.
Activation of Keap1-dependent Nrf2 promotes anti-oxidant Trx1 and Akt/HIF-1 signaling in hypoxic hepatocytes
Our in vivo data has implicated the role of hepatocyte-specific Keap1 in mediating Nrf2 activation in IR-stressed OLTs. To further elucidate the regulatory mechanism of Keap1-Nrf2 signaling, we used primary hepatocyte culture system. Hepatocyte Keap1 deficiency or Keap1 siRNA transfection augmented Western–assisted expression (AU) of Nrf2, Trx1, and p-Akt (Fig. 4D, 3.2-3.4, 3.3-3.5, 3.1-3.3; and Suppl. Fig. 3A, Fig. 3A, 3.0-3.2, 2.7-2.9, 2.6-2.8, respectively) in H2O2-stressed hepatocytes, as compared with WT controls (0.5-0.9). In contrast, Nrf2 deficiency or siRNA silencing reduced hepatocyte expression of Trx1 and p-Akt (0.2-0.4) under hypoxia in vitro conditions. Furthermore, Keap1 deficiency or Keap1 siRNA silencing, but not Nrf2 ablation, increased HIF-1 expression (supplementary Fig. 3A, 2.6-2.8 vs 0.4-0.6). PI3K inhibition diminished p-Akt (Fig. 4E, 0.5-0.7) and HIF-1 (0.4-0.6) expression in Keap1-deficient hepatocyte cultures. Consistent with these findings, PI3K inhibition has led to decreased mRNA levels coding for HIF-1 , HO-1, and Cyclin D1 in H2O2-stressed hepatocytes (Fig. 4F). Keap1 HKO or Keap1 siRNA silencing increased cell viability (Suppl. Fig. 3B, 72.8 9.2%, p<0.005) and decreased LDH release (U/L; Fig. 4G, 6.4±1.35; p<0.0005). In contrast, Nrf2 deficiency or PI3K inhibition reversed hepatoprotection seen otherwise in Keap1-deficient hepatocyte cultures, evidenced by decreased cell viability (27.65.2%), and increased LDH levels (20.6±1.93). Moreover, HIF-1 inhibition (YC-1 pretreatment) decreased survival of Keap1-siRNA-transfected hypoxic hepatocytes (Suppl. Fig. 3C, 23.5 9.1, p<0.005), as compared with DMSO controls (70.4 8.9).
Discussion
Although the importance of Nrf2 transcription factor as a master switch of redox homeostasis in a variety of liver pathologies has been established [26], its role in liver IRI remains to be elucidated. In this study, we have identified a novel cytoprotective regulatory mechanism of Keap1-Nrf2 complex in a clinically-relevant mouse model of IR-stressed OLT damage (Suppl. Fig. 1). Although hepatocyte-specific disruption of Keap1 signaling in livers subjected to cold storage imposed IR-resistance and improved post-transplant survival, Nrf2 knock-out exacerbated hepatic IRI, implicating Nrf2 is tightly negatively -regulated by Keap1, which is essential for promoting Nrf2-mediated cytoprotection in IR-stressed OLTs.
Since Nrf2 activation orchestrates an array of cell defensive functions, the regulatory mechanism of Keap1-Nrf2 complex may involve multiple intercellular signaling pathways. We found that hepatocyte disruption of Keap1 activated Nrf2, which then increased induction of Trx1, a key redox regulation component, whereas Nrf2 HKO abolished Trx1 expression. Indeed, Trx1 may activate a number of transcription factors that contribute to cell growth/survival [27]. For instance, inhibition of cardiac Trx1 increased myocardial oxidative stress leading to heart failure [28], suggesting an important anti-oxidant regulatory role of Trx1. Our study has revealed that Keap1-dependent Nrf2 activation enhanced Akt phosphorylation and HIF-1 signaling, leading to increased transcription of Nqo1/Gclc genes. This indicates that Keap1-Nrf2 positively regulates the cellular anti-oxidant network in IR-stressed OLTs. Consistent with the ability of Trx1 to stimulate cell survival by PI3K/Akt [29] and HIF-1 signaling in cancer cells [30], we now show that Keap1-dependent Nrf2 augmented Trx1, as well as promoted PI3K/Akt and HIF-1 activation.
The question arises as to whether Nrf2-induced Akt can mediate HIF-1 signaling in our model? It has been reported that disruption of prolyl-hydroxylases (PHD1), a hydroxylase leading to degradation of HIF-1, increased hypoxia tolerance by reducing oxidative stress and reprogramming hepatocellular metabolism [31]. In addition, activation of HIF-1 promoted hepatocyte survival via Wnt- -catenin - HIF-1 interaction [32]. Our data suggests that HIF-1 activity was mediated by Akt pathway, as PI3K/Akt blockade in the donor liver impeded HIF-1 expression, resulting in IR-damage in both WT and Keap1HKO transplants.
Next, we used mouse H2O2-stressed primary hepatocyte cultures to analyze downstream molecular mechanisms by which Keap1-Nrf2 may regulate PI3K/Akt signaling in liver IRI. First, we found that Keap1 deficiency or siRNA silencing resulted in enhanced hepatocyte expression of Nrf2, Trx1, p-Akt, and HIF-1α, as well as increased cell survival (Suppl. Fig. 3A/B). In marked contrast, disruption of Nrf2 or knockdown of both Keap1 and Nrf2 diminished expression of these genes and decreased hepatocyte viability, suggesting that Keap1-dependent Nrf2 induced Trx1, and promoted PI3K/Akt signaling under hypoxia conditions. Second, blocking PI3K/Akt suppressed HIF-1 , which is critical for cell survival, as evidenced by decreased cell viability after pretreatment of Keap1-silenced hepatocytes with HIF inhibitor (Suppl. Fig. 3C). Third, inhibition of PI3K/Akt decreased mRNA levels coding for HO-1, one of HIF-1 target genes known to exert multiple regulatory functions in oxidative stress, inflammation, and apoptosis [3, 4]. Consistent with our findings, HO-1 induction in hepatocytes increased the resistance to cell death during endotoxemia [33], whereas hepatocyte-specific HO-1 deletion disrupted redox homeostasis in basal and oxidative conditions [34]. We have shown that HO-1 overexpression induced Stat3-mediated -catenin, which in turn inhibited TLR4 innate inflammatory response via a negative feedback regulation [35]. Although in the present study, the hepatocellular damage by 1h post-OLT was comparable in all animal groups, a sharp increase in sALT levels at 6h and 24h correlates with enhanced inflammatory cell infiltration in OLTs. It is likely that Keap1-deficient cells are more resistant to oxidative stress generated by inflammatory cells. Indeed, HIF-1 induced HO-1 in Keap1-deficient cells diminished both macrophage/neutrophil trafficking, and proinflammatory cytokines in IR-stressed OLTs. In addition, PI3K/Akt blockade reduced HO-1 expression, leading to increased LDH release in hepatocyte cultures. Hence, HIF-1 -induced HO-1 upregulation represents one of the important cytoprotective mechanisms against hepatic IRI.
We found that Keap1-Nrf2 mediated Akt activation enhanced Cyclin D1 expression, accompanied by increased cell survival (Suppl. Fig. 3B) and decreased LDH release in hypoxic hepatocyte cultures. However, blocking PI3K/Akt has led to decreased Cyclin D1 transcription. Indeed, Akt, also known as Protein Kinase B, promotes cell survival by phosphorylating Bcl-2/ Bcl-xL–associated death promoter (BAD), a pro-apoptotic protein of the Bcl-2 family, and inhibiting caspase-mediated cell death program [36]. Moreover, Akt regulates Cyclin D1 during cell proliferation and apoptosis [37]. Overexpression of Cyclin D1 was also shown to increase tolerance to apoptosis and to promote cancer cell survival [38]. Consistent with in vitro data, our in vivo results support the regulatory role of Keap1-Nrf2 mediated PI3K/Akt axis in hepatocyte death. Keap1HKO upregulated Bcl-2/Bcl-xL but downregulated cleaved caspase-3 expression/ activity, which in turn decreased apoptotic cell death in IR-stressed OLTs. Our results highlight the regulatory function of Keap1-Nrf2 to trigger PI3K/Akt signaling and prevent IR-induced liver cell apoptosis. In agreement with our finding, active PI3K-Akt pathway enabled Nrf2 to promote metabolic activities that support cell proliferation in addition to enhancing cytoprotection [39].
In conclusion, Keap1-Nrf2 complex ameliorated hepatic IRI in OLTs through Keap1 negatively regulating Nrf2 activity (Suppl. Fig. 1). Hepatocyte Keap1 deficiency facilitated Nrf2 nuclear translocation and activated Trx1, an ARE targeting gene. In turn, Trx1 promoted PI3K/Akt, crucial for HIF-1 signaling. HIF-1 -mediated antioxidant HO-1 and CyclinD1 expression resulted in cytoprotection by downregulating hepatic inflammation and apoptosis. By identifying new molecular pathways of Keap-1-Nrf2 regulation, the novel therapeutic strategy could be established to develop Nrf2 pharmacological inducers in the management of inflammatory injury in liver transplant recipients.
Supplementary Material
Acknowledgments
Financial support: NIH Grant DK 062357; The Diann Kim and The Dumont Research Foundations.
List of abbreviations
- ARE
anti-oxidant response element
- HIF-1
hypoxia inducible factor-1
- HKO
hepatocyte knock-out
- HO-1
heme-oxygenase-1
- HRE
hypoxia response element
- H2O2
hydrogen peroxide
- IRI
ischemia/reperfusion injury
- Keap1
Kelch-like ECH-associated protein 1
- LDH
lactate dehydrogenase
- Nrf2
nuclear factor erythroid 2-related factor 2
- OLT
orthotopic liver transplantation
- PI3K
phosphoinositide 3-kinase
- sALT
serum alanine aminotransferase
- Trx1
Thioredoxin 1
- TUNEL
terminal deoxyribonucleotidyl transferase (TdT)-mediated dUTP-digoxigenin nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 3.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905–912. doi: 10.1097/00007890-200210150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 7.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, et al. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med. 2011;51:216–224. doi: 10.1016/j.freeradbiomed.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao HD, Zhang F, Shen G, Li YB, Li YH, Jing HR, et al. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16:3002–3010. doi: 10.3748/wjg.v16.i24.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaman MB, Leonard MO, Ryan EJ, Nolan NP, Hoti E, Maguire D, et al. Lower expression of Nrf2 mRNA in older donor livers: a possible contributor to increased ischemiareperfusion injury? Transplantation. 2007;84:1272–1278. doi: 10.1097/01.tp.0000288229.53064.e2. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 16.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 17.Reisman SA, Yeager RL, Yamamoto M, Klaassen CD. Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol Sci. 2009;108:35–47. doi: 10.1093/toxsci/kfn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 20.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 21.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56:359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. Mouse liver cell culture. I. Hepatocyte isolation. In Vitro. 1981;17:913–925. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- 26.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, et al. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Im JY, Lee KW, Woo JM, Junn E, Mouradian MM. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet. 2012;21:3013–3024. doi: 10.1093/hmg/dds131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- 31.Schneider M, Van Geyte K, Fraisl P, Kiss J, Aragones J, Mazzone M, et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 2010;138:1143–1154. e1141–1142. doi: 10.1053/j.gastro.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 32.Lehwald N, Tao GZ, Jang KY, Sorkin M, Knoefel WT, Sylvester KG. Wnt-beta-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology. 2011;141:707–718. 718, e701–705. doi: 10.1053/j.gastro.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorman RB, Bajt ML, Farhood A, Mayes J, Jaeschke H. Heme oxygenase-1 induction in hepatocytes and non-parenchymal cells protects against liver injury during endotoxemia. Comp Hepatol. 2004;3(Suppl 1):S42. doi: 10.1186/1476-5926-2-S1-S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamiya T, Katsuoka F, Hirayama A, Nakajima O, Kobayashi A, Maher JM, et al. Hepatocyte-specific deletion of heme oxygenase-1 disrupts redox homeostasis in basal and oxidative environments. Tohoku J Exp Med. 2008;216:331–339. doi: 10.1620/tjem.216.331. [DOI] [PubMed] [Google Scholar]
- 35.Ke B, Shen XD, Kamo N, Ji H, Yue S, Gao F, et al. beta-catenin regulates innate and adaptive immunity in mouse liver ischemia-reperfusion injury. Hepatology. 2013;57:1203–1214. doi: 10.1002/hep.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 37.Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, et al. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes. 2006;55:318–325. doi: 10.2337/diabetes.55.02.06.db05-0757. [DOI] [PubMed] [Google Scholar]
- 38.Roue G, Pichereau V, Lincet H, Colomer D, Sola B. Cyclin D1 mediates resistance to apoptosis through upregulation of molecular chaperones and consequent redistribution of cell death regulators. Oncogene. 2008;27:4909–4920. doi: 10.1038/onc.2008.126. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.