Abstract
Introduction
Ethanol infusion was an early mode of ablative treatment for cardiac arrhythmias. Its initial descriptions involved coronary intra-arterial delivery, targeting arrhythmogenic substrates in drug-refractory ventricular tachycardia or the atrioventricular node. Largely superseded by radiofrequency ablation (RFA) and other contact-based technologies as a routine ablation strategy, intracoronary arterial ethanol infusion remains as an alternative option in the treatment of ventricular tachycardia when conventional ablation fails. Arrhythmic foci that are deep-seated in the myocardium may not be amenable to catheter ablation from either the endocardium or the epicardium by RFA, but they can be targeted by an ethanol infusion.
Recent findings
Recently, we have explored ethanol injection through cardiac venous systems, in order to avoid the risks of complications and limitations of coronary arterial instrumentation. Vein of Marshall ethanol infusion is being studied as an adjunctive procedure in ablation of atrial fibrillation, and coronary venous ethanol infusion for ventricular tachycardia.
Conclusion
Ethanol ablation remains useful as a bail-out technique for refractory cases to RFA, or as an adjunctive therapy that may improve the efficacy of catheter ablation procedures.
Keywords: cardiac arrhythmia, catheter ablation, ethanol
INTRODUCTION
Radiofrequency ablation (RFA) has become the standard of care for catheter ablation of cardiac arrhythmias. Other ablative technologies are available depending on the tissue being targeted, and include cryoablation, [1] laser [2], and high-intensity focused ultrasound [3], among others. Common to all is the requirement of some degree of contact between an intracardiac catheter and a destructive energy of physical nature. The historical evolution of catheter ablation, however, commenced with the concept of delivering a cytotoxic agent through the arterial vasculature, supplying the targeted myocardium, which was pioneered in Zipes’ laboratory [4,5]. The most common type of alcohol utilized for this purpose is ethanol, which is highly cytotoxic and has multiple other uses in humans. Today, arrhythmogenic foci that are deep-seated in the myocardium and are not amenable to catheter ablation from either the endocardium or the epicardium using contact-based physical forms of ablative energy can still be targeted with intravascular ethanol injection.
The purpose of this review is to discuss the basic principles of ethanol ablation, summarize the clinical results achieved to date, and propose future implications.
ETHANOL MECHANISMS OF ACTION, DOSE, AND RATE OF INFUSION
Ethanol (CH3CH2OH) is a short-chain alcohol, water-soluble compound that rapidly crosses the cell membranes. When cells are exposed to high concentrations, ethanol solubilizes the cell membranes and alters the tertiary protein structures, leading to immediate cell destruction [6,7]. Most of the fluid membranes, including those that are low in cholesterol, are the most easily solubilized by ethanol. Ethanol interferes with the packing of molecules in the phospholipid bilayer of the cell membrane, thus increasing membrane fluidity. At lower concentrations, such as those achieved after oral intake, cytotoxicity is less immediate. The nonoxidative metabolism of alcohol with the formation of phosphatidilethanol and fatty acid alcohol ester disrupt the intracellular signal transduction. Impairment of intracellular transduction, degradation of proteins, and mitochondrial injury are other mechanisms associated with the cytotoxicity of short-chain alcohols [6]. Production of reactive oxygen species like superoxide anion, lipid peroxy radical, and alcohol radicals attached amines and sulfhydryl groups, which degrade proteins and cytochromes [8]. A variety of mechanisms may account for the biochemical alterations of mitochondria that are induced by alcohols. These effects are related to the hydrophobicity of the alcohols and are accompanied by decreases in mitochondrial ATPase activity [9]. Although effects of ethanol to decrease mitochondrial function and depress energy balance within the liver are clear, such effects in cardiac mitochondria are not well studied. Data are lacking that indicate the effect of ethanol on heart mitochondria, but its association has been reported with ethanol-induced cardiomyopathy [10], and it may not be significant in the acute setting [11].
Absolute ethanol was first reported in the anesthesiology literature for the purposes of pain control, using a trans-sphenoidal injection approach to ethanol-induced hypophysectomy [12-15]. Although the exact mechanisms of neuro-adenolysis of the pituitary for cancer pain control remained unclear, autopsy studies revealed necrosis of the pituitary gland [12]. Ethanol-induced neurolysis remains a valuable therapeutic strategy for intractable cancer pain [16], and it is under this indication that it is labeled in the manufacturer’s package insert. The strategy of using intra-arterial ethanol infusion for tissue ablation was developed for ablating renal tissue. It was observed that absolute ethanol injected into the renal artery at a rate approximating renal arterial blood flow, distributed rapidly to the renal parenchyma, causing cell necrosis [12,15].
The cytotoxicity of ethanol at intravascular concentrations can be attributed to various mechanisms. Ethanol, when injected at slower rates – at which it is assumed to undergo some degree of dilution – produces denaturation of erythrocytes; activation of the coagulation cascade and thrombosis; aberrations in phospholipid and fatty acid metabolism; changes in the cellular redox state; disruption of energy state; and an increase of reactive metabolites [6]. The optimal ethanol concentration and rate of infusion for arrhythmia ablation are controversial and have yet to be clarified. Additionally, vascular damage with sclerosis of the injected vessel follows routinely after infusion, and therefore, with intra-arterial infusions, tissue ischemia and infarction of the injected territory are expected to play a role in ethanol’s therapeutic effect.
ATRIOVENTRICULAR NODAL ABLATION WITH ETHANOL
In humans, the atrioventricular nodal (AVN) artery can arise from either the right coronary artery (most common) or the left circumflex artery. Selective cannulation of the readily identifiable AVN artery is possible. Brugada et al. [17] were the first to demonstrate termination of atrioventricular re-entry by cannulating the AVN artery and infusing cold saline into it. Proof-of-concept canine studies showed AVN block after absolute ethanol infusion in the AVN artery [18]. The feasibility of achieving complete, durable AVN block by ethanol infusion in the AVN artery was then proven in six patients [12]. After selective engagement of the AVN artery, isotonic cold saline infusion was shown to produce transient AVN block, confirming irrigation of the AVN by the target vessel (see Fig. 1). The usual dose implemented for AVN ablation with selective intracoronary injection was ethanol 95%, as a bolus of 0.5–2 ml per dose over 1–4 s and up to three times. Efficacy and safety of AVN ablation with ethanol was rapidly confirmed by other groups [19,20], although the long-term success appeared to be variable [18-21]. The rate of success ranges between 58 and 72%. If acute success is not achieved with an initial ethanol infusion, a repeat ablation may be attempted, but it could be technically challenging due to occlusion of the AVN artery after the first procedure. The durability of the lesion originated by this method was studied in 1991 by de Swart et al. Eleven patients were followed for 10 months, three of which regained AVN conduction within the first 30 days [18]. Predicting the degree of AVB or recovery of AVN function over time was not possible, even by carefully controlling the infusion rate and ethanol concentration. Slow pathway ablation for AVN or AVN modification was reported in dogs by using slow ethanol infusions or diluted ethanol (25 or 50%) [20], but the inability to predict complete atrioventricular block with this technique would make it unsuitable for AVN re-entry. Table 1 summarizes the reported results of ethanol ablation of the AVN.
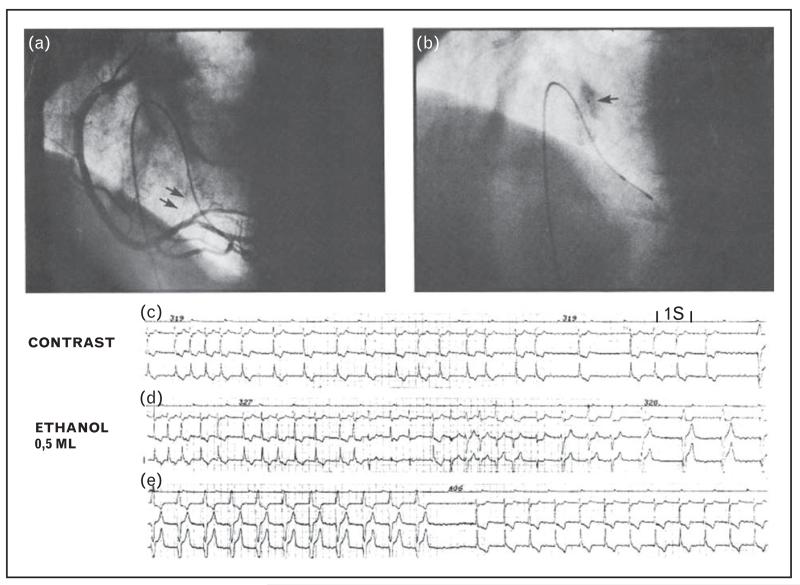
FIGURE 1.
Ethanol ablation of the atrioventricular node. Panel a shows a right coronary angiogram in left anterior oblique projection. Atrioventricular nodal artery is indicated by arrow. Panel b shows selective catheterization of atrioventricular nodal artery with 2.5F catheter (arrow). Contrast is given showing myocardial staining and no backflow. Panel c shows effects of contrast material on ventricular rate during atrial fibrillation. Transient complete atrioventricular block and pacemaker rhythm is observed in right part of electrocardiographic strip. Leads I, II, and III are shown. Panel d shows effects of administration of 0.5 ml 96% ethanol. After a series of premature ventricular beats, complete atrioventricular block and pacemaker rhythm occur. Panel e shows escape rhythm after interruption of pacing (from Brugada et al. [18]).
Table 1.
Results of transcoronary ethanol ablation of the atrioventricular node
| Study reference |
Year | Study design |
Patients (n) |
Age (years) |
Indications | Follow-up | Results | Complications | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Brugada et al. [18] |
1990 | Intracoronary 96% ethanol injection |
7 | 64.3 ± 5.4 | Atrial fibrillation with rapid ventricular rates. Normal coron- aries. Free from struc- tural heart disease. |
1–7 months (mean 4) |
Complete AV block: 5 patients (71%) |
100% transient chest pain |
Successful approach. Delivering catheter should be appropri- ately wedged to pre- vent ethanol backflow. |
| Modified AV conduc- tion and symptom control: 2 patients (29%) |
Inferior wall MI: 1 patient (14%) |
||||||||
| Minimal enzyme rise (AST): 6 patients (86%) |
|||||||||
|
| |||||||||
| Kay et al. [19] |
1991 | Intracoronary 96% ethanol injection |
12 (10 received ethanol) |
57.9 ± 13.3 | Atrial fibrillation 4, atrial flutter 4, AV node re-entrant tachy- cardia 3, ectopic atrial tachycardia 1 |
48–216 days (mean 134.8) |
Complete AV block at discharge: 10 patients (100%) |
100% transient chest pain |
Feasible and low-risk procedure. Reflux into the distal right coron- ary artery may occur. |
| Recovery of AV conduc- tion at follow-up: 3 patients (30%) |
ST-segment elevation inferior leads (reflux into the distal RCA): 2 patients (20%) with enzyme rise (CKMB) |
||||||||
|
| |||||||||
| Sneddon et al. |
1991 | Intracoronary dehy- drated alcohol injection |
14 (10 received ethanol) |
54–75 (mean 64) |
Refractory atrial flutter or fibrillation |
4–7months (mean 5.35) |
Complete AV block immediately after injection in all the patients |
Modest transient rise in CK-MB |
Feasible approach. Modest success rate and potential compli- cations reserve this technique as an alternative after fail- ure of other ablative treatments. |
| AV conduction returned in 4 patients (40%) |
Chest discomfort. | ||||||||
| VFib in 2 patients (20%) with contrast injection |
|||||||||
| Coronary artery spasm in 3 patients after cold saline injection (21%) |
|||||||||
|
| |||||||||
| Strickberger et al. [21] |
1993 | Intracoronary 25% ethanol injection |
11 (9 received ethanol) |
56.5 ± 8.1 | Atrial fibrillation and rapid ventricular response |
19–25 months (mean of 22.2 ±2.2) |
‘Clinical success’ (ventri- cular rate control or complete AV block in) 7 patients (78%) |
Transient modest rise in CK-MB |
Acute effects of diluted ethanol on the AV node did not predict long-term outcome. Similar effect to rapid administration of 96% ethanol. |
| Permanent ventricular rate control without causing AV block in 4 patients (44%) |
Fever and positive blood cultures after placement of tempor- ary transvenous pace- maker following ethanol injection |
||||||||
| No significant change in LVEF |
|||||||||
AST, aspartate aminotransferase; AV, Atrioventricular; CK, creatine kinase; CK-MB, creatine kinase MB; LVEF, Left ventricular ejection fraction; Ml, myocardial infarction.
With the advent of transvenous (endocardial) catheter ablation of the AVN, ablation or modification of the AVN conduction by selective AVN artery ethanol infusion was largely abandoned. It remained as a suitable option when standard catheter approaches were not possible as in tricuspid atresia [22]. It seemed to be a well tolerated procedure in individuals treated in experienced centers. Described complications are: chest pain, myocardial infarction, coronary artery spasm, and occlusion or dissection of the targeted vessel. Currently, intracoronary ethanol for the ablation of the AVN is of historical interest, given the high success rates of radiofrequency [23] and other technologies such as cryoablation [10].
VENTRICULAR TACHYCARDIA ABLATION WITH ETHANOL
The first successful ventricular tachycardia ablation with ethanol was reported in dogs by Chilson et al. [5] in 1986 and transcoronary by Inoue et al. [4] in 1987, both in Zipes’ animal laboratory. In their animal study, focal ventricular tachycardia induced by intramyocardial injection of aconitine was suppressed by ethanol (at least 50% concentration) or phenol injection delivered into the artery supplying the aconitine-injected myocardial tissue. Successful ventricular tachycardia elimination was correlated with myocardial necrosis and arterial thrombus formation, which were not achieved by lower (25%) ethanol concentrations. In 1988, Brugada et al. [17] described the first clinical observation showing the effect of a focal ventricular tachycardia temporary with termination after transvascular interventions such as occlusion of a vessel or rapid ice-cold saline injection. This observation was followed by the demonstration of an effective cure of ventricular tachycardia by intracoronary ethanol infusion in three patients who had remained in incessant ventricular tachycardia refraction to multiple treatment modalities [24] (see Fig. 2). Kay et al. [25] prospectively evaluated the clinical utility of intra-arterial ethanol infusion for ventricular tachycardia in 23 patients. They found that ventricular tachycardia could be terminated by injections of saline solution or contrast medium in 11 of the 21 patients in whom the protocol could be completed. Ethanol was infused in 10 of these patients and led to acute elimination of ventricular tachycardia inducibility in 90% of them [25]. After repeating the electrophysiology study, inducibility recovered in two other patients, yielding an overall success of 70%. Associated complications included complete atrio-ventricular block in four patients (40%) and pericarditis in one patient. Initial ethanol dose and concentration (‘absolute’ or 96–98%) appeared to be arbitrarily selected. Haines et al. [26] performed a systematic study in dogs addressing these issues, testing different concentrations (0, 10, 25, 50, 75, and 100%): as the ethanol concentration increased, the ablation vessels were more persistently occluded and the size of identifiable myocardial lesions is increased significantly with increasing ethanol concentration, although there was significant variability within groups. Table 2 summarizes the reported results of ethanol ablation of ventricular tachycardia.
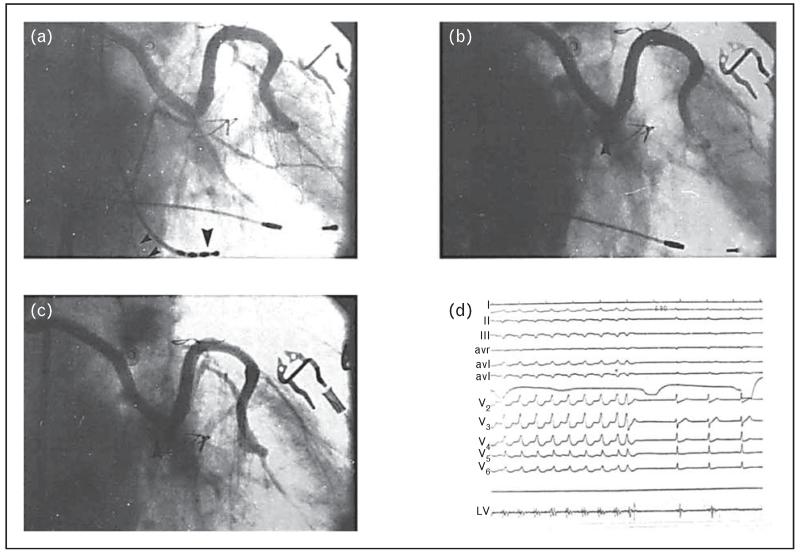
FIGURE 2.
Ethanol ablation of ventricular tachycardia. Right anterior oblique projection of the sequential vein graft. Panel a: Before the injection of ethanol, showing all three marginal branches of the circumflex artery (small arrowheads indicate the tachycardia-related artery, whereas large arrowhead is the mapping catheter localized on the site of origin of the tachycardia). Panel b shows immediately after the injection of ethanol, there is total occlusion of the circumflex (arrowhead). Panel c shows a 14-day follow-up angiogram showing persistent occlusion of the circumflex with minimal recanalization of the third marginal branch (arrowhead). Panel d shows termination of ventricular tachycardia immediately after the injection of ethanol. Left ventricular bipolar recordings obtained from the distal two poles of the quadripolar mapping electrode, with the catheter placed at the site of origin of the ventricular tachycardia. Note the marked splitting of the potentials (from Brugada et al. [24]).
Table 2.
Results of transcoronary arterial ethanol ablation of ventricular tachycardia
| Study reference |
Year | Study design |
Patients (n) |
Age (years) |
Indications | Follow-up | Results | Complications | Observations |
|---|---|---|---|---|---|---|---|---|---|
| Brugada et al. [24] |
1989 | Intracoronary 96% ethanol injection |
3 | 44, 61, 62 | Incessant tachycar- dia postmyocar- dial infarction |
2–9 months (mean 5.7) |
Cease of arrhythmia in 100% (one recur- rence at 1 month, repeating procedure successfully) |
Short-lasting chest pain in all the patients |
Chemical ablation of the arrhythmogenic resulted in cure of the disorder. Size of the ablation should be as limited as possible. |
| Temporary complete AV block with pacemaker implantation in 1 patient (33%) |
New collateral blood supply may lead to recurrence. |
||||||||
| Small to moderate AST rise |
|||||||||
|
| |||||||||
| Kay et al. [25] |
1992 | Intracoronary 96% ethanol injection |
23 (10 received ethanol) |
59 ± 12 | Sustained monomor- phic ventricular tachycardia related to prior MI |
102–788 days (mean 372) |
Cease in 100%, with inducible VT in 3 patients (30%) |
Complete AV block in 4 patients (40%) |
Moderate degree of efficacy and potential for complications. Lo- ng-term control for particular patients. Limited by the fre- quent inability to localize the arrhyth- mia-related vessel. |
| Little or no change in LVEF. |
Pericarditis (Dressler’s syndrome) in 1 patient (10%) |
||||||||
|
| |||||||||
| Tokuda et al. [28] |
2011 | Intracoronary 96% ethanol injection |
27 – out of these, 22 received ethanol |
63 ± 13 | Symptomatic mono- morphic VT refrac- tory to RFA; structural heart disease |
20 ± 11 months |
VT was no inducible after ablation in 18 patients (82%) |
Complete heart block in 5 patients (38% of 13 patients with intact AV conduction) |
Important role for diffi- cult VTs in high-risk patients. Prevents recurrences in 36% and improves arrhyth- mia control in an additional 27%. |
| VT recurrence in 14 patients (64%) |
Temporary coronary spasm 1 patient (5%) |
||||||||
| Nine out of 11 were free from VT storm |
Total mortality 32%, early mortality (within 30 days) 14% and late mortality 18% |
||||||||
| CK, CK-MB and Trop I elevation |
|||||||||
|
| |||||||||
| Sacher et al. [32] |
2008 | Intracoronary 96% ethanol injection |
9 | 55 ± 9 | Refractory monomor- phic VT due to scar-related re- entry; ischemic cardiomyopathy in 6 patients (67%) |
29 ± 23 months |
No VT recurrence in 67% |
Transient ST-elevation during injection in 5 patients (55%) |
Applicable alternative especially in cases of septal scar (VT cir- cuits deep intramyo- cardial). TCEA rarely used (1.4%). |
| No significant change in LVEF |
Immediate: transient severe hypotension in 2 patients (22%) and bilateral groin hemato- mas in 1 patient (11%) |
||||||||
| CK-MB and Trop. I rise with injection |
-Three patients died in the follow-up from refrac- tory HF (33%). |
||||||||
AST, aspartate aminotransferase; AV, Atrioventricular; CK, creatine kinase; CK-MB, creatine kinase MB; HF, heart failure; LVEF, Left ventricular ejection fraction; RFA, radiofrequency ablation; TCEA, transcoronary ethanol ablation; Trop. I, troponin I; VT, ventricular tachycardia.
In the current ablation era, ethanol ablation strategies have been limited to patients with previously failed RFAs. In a small series of nine such patients, clinical success (freedom from clinical ventricular tachycardia) of transcoronary ethanol infusion was 67% on long-term-follow-up, a rate that does not appear radically different to the success rate of radiofrequency [27]. In the largest experience collected to date of transcoronary ethanol ablation [28], a total of 27 patients underwent ethanol ablation attempts. In 5 of them, the coronary anatomy was not suitable. After ethanol ablation in 22 patients, ventricular tachycardia was no longer inducible in 82% of the patients. Clinical responses were achieved in 9 out of the 11 patients with ventricular tachycardia storm. However, ventricular tachycardia recurred in 64% of the patients. Complete atrioventricular block occurred in five patients. Although far from uniformly effective, ethanol ablation continues to represent a viable treatment option for patients with prior RFA failures, and periodically, case reports of the bail-out use of ethanol ablation for ventricular tachycardia refractory to RFA continue to appear in the literature [29-31]. The success of the procedure critically relies on the accurate identification of an arterial branch that supplies the arrhythmogenic ventricular tissue and the suitability of that branch to selective cannulation.
The targeted arrhythmogenic myocardial territory can have collateral circulation which may affect the efficacy of the ablation. Once the appropriate vessel is identified, the engagement should be as distal as possible to avoid injury from side branches. The usual dose is 1–2 ml of ethanol bolus over 1–4 min, with a balloon inflated up to five times if necessary. In the literature, noninducibility ventricular tachycardia rates after ethanol ablation range from 56 to 84% [27,32], with a 64% of recurrence for any ventricular tachycardia [28]. Table 2 summarizes the results reported to date using transarterial coronary ethanol infusion for the treatment of ventricular tachycardia.
Ethanol ablation may also fail to produce a complete lesion and it can leave surviving myocardial cells that lead to re-entrant circuits and ventricular tachycardia. This phenomenon has been described following ventricular tachycardia ethanol septal ablation for hypertrophic obstructive cardio-myopathy [33]. Additional restrictions of the technique involve areas with severe coronary artery disease – a condition that limits access to the target vessel in ischemic ventricular tachycardia. Direct intra-myocardial ethanol injection has been tested in animal models with success [34-37]. The efficacy and safety of this procedure may change significantly from patient to patient, and is also dependent on the clinical experience of the practitioner [28,32]. Considering the uncertainties that still remain, the use in the current medical practice has been reserved to special situations.
USING THE VENOUS ROUTE FOR ETHANOL DELIVERY
There are several important limitations to the transcoronary arterial ethanol delivery for ventricular tachycardia ablation. Technical difficulties cannulating the target arterial branch are common (up to 18.5% of attempted patients) [28], and this is a problem that appears unavoidable, given that in ischemic ventricular tachycardia, obstructive coronary artery disease is expected in arteries that supply the substrate of ventricular tachycardia. Reflux of ethanol has been associated with unintended lesion to proximal or surrounding tissue [38]. Slow infusion rates through a catheter properly wedged in a small vessel may reduce the risk of this complication. Other variables are the size and flow rate of the vessel cannulated, which would affect the ethanol cytotoxicity [21,29,39]. Additionally, catastrophic complications derived from complex coronary artery manipulation, such as coronary artery dissection, can always occur. These issues have led investigators to consider the venous route for ethanol delivery: retrograde infusions would not be subject to certain limitations of anterograde delivery through the arterial system.
Ventricular venous ethanol delivery for ventricular tachycardia
Recognizing these limitations of intra-arterial delivery, Inoue et al. [4] had originally described that coronary sinus phenol infusion in dogs led to ‘considerable’ subendocardial necrosis, but there were no additional descriptions. Wright et al. [40] explored the retrograde venous approach in a canine model. Balloon occlusion of the distal anterior inter-ventricular vein or the distal great cardiac vein was performed and then ethanol was infused at 1.5, 3, and 5 ml. They found that transmural lesions could be achieved when infused ethanol volumes were at least 3 ml, and hypothesized that for smaller volumes, collateral flow via Thebesian veins into the left ventricular cavity could prevent ethanol from reaching the capillaries, where its ablative action would reach the myocardial cells.
We recently reported two cases of successful ventricular tachycardia ablation in humans using this approach [41]. Both patients had idiopathic focal ventricular tachycardia in the setting of non-ischemic cardiomyopathies and had undergone prior failed RF ablations in multiple procedures. In the first case, ventricular tachycardia originated from the superior septal region of the left ventricular outflow tract. Mapping was performed, as routinely done, using a multipolar catheter in the anterior interventricular vein, where the earliest activations were found in a septal branch and a pace-map showed a perfect QRS match. Subsequently, radio-frequency applications were delivered in the vein, as well as in the adjacent epicardium and the adjacent endocardium, all without success. Given that cannulation of the anterior interventricular vein – including the first septal branch – had already been achieved for mapping purposes, cannulation of the first septal branch with an angioplasty balloon and wire was straightforward. One millilitre of 98% ethanol was delivered via the angioplasty balloon in the septal branch of the anterior interventricular vein. After ethanol infusion, ventricular tachycardia was eliminated. A large proximal septal scar was created as demonstrated by MRI (see Fig. 3). The patient recovered well, but developed a pleural effusion requiring thoracentesis, which was attributed to Dressler’s syndrome due to pericardial manipulation. Ventricular tachycardia did not recur on follow-up. This demonstrated the safety and feasibility of this approach.
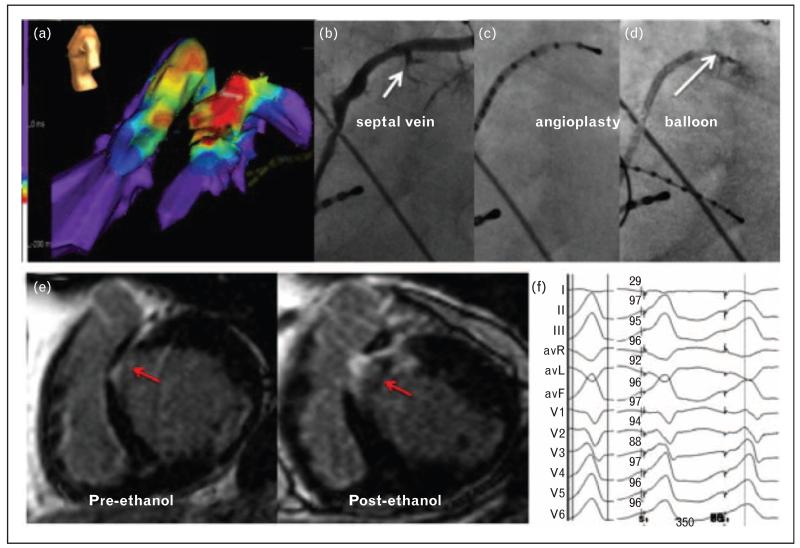
FIGURE 3.
Coronary venous mapping and ethanol ablation of VT. (a) Activation maps of VT in the AIV and in RV and LV cavities. The earliest activation site is shown coming from within the AIV. (b) Selective venogram of the AIV showed two septal branches. (c) The AIV was then cannulated with a decapolar catheter, and the distal poles were inserted into the septal branch. Pace maps were obtained that matched the QRS morphology of the clinical tachycardia as shown in (f). (d) The septal branch was cannulated with an angioplasty wire and occluded with a 1.5 6 mm balloon. Ethanol (98%, 1 cm3) was then injected into the septal vein. (e) CMR at baseline showed a pre-existing midmyocardial septal area of delayed enhancement (pre-ethanol) that was significantly expanded after ethanol injection. (f) Twelve-lead electrocardiogram of the spontaneous VT (left) and paced QRS from the septal branch of the AIV (right), along with automated calculation of the percentage of similarity by using Bard software (Bard Electrophysiology, Boston, Massachusetts, USA). AIV, anterior interventricular vein; CMR, cardiac magnetic resonance; LV, left ventricle; RV, right ventricle; VT, ventricular tachycardia (from Baher et al. [41]).
A second case of an inferoseptal ventricular tachycardia was targeted successfully cannulating a septal branch of the middle cardiac vein and infusing ethanol there [41].
Vein of Marshall ethanol infusion for atrial fibrillation
There is a plethora of data supporting the role of the ligament of Marshall in atrial arrhythmogenesis, including the abundant sympathetic and parasym-pathetic innervation; its role in ectopic beat generation leading to atrial fibrillation; and its epicardial electrical connections with the pulmonary veins [42]. Despite these, therapeutic approaches to target the ligament of Marshall had been limited to epicardial surgical ligation or sectioning of the ligament. Since the ligament of Marshall continues as an atrial vein [vein of Marshall (VOM)], connected to the coronary sinus, it is amenable to selective retrograde cannulation [43]. We demonstrated the feasibility and ablative efficacy of ethanol infusion in the VOM first in canines and later in humans [38,44].
The VOM is a true atrial vein with capillary connections with the neighboring atrial myo-cardium [45], which implies that VOM ethanol infusion can lead to myocardial ablation in the neighboring tissues. We showed the role of VOM in providing epicardial connections to the left pulmonary veins leading to reconnections and clinical failures of catheter ablation of atrial fibrillation with pulmonary vein isolation [45]. Additionally, the VOM sits on the epicardial aspect of the left atrial ridge and the mitral isthmus – a critical region sustaining perimitral flutter. We showed that VOM ethanol infusion can lead to effective ablation of this region and cure of perimitral flutter [46]. Finally, we demonstrated that the abundant para-sympathetic innervation of the VOM and the neighboring atrial epicardium can be ablated by VOM ethanol infusion [47■] (see Fig. 4). However encouraging these findings are, a therapeutic role of VOM ethanol infusion can only be justified if it leads to improved outcomes in a controlled clinical trial. We are currently conducting an NIH-sponsored randomized controlled multicenter clinical trial to delineate the possible outcome benefits of VOM ethanol infusion in persistent atrial fibrillation.
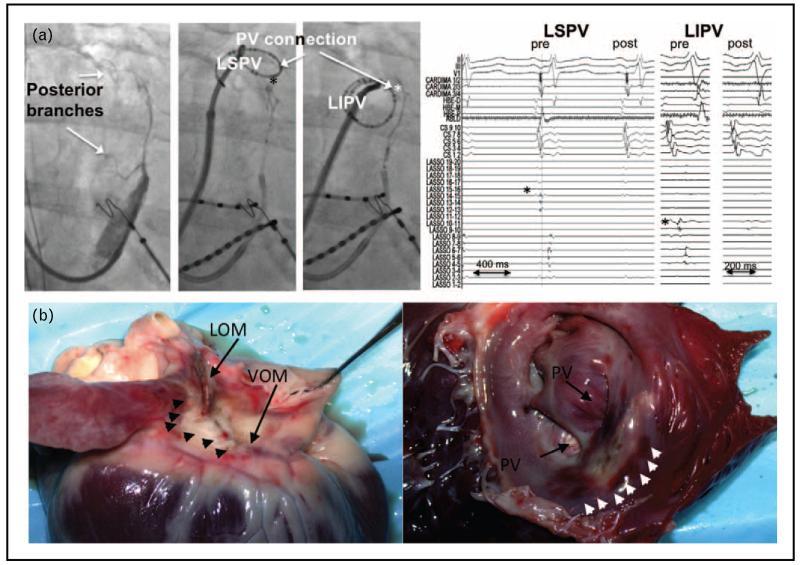
FIGURE 4.
Pulmonary vein isolation after ethanol infusion in the vein of Marshall (VOM). Panel a shows simultaneous LIPV and LSPV disconnection by VOM ethanol. Left image shows venograms with large VOM posterior branches, directed toward the LSPV and LIPV, and their reconnection sites (asterisks). Right image shows ethanol infusion led to disconnection of both veins. Panel b shows ethanol ablation lesion. Left heart shows epicedial aspect, showing the VOM and ligament of Marshall area, with pale discoloration of the ablated areas (arrowheads). Right heart shows endocardial aspect after incision in the left atrial appendage. A pale area of discoloration is shown anterior to the left pulmonary veins, surrounded by small area of tissue hemorrhage (arrowheads) (from Valderrábano et al. [44] and Dave et al. [45]).
CONCLUSION
Use of ethanol injection for ablation of cardiac arrhythmias is technically complex, dependent on the cardiac vascular system, and is unlikely that ethanol injection would be preferred over RFA as a first line of therapy. The complexity of ventricular tachycardia ablation in the setting of structural heart disease is likely to continue to generate difficult cases that fail to respond to standard RFA and may benefit from ethanol infusion. In this setting, transcoronary arterial ethanol has established itself as a potentially useful therapeutic alternative. The transcoronary venous approach needs further validation, but offers several theoretical advantages. AVN ablation is solidly achieved by RFA and it is unlikely that ethanol will be used again for this purpose. The conventional RFA approach to atrial fibrillation ablation has significant limitations in its mechanistic basis, its technical feasibility, and, most importantly, its clinical success, particularly in persistent atrial fibrillation. The VOM appears as a legitimate target to improve outcomes, and ethanol ablation has a potential clinical use as an adjunctive therapy for standard RFA. Further ethanol ablation studies are necessary to elucidate its role and clinical implications.
KEY POINTS.
Intracoronary ethanol infusion was an early mode of ablation that showed efficacy for the atrioventricular node and ventricular tachycardia ablation.
Although its clinical use has been largely superseded by radiofrequency, it remains a valuable tool for ablating substrates that can not be reached via contact-dependent methods of catheter ablation.
Ethanol infusion in the coronary veins lacks the risks of arterial infusion. Its use in the VOM for atrial fibrillation and in coronary veins for ventricular tachycardia ablation shows promise.
Acknowledgements
We would like to thank Dr Douglas Zipes for his contributions with the study.
Financial support and sponsorship
The study was supported by the Department of Cardiac Electrophysiology, Methodist Hospital, Houston, Texas, USA.
Dr Valderrábano is currently receiving a grant (NIH-NHLBI grant R01HL115003).
Footnotes
Conflicts of interest
Dr Schurmann and Dr Peñalver have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■ of outstanding interest
- 1.Alhumaid F, Cheng A, Calkins H, Berger RD. Successful cryothermal ablation for atrioventricular nodal reentry tachycardia after radiofrequency ablation failure. J Interven Cardiac Electrophysiol. 2012;34:89–92. doi: 10.1007/s10840-011-9628-3. [DOI] [PubMed] [Google Scholar]
- 2.Dukkipati SR, Kuck KH, Neuzil P, et al. Pulmonary vein isolation using a visually guided laser balloon catheter: the first 200-patient multicenter clinical experience. Circ Arrhyth Electrophysiol. 2013;6:467–472. doi: 10.1161/CIRCEP.113.000431. [DOI] [PubMed] [Google Scholar]
- 3.Metzner A, Chun KR, Neven K, et al. Long-term clinical outcome following pulmonary vein isolation with high-intensity focused ultrasound balloon catheters in patients with paroxysmal atrial fibrillation. Europace. 2010;12:188–193. doi: 10.1093/europace/eup416. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Waller BF, Zipes DP. Intracoronary ethyl alcohol or phenol injection ablates aconitine-induced ventricular tachycardia in dogs. J Am Coll Cardiol. 1987;10:1342–1349. doi: 10.1016/s0735-1097(87)80139-0. [DOI] [PubMed] [Google Scholar]
- 5.Chilson DA, Peigh PS, Mahomed Y, et al. Chemical ablation of ventricular tachycardia in the dog. Am Heart J. 1986;111:1113–1118. doi: 10.1016/0002-8703(86)90014-1. [DOI] [PubMed] [Google Scholar]
- 6.Baker Kramer. Cytoxicity of short-chain alcohols. Ann Rev Pharmacol Toxicol. 1999;15:1013–1018. doi: 10.1146/annurev.pharmtox.39.1.127. [DOI] [PubMed] [Google Scholar]
- 7.Lasner M, Roth LG, Chen CH. Structure-functional effects of a series of alcohols on acetylcholinesterase-associated membrane vesicles: elucidation of factors contributing to the alcohol action. Arch Biochem Biophys. 1995;317:391–396. doi: 10.1006/abbi.1995.1179. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12:219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 9.Lenaz G, Parenti-Castelli G, Sechi AM. Lipid-protein interactions in mitochondria. Changes in mitochondrial adenosine triphosphatase activity induced by n-butyl alcohol. Arch Biochem Biophys. 1975;167:72–79. doi: 10.1016/0003-9861(75)90442-7. [DOI] [PubMed] [Google Scholar]
- 10.Das AM, Harris DA. Regulation of the mitochondrial atp synthase is defective in rat heart during alcohol-induced cardiomyopathy. Biochim Biophys Acta. 1993;1181:295–299. doi: 10.1016/0925-4439(93)90035-y. [DOI] [PubMed] [Google Scholar]
- 11.Auffermann W, Camacho SA, Wu S, et al. 31p and 1 h magnetic resonance spectroscopy of acute alcohol cardiac depression in rats. Magn Reson Med. 1988;8:58–69. doi: 10.1002/mrm.1910080107. [DOI] [PubMed] [Google Scholar]
- 12.Trouwborst A, Yanagida H, Erdmann W, Kok A. Mechanism of neuroadenolysis of the pituitary for cancer pain control. Appl Neurophysiol. 1984;47:97–110. doi: 10.1159/000101210. [DOI] [PubMed] [Google Scholar]
- 13.Corssen G, Holcomb MC, Moustapha I, et al. Alcohol-induced adenolysis of the pituitary gland: a new approach to control of intractable cancer pain. Anesth Analg. 1977;56:414–421. doi: 10.1213/00000539-197705000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Katz J, Levin AB. Treatment of diffuse metastatic cancer pain by instillation of alcohol into the sella turcica. Anesthesiology. 1977;46:115–121. doi: 10.1097/00000542-197702000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Levin AB, Benson RC, Jr, Katz J, Nilsson T. Chemical hypophysectomy for relief of bone pain in carcinoma of the prostate. J Urol. 1978;119:517–521. doi: 10.1016/s0022-5347(17)57534-5. [DOI] [PubMed] [Google Scholar]
- 16.Wyse JM, Carone M, Paquin SC, et al. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–3546. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 17.Brugada P, de Swart H, Smeets JL, et al. Termination of tachycardias by interrupting blood flow to the arrhythmogenic area. Am J Cardiol. 1988;62:387–392. doi: 10.1016/0002-9149(88)90964-2. [DOI] [PubMed] [Google Scholar]
- 18.Brugada P, de Swart H, Smeets J, Wellens HJ. Transcoronary chemical ablation of atrioventricular conduction. Circulation. 1990;81:757–761. doi: 10.1161/01.cir.81.3.757. [DOI] [PubMed] [Google Scholar]
- 19.Kay GN, Bubien RS, Dailey SM, et al. A prospective evaluation of intracoronary ethanol ablation of the atrioventricular conduction system. J Am Coll Cardiol. 1991;17:1634–1640. doi: 10.1016/0735-1097(91)90659-w. [DOI] [PubMed] [Google Scholar]
- 20.Wang PJ, Ursell PC, Sosa-Suarez G, et al. Permanent av block or modification of av nodal function by selective av nodal artery ethanol infusion. Pacing Clin Electrophysiol. 1992;15:779–789. doi: 10.1111/j.1540-8159.1992.tb06845.x. [DOI] [PubMed] [Google Scholar]
- 21.Strickberger SA, Foster PR, Wang PJ, et al. Intracoronary infusion of dilute ethanol for control of ventricular rate in patients with atrial fibrillation. Pacing Clin Electrophysiol. 1993;16:1984–1993. doi: 10.1111/j.1540-8159.1993.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 22.Peters NS, Coromilas J, Hanna MS, et al. Characteristics of the temporal and spatial excitable gap in anisotropic reentrant circuits causing sustained ventricular tachycardia. Circ Res. 1998;82:279–293. doi: 10.1161/01.res.82.2.279. [DOI] [PubMed] [Google Scholar]
- 23.Trouton TG, O’Nunain SS, Kim YH, et al. Curative transcatheter radiofre-quency current ablation for atrioventricular nodal reentry tachycardia. Arch Intern Med. 1994;154:1226–1231. [PubMed] [Google Scholar]
- 24.Brugada P, de Swart H, Smeets JL, Wellens HJ. Transcoronary chemical ablation of ventricular tachycardia. Circulation. 1989;79:475–482. doi: 10.1161/01.cir.79.3.475. [DOI] [PubMed] [Google Scholar]
- 25.Kay GN, Epstein AE, Bubien RS, et al. Intracoronary ethanol ablation for the treatment of recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1992;19:159–168. doi: 10.1016/0735-1097(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 26.Haines DE, Whayne JG, DiMarco JP. Intracoronary ethanol ablation in swine: effects of ethanol concentration on lesion formation and response to programmed ventricular stimulation. J Cardiovasc Electrophysiol. 1994;5:422–431. doi: 10.1111/j.1540-8167.1994.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 28.Tokuda M, Sobieszczyk P, Eisenhauer AC, et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhyth Electrophysiol. 2011;4:889–896. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]
- 29.Miller MA, Kini AS, Reddy VY, Dukkipati SR. Transcoronary ethanol ablation of ventricular tachycardia via an anomalous first septal perforating artery. Heart Rhythm. 2011;8:1606–1607. doi: 10.1016/j.hrthm.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Atienza F, Arenal A, Perez-David E, et al. New diagnostic and therapeutic approaches to treat ventricular tachycardias originating at the summit of the left ventricle: Role of merged hemodynamic-mri and alternative ablation sources. Circ Arrhyth Electrophysiol. 2013;6:e80–e84. doi: 10.1161/CIRCEP.113.000430. [DOI] [PubMed] [Google Scholar]
- 31.Gabus V, Jeanrenaud X, Eeckhout E, Pruvot E. Transcoronary ethanol for incessant epicardial ventricular tachycardia. Heart Rhythm. 2014;11:143–145. doi: 10.1016/j.hrthm.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 32.Sacher F, Sobieszczyk P, Tedrow U, et al. Transcoronary ethanol ventricular tachycardia ablation in the modern electrophysiology era. Heart Rhythm. 2008;5:62–68. doi: 10.1016/j.hrthm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Simon RD, Crawford FA, 3rd, Spencer WH, 3rd, Gold MR. Sustained ventricular tachycardia following alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Pacing Clin Electrophysiol. 2005;28:1354–1356. doi: 10.1111/j.1540-8159.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 34.Weismuller P, Mayer U, Richter P, et al. Chemical ablation by subendocardial injection of ethanol via catheter: preliminary results in the pig heart. Eur Heart J. 1991;12:1234–1239. doi: 10.1093/eurheartj/12.11.1234. [DOI] [PubMed] [Google Scholar]
- 35.Goette A, Hartung W, Lesh M, et al. Transcatheter subendocardial infusion. A novel technique for mapping and ablation of ventricular myocardium. Circulation. 1996;94:1449–1455. doi: 10.1161/01.cir.94.6.1449. [DOI] [PubMed] [Google Scholar]
- 36.Callans DJ, Ren JF, Narula N, et al. Left ventricular catheter ablation using direct, intramural ethanol injection in swine. J Interven Cardiac Electrophysiol. 2002;6:225–231. doi: 10.1023/a:1019505703083. [DOI] [PubMed] [Google Scholar]
- 37.Reek S, Geller JC, Schildhaus HU, et al. Catheter ablation of ventricular tachycardia by intramyocardial injection of ethanol in an animal model of chronic myocardial infarction. J Cardiovasc Electrophysiol. 2004;15:332–341. doi: 10.1046/j.1540-8167.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 38.Valderrabano M, Chen HR, Sidhu J, et al. Retrograde ethanol infusion in the vein of marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circ Arrhyth Electrophysiol. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okishige KFP. Alcohol ablation for tachycardia therapy. J Cardiovasc Electrophysiol. 1992;3:354–364. [Google Scholar]
- 40.Wright KN, Morley T, Bicknell J, et al. Retrograde coronary venous infusion of ethanol for ablation of canine ventricular myocardium. J Cardiovasc Electrophysiol. 1998;9:976–984. doi: 10.1111/j.1540-8167.1998.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 41.Baher A, Shah DJ, Valderrabano M. Coronary venous ethanol infusion for the treatment of refractory ventricular tachycardia. Heart Rhythm. 2012;9:1637–1639. doi: 10.1016/j.hrthm.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang C, Chen PS. Ligament of marshall: why it is important for atrial fibrillation ablation. Heart Rhythm. 2009;6:S35–40. doi: 10.1016/j.hrthm.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 43.Hwang C, Wu TJ, Doshi RN, et al. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 44.Valderrábano M, Liu X, Sasaridis C, et al. Ethanol infusion in the vein of marshall: adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6:1552–1558. doi: 10.1016/j.hrthm.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dave AS, Baez-Escudero JL, Sasaridis C, et al. Role of the vein of marshall in atrial fibrillation recurrences after catheter ablation: therapeutic effect of ethanol infusion. J Cardiovasc Electrophysiol. 2012;23:583–591. doi: 10.1111/j.1540-8167.2011.02268.x. [DOI] [PubMed] [Google Scholar]
- 46.Baez-Escudero JL, Morales PF, Dave AS, et al. Ethanol infusion in the vein of marshall facilitates mitral isthmus ablation. Heart Rhythm. 2012;9:1207–1215. doi: 10.1016/j.hrthm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47■.Baez-Escudero JL, Keida T, Dave AS, et al. Ethanol infusion in the vein of marshall leads to parasympathetic denervation of the human left atrium: implications for atrial fibrillation. J Am Coll Cardiol. 2014;63:1892–1901. doi: 10.1016/j.jacc.2014.01.032. This article supports the precense of parasympathethic innervation in the VOM area, which could lead to an area of interest for atrial fibrillation ablation and new use of ethanol for ablation