Abstract
Past research suggests that as many as 50% of onward human immunodeficiency virus (HIV) transmissions occur during acute and recent HIV infection. It is clearly important to develop interventions which focus on this highly infectious stage of HIV infection to prevent further transmission in the risk networks of acutely and recently infected individuals. Project Protect tries to find recently and acutely infected individuals and prevents HIV transmission in their risk networks. Participants are recruited by community health outreach workers at community-based HIV testing sites and drug users’ community venues, by coupon referrals and through referrals from AIDS clinics. When a network with acute/recent infection is identified, network members are interviewed about their risky behaviors, network information is collected, and blood is drawn for HIV testing. Participants are also educated and given prevention materials (condoms, syringes, educational materials); HIV-infected participants are referred to AIDS clinics and are assisted with access to care. Community alerts about elevated risk of HIV transmission are distributed within the risk networks of recently infected. Overall, 342 people were recruited to the project and screened for acute/recent HIV infection. Only six index cases of recent infection (2.3% of all people screened) were found through primary screening at voluntary counseling and testing (VCT) sites, but six cases of recent infection were found through contact tracing of these recently infected participants (7% of network members who came to the interview). Combining screening at VCT sites and contact tracing the number of recently infected people we located as compared to VCT screening alone. No adverse events were encountered. These first results provide evidence for the theory behind the intervention, i.e., in the risk networks of recently infected people there are other people with recent HIV infection and they can be successfully located without increasing stigma for project participants.
Keywords: HIV, acute infection, recent infection, prevention, social networks intervention
Introduction
Early human immunodeficiency virus (HIV) infection accounts for a large share of transmission events. Even in the long-established epidemic among Montreal men who have sex with men (MSM), half of transmission events came from a recently infected transmitter (Ambrosioni et al., 2012; Brenner et al., 2007, 2011). The HIV outbreak among injection drug users (IDUs) in Athens is driven by recent HIV infections (Fotiou et al., 2012; Paraskevis et al., 2011). Studies of serodiscordant couples in Malawi (Powers et al., 2010) and Uganda (Wawer et al., 2005) report about 40–50% of heterosexual transmission of HIV to be attributed to sexual contacts with recently infected individuals. HIV transmission from the recently infected can be decreased by early administration of antiretroviral treatment soon after infection (Cohen et al., 2011). Finding people early in infection is challenging since even education about its symptoms does not increase case-finding (Stekler, Baldwin, Louella, Katz, & Golden, 2013).
Thus, finding recently infected people and linking them to care are an emerging goal of HIV prevention.
The Project Protect intervention aims to reduce HIV infections by developing novel ways to locate recently infected people by using social and risk network techniques and to reduce transmission even by those who cannot be located and who are not aware of their status (Friedman, Vasylyeva, & Smyrnov, 2012). We expected that networks with an acute/recent infection case would include others who have recently become infected, and that by reaching them rapidly we might prevent further transmission. Project Protect has mostly targeted IDUs but also intervened in their extended sexual and social networks. It has been implemented in six cities of Ukraine at different times. Here we describe Project Protect as it was implemented in cities of Krivoy Rog and Lviv, Ukraine, from November 2011 to August 2012.
Intervention design
The intervention started with community education about recent HIV infection and its risks conducted at small group meetings with IDUs and with community distribution of informational materials. Intervention protocol was reviewed and approved by Institutional Review Board of Gromashevsky Institute of Epidemiology and Infectious Diseases of the Academy of Medical Science of Ukraine. Informed consent was obtained from each participant.
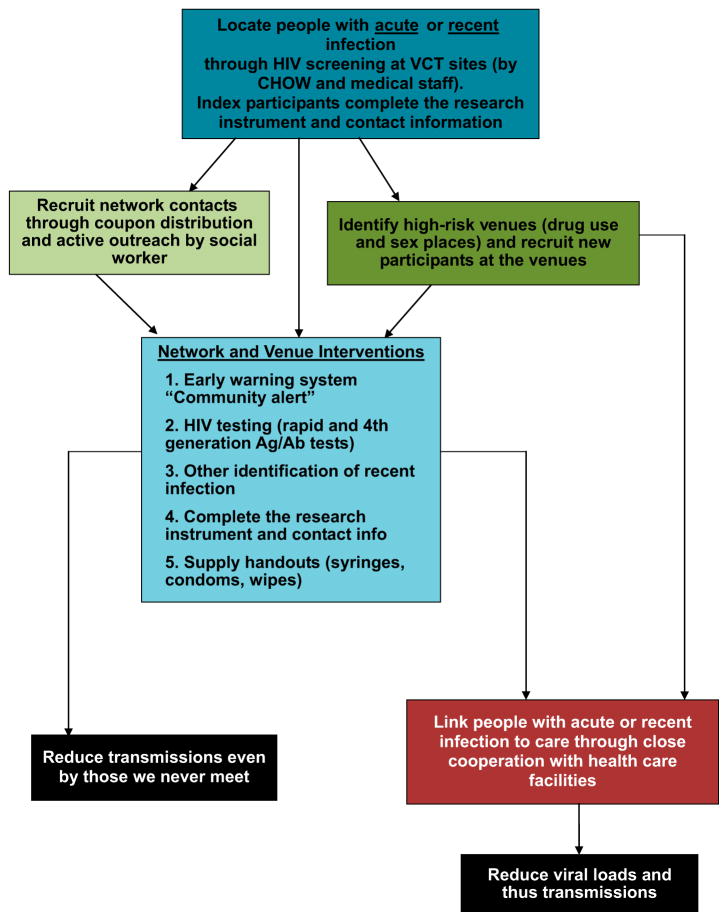
Primary screening was conducted at community-based HIV testing sites and government AIDS clinics (Figure 1), where people who were tested were subsequently invited to participate in the project and, if agreed, signed informed consents. They provided contact information, previous HIV test result, took a rapid HIV test and, if negative, provided a blood sample for additional testing (Genscreen ultra HIV Ag/Ab test to identify acute infection). Participants received no incentive for this initial enrollment.
Figure 1.
Intervention flow.
Although some studies combine rapid HIV-1/2 (HIV) antibody testing with pooled HIV-1 RNA testing to find acute HIV infection cases (Christopoulos et al., 2013; Martin et al., 2013), this is not feasible in many localities. We used a combination of rapid and Ag/Ab and Ag tests (Centers for Disease Control and Prevention, 2013; Gilbert et al., 2013) to define acute infection. Recent infection was defined as having positive rapid test result and negative result within prior 6 months or being younger than 21 years old and never having had an HIV-positive test result before. We used 21 years old as a cut point because HIV-positive younger IDUs are fairly likely to be recently infected with HIV and we wanted to prevent onward transmission if that was the case. All index and tracing-derived acute/recent cases were invited to have in-depth interviews with informed consent and to provide contact and venue information. All interviewed participants received 40 hryvnas (5 USD).
Interviewers asked participants about people they inject or have sex with (risk partners), other people who were present when they had sex or injected drugs, and people from venues where participants practiced risky behavior (injected together, met sex partners or had sex) during the last 6 months. They were given coupons to invite network members for interviews (coupon referral). If participants refused to distribute coupons, interviewer asked about other ways to recruit their network members to the project such as giving us the contact’s address/phone or organizing an “accidental” meeting with a community health outreach worker (CHOW).
All participants were informed about the risks of acute/recent HIV infection and were asked to distribute community alert leaflets among their risk partners and at their venues. Community alerts stated that a highly infectious HIV case was identified in a particular community and warned readers to be “super careful” during the next 6 months to stay safe. Alerts explained how people can protect themselves and others in this period and included antistigma messages to prevent violence toward HIV-positive participants. If participants did not want to distribute community alerts themselves, other ways to distribute community alerts were often worked out.
After the interview, CHOWs organized venue visits to reach participant’s risk contacts and distribute community alerts.
All HIV-positive participants were referred to AIDS clinics for diagnosis confirmation.
Results
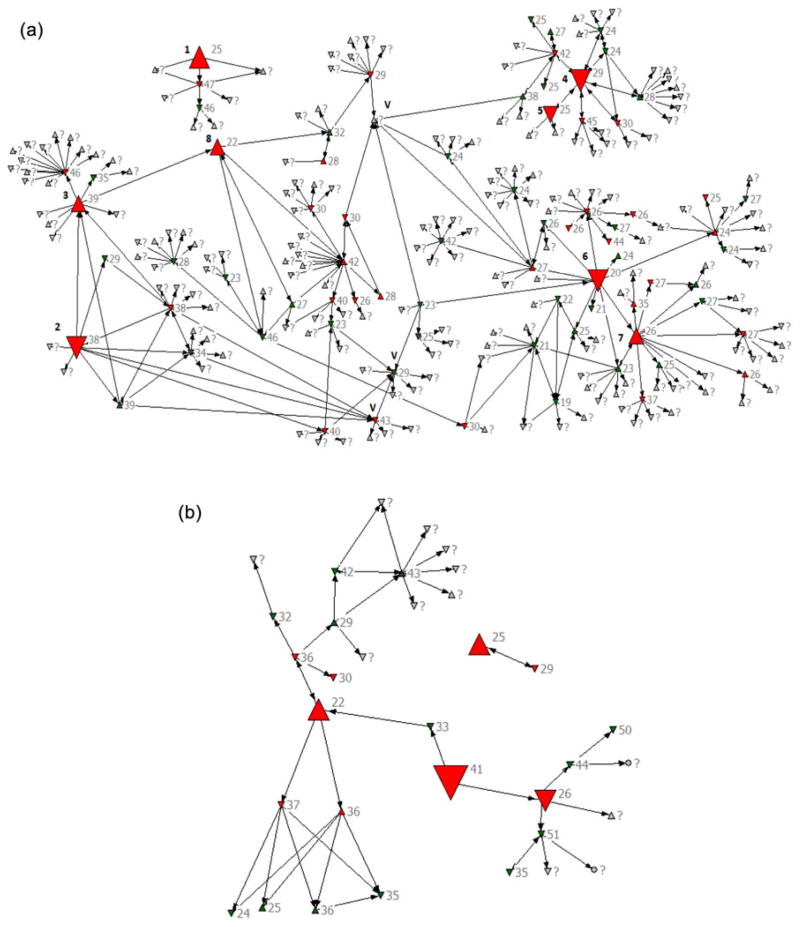
In Krivoy Rog (Figure 2a), four index cases with recent infection were found through routine screening at voluntary counseling and testing (VCT) sites; four additional recent cases were found through tracing of the index participants’ contacts (Table 1).
Figure 2.
Network recruitments: (a) Kriviy Rig and (b) Lviv.
Note: Up triangle indicates female; green color indicates HIV negative; labels indicate age. Down triangle indicates male; red color indicates HIV positive; “?” indicates unknown age. Circle indicates unknown sex; grey color indicates unknown HIV status. The largest triangles indicate index cases (recent or acute HIV infection), big triangles indicate network-recruited recent or acute HIV infection. V – volunteers on Kriviy Rig who helped CHOWs to recruit contacts named at the interview. [To view this figure in colour, please see the online version of this journal.]
Table 1.
Description of recruitment, network/venue tracing, and recent infections.
| # | Category of participants | Number in Kriviy Rig | Number in Lviv | Total number |
|---|---|---|---|---|
| 1. | Total number of people screened for HIV, whether indexa cases or as network/venue recruits | 217 | 125 | 342 |
| 2. | Total number of participants who got positive result of rapid HIV antibody test | 87 | 14 | 101 |
| 3. | Total number of recently infected individuals | 8 | 4 | 12 |
| • Had preceding negative HIV test result | 6 | 4 | 10 | |
| • Were younger than 21 years old | 2 | 0 | 2 | |
| 4. | Indexa participants who were initially selected for interviews and interviewed because they were recently HIV infected | 4 | 2 | 6 |
| 5. | People named at interviews of recently infected participants as members of their risk networks | 251 | 42 | 293 |
| 6. | Number of risk network members who were recruited to the project (tested and interviewed) | 66 | 20 | 86 |
| 7. | Uninfected risk network members who were recruited to the project | 30 | 9 | 39 |
| 8. | Recently infected risk network members who were recruited to the project | 4 | 2 | 6 |
| 9. | Long-termb infected risk network members who were recruited to the project | 32 | 9 | 41 |
| 10. | Number of venues visited by project team | 3 | 3 | 6 |
| 11. | Number of community alerts distributed through risk networks and at the venues of recent cases | 246 | 92 | 338 |
Index cases are those recruited to the project at community-based HIV-testing sites or government AIDS clinics at primary screening.
Long-term infected members are those who have HIV Ab-positive test and do not meet the recent/acute infection case criteria.
In Krivoy Rog, two risk network clusters of 9 and 187 members were observed, including those named but not recruited; 25% of named contacts were recruited into the project (n = 3 and 47 for each network cluster, respectively). Eight participants (4%) were acute/recent cases (n = 1 and 7, respectively).
In Lviv (Figure 2b), one index case with recent infection was found through screening at VCT sites, one through referral from an AIDS clinic (non-IDU), and two recent cases were found through tracing index participants’ contacts.
In Lviv, there were two risk network clusters (n = 24 and 2); 85% of contacts named at the interviews were successfully recruited into the project (n = 20 and 2, for each network cluster respectively). Four participants (15%) were identified as recent cases (n = 3 and 1, respectively).
In Lviv, fewer contacts were named during each interview. Consequently, CHOWs put greater efforts to invite every named risk partner for an interview which resulted in higher rates of contact tracing.
In Krivoy Rog and Lviv, CHOWs organized six venue visits. All people present at the venue were tested (both rapid and fourth-generation tests), interviewed (or invited for an interview at the project site), and informed about risks of acute HIV infection (community alerts were distributed). None were recent cases but all are included in Figure 2.
Discussion
Twelve cases of recent infection were found (3.5% of all participants who were tested as part of primary screening or network members recruitment) if we include two IDUs who were less than 21 years old. Six index recent infection cases (2.3% of all people screened) were found through primary screening at VCT sites and six recent infection cases (including two IDUs who were less than 21 years old) were found through contact tracing of recently infected participants (7% of network members who came to the interview). This strongly supports the underlying hypothesis of this intervention: combining VCT and network recruitment can increase efficiency of locating people who have recently been infected at VCT sites. This “yield” could be further increased by using assays for recent infection (such as Limiting Antigen-Avidity (LAg) test; Duong et al., 2012).
There were no reported cases of cruelty, violence, or stigmatization resulting from project activities.
Primary screening for recent cases including venous blood sampling can be successfully done at VCT sites and at AIDS clinics. Recruitment of the risk contacts of index cases through coupon referrals, contact tracing and venue visits is possible and can be successful. The opportunity to have the “acute infection” test and receive the most up-to-date information about HIV status is a strong motivation to participate.
Prior community education about the project played an important role and let it both screen more people and minimize stigmatization of project participants.
The project faced several problems. Contact tracing took time: at least one week to get risk contacts to an interview and two weeks to organize a venue visit. Such delay might allow preventable HIV transmission to occur. Furthermore, since some participants were reluctant to name and describe members of their risk networks, not all risk contacts could be followed.
Conclusions
The project was successful in recruiting people with recent infection. As expected, we found a relatively high density of recently infected people in risk networks of recently infected index participants. No resources were available to study the extent to which infections were averted or participants successfully linked to care. Our team has begun research to study these outcomes (Friedman et al., 2013).
Acknowledgments
We would like to thank Konstantin Dumchev of CDC-Ukraine and Georgios Nikolopoulos of NDRI for their assistance.
Funding
We gratefully acknowledge support from National Institute on Drug Abuse grants [grant number P30 DA11041] (Center for Drug Use and HIV Research); [grant number DP1 DA034989] (Preventing HIV Transmission by Recently-Infected Drug Users); and National Institutes of Health [grant number D43TW000233] funded by the Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Ambrosioni J, Junier T, Delhumeau C, Calmy A, Hirschel B, Zdobnov E, Yerly S. Impact of highly active anti-retrovival therapy on the molecular epidemiology of newly diagnozed HIV infections. AIDS. 2012;26:2079–2086. doi: 10.1097/QAD.0b013e32835805b6. [DOI] [PubMed] [Google Scholar]
- Brenner BG, Roger M, Routy JP, Moisy D, Ntemgwa M, Matte C, Wainberg MA. High rates of forward transmission events after acute/early HIV-1. The Journal of Infectious Diseases. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- Brenner BG, Roger M, Stephens D, Moisi D, Hardy I, Weinberg J, Wainberg MA. Transmission clustering drives the onward spread of the HIV epidemic among men who have sex with men in Quebec. The Journal of Infectious Diseases. 2011;204:1115–1119. doi: 10.1093/infdis/jir468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm – United States. Morbidity and Mortality Weekly Report. 2013 Jun 21;62(24):489–494. [PMC free article] [PubMed] [Google Scholar]
- Christopoulos KA, Zetola NM, Klausner JD, Haller B, Louie B, Hare CB, Pilcher CD. Leveraging a rapid, round-the-clock HIV testing system to screen for acute HIV infection in a large urban public medical center. Journal of Acquired Immune Deficiency Syndromes. 2013;62(2):e30–e38. doi: 10.1097/QAI.0b013e31827a0b0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong YT, Maofeng Q, Anindya KD, Jackson K, Dobbs T, Kim AA, Parekh BS. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: Potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7:e33328. doi: 10.1371/journal.pone.0033328.t003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiou A, Micha K, Paraskevis D, Terzidou M, Malliori MM, Hatzakis A. HIV outbreak among injecting drug users in Greece. An updated report for the EMCDDA on the recent outbreak of HIV infections among drug injectors in Greece. Lisbon: EMCDD; 2012. [Google Scholar]
- Friedman SR, Downing MJ, Jr, Smyrnov P, Nikolopoulos G, Schneider JA, Livak B, Hatzakis A. Socially-integrated transdisciplinary HIV prevention. AIDS and Behavior. 2013 doi: 10.1007/s10461-013-0643-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Vasylyeva T, Smyrnov P. Recently-funded transdisciplinary integrated HIV prevention project: overview and challenges. 2012 Retrieved from http://somatosphere.net/2012/10/recently-funded-transdisciplinary-integrated-hiv-prevention-project-overview-and-challenges.html.
- Gilbert M, Cooka D, Steinberga M, Kwaga M, Roberte W, Doupea G, Rekart M. Targeting screening and social marketing to increase detection of acute HIV infection in men who have sex with men in Vancouver, British Columbia. AIDS. 2013;27:2649–2654. doi: 10.1097/QAD.0000000000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EG, Salaru G, Mohammed D, Coombs RW, Paul SM, Cadoff EM. Finding those at risk: acute HIV infection in Newark, NJ. Journal of Clinical Virology. 2013;58(1):e24–e28. doi: 10.1016/j.jcv.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas M, Hatzakis A. HIV-1 outbreak among injection drug users in Greece, 2011: a preliminary report. Euro Surveillance. 2011;16(36) doi: 10.2807/ese.16.36.19962-en. pii–19962. [DOI] [PubMed] [Google Scholar]
- Powers K, Ghani A, Miller WC, Hoffman I, Pettifor A, Kamanga J, Cohen MS. The contribution of early HIV infection to HIV spread in Lilongwe, Malawi: Implications for transmission prevention strategies. Presented at the XVIII International AIDS Conference; Vienna, Austria. 2010. [Google Scholar]
- Stekler JD, Baldwin HD, Louella MW, Katz DA, Golden MR. ru2hot? A public health education campaign for men who have sex with men to increase awareness of symptoms of acute HIV infection. Sexually Transmitted Infections. 2013;89:409–414. doi: 10.1136/sextrans-2012-050730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, Rakai, Uganda. The Journal of Infectious Diseases. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]