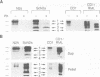
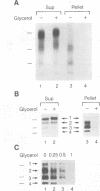
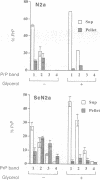
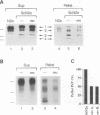
Abstract
The fundamental event in prion diseases involves a conformational change in one or more of the alpha-helices of the cellular prion protein (PrP(C)) as they are converted into beta-sheets during the formation of the pathogenic isoform (PrP(Sc)). Here, we show that exposure of scrapie-infected mouse neuroblastoma (ScN2a) cells to reagents known to stabilize proteins in their native conformation reduced the rate and extent of PrP(Sc) formation. Such reagents include the cellular osmolytes glycerol and trimethylamine N-oxide (TMAO) and the organic solvent dimethylsulfoxide (DMSO), which we refer to as 'chemical chaperones' because of their influence on protein folding. Although the chemical chaperones did not appear to affect the existing population of PrP(Sc) molecules in ScN2a cells, they did interfere with the formation of PrP(Sc) from newly synthesized PrP(C). We suggest that the chemical chaperones act to stabilize the alpha-helical conformation of PrP(C) and thereby prevent the protein from undergoing a conformational change to produce PrP(Sc). These observations provide further support for the idea that prions arise due to a change in protein conformation and reveal potential strategies for preventing PrP(Sc) formation.
Full text
PDF

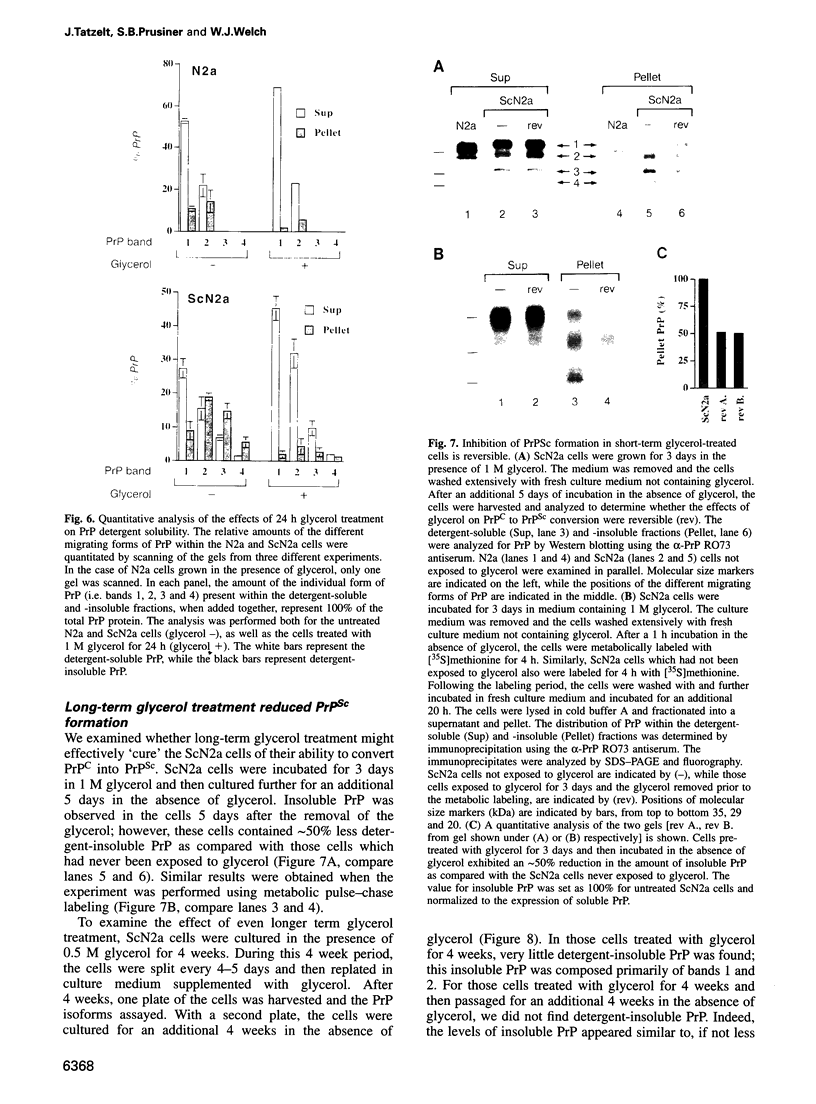





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back J. F., Oakenfull D., Smith M. B. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry. 1979 Nov 13;18(23):5191–5196. doi: 10.1021/bi00590a025. [DOI] [PubMed] [Google Scholar]
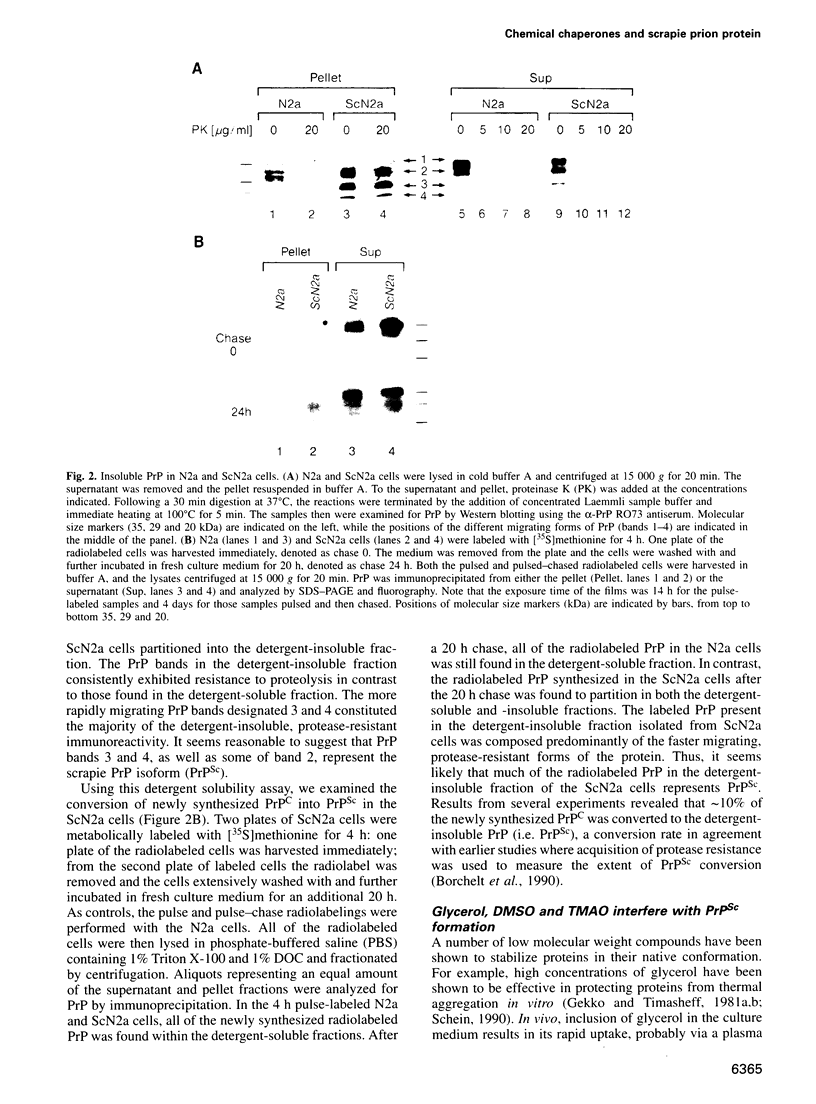
- Borchelt D. R., Scott M., Taraboulos A., Stahl N., Prusiner S. B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990 Mar;110(3):743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt D. R., Taraboulos A., Prusiner S. B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem. 1992 Aug 15;267(23):16188–16199. [PubMed] [Google Scholar]
- Brown C. R., Hong-Brown L. Q., Biwersi J., Verkman A. S., Welch W. J. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones. 1996 Jun;1(2):117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Butler D. A., Scott M. R., Bockman J. M., Borchelt D. R., Taraboulos A., Hsiao K. K., Kingsbury D. T., Prusiner S. B. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988 May;62(5):1558–1564. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B. W., Dong A., Bhat K. S., Ernst D., Hayes S. F., Caughey W. S. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochemistry. 1991 Aug 6;30(31):7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- Caughey B., Kocisko D. A., Raymond G. J., Lansbury P. T., Jr Aggregates of scrapie-associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem Biol. 1995 Dec;2(12):807–817. doi: 10.1016/1074-5521(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J., Ernst D., Race R. E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991 Dec;65(12):6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991 Sep 25;266(27):18217–18223. [PubMed] [Google Scholar]
- Cerneus D. P., Ueffing E., Posthuma G., Strous G. J., van der Ende A. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. Role of cholesterol. J Biol Chem. 1993 Feb 15;268(5):3150–3155. [PubMed] [Google Scholar]
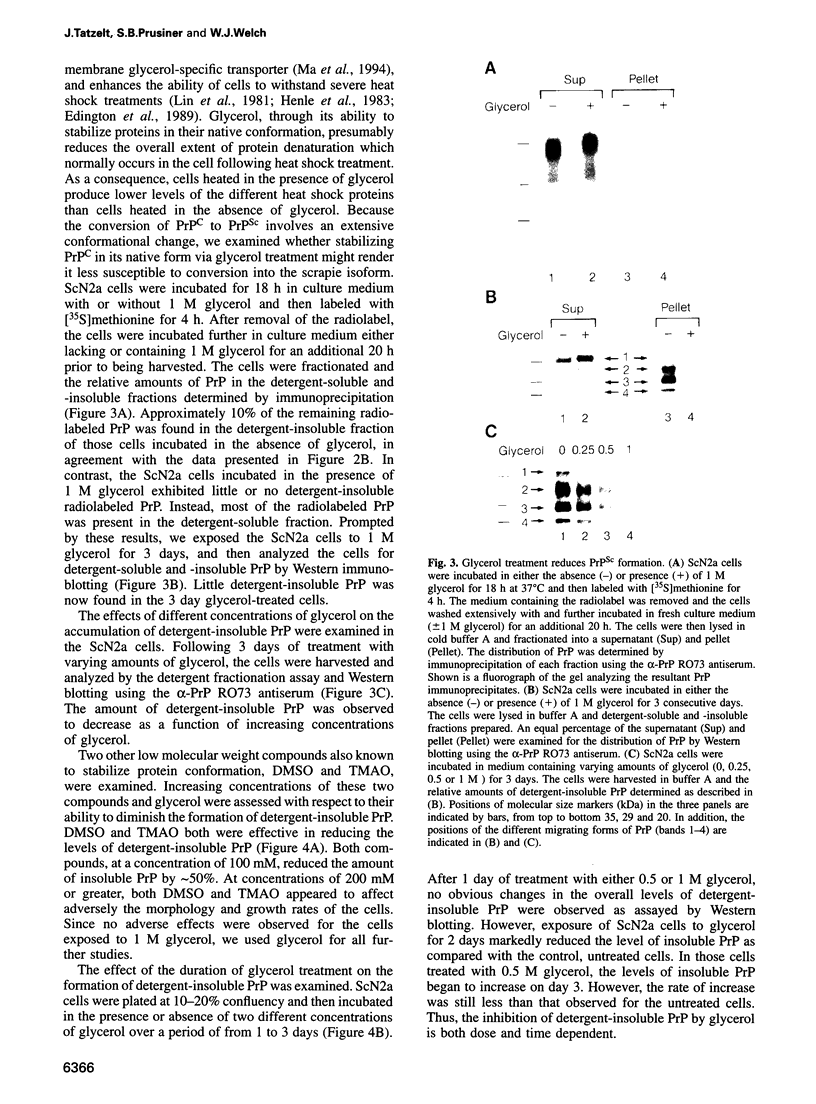
- Cinek T., Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992 Oct 1;149(7):2262–2270. [PubMed] [Google Scholar]
- Cohen F. E., Pan K. M., Huang Z., Baldwin M., Fletterick R. J., Prusiner S. B. Structural clues to prion replication. Science. 1994 Apr 22;264(5158):530–531. doi: 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- Edington B. V., Whelan S. A., Hightower L. E. Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: additional support for the abnormal protein hypothesis of induction. J Cell Physiol. 1989 May;139(2):219–228. doi: 10.1002/jcp.1041390202. [DOI] [PubMed] [Google Scholar]
- Gabizon R., Telling G., Meiner Z., Halimi M., Kahana I., Prusiner S. B. Insoluble wild-type and protease-resistant mutant prion protein in brains of patients with inherited prion disease. Nat Med. 1996 Jan;2(1):59–64. doi: 10.1038/nm0196-59. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Genetic control of nucleation and polymerization of host precursors to infectious amyloids in the transmissible amyloidoses of brain. Br Med Bull. 1993 Oct;49(4):913–931. doi: 10.1093/oxfordjournals.bmb.a072653. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Transmissible and non-transmissible amyloidoses: autocatalytic post-translational conversion of host precursor proteins to beta-pleated sheet configurations. J Neuroimmunol. 1988 Dec;20(2-3):95–110. doi: 10.1016/0165-5728(88)90140-3. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gasset M., Baldwin M. A., Fletterick R. J., Prusiner S. B. Perturbation of the secondary structure of the scrapie prion protein under conditions that alter infectivity. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):1–5. doi: 10.1073/pnas.90.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekko K., Koga S. Increased thermal stability of collagen in the presence of sugars and polyols. J Biochem. 1983 Jul;94(1):199–205. doi: 10.1093/oxfordjournals.jbchem.a134330. [DOI] [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Thermodynamic and kinetic examination of protein stabilization by glycerol. Biochemistry. 1981 Aug 4;20(16):4677–4686. doi: 10.1021/bi00519a024. [DOI] [PubMed] [Google Scholar]
- Hemström C., Virtanen A., Bridge E., Ketner G., Pettersson U. Adenovirus E4-dependent activation of the early E2 promoter is insufficient to promote the early-to-late-phase transition. J Virol. 1991 Mar;65(3):1440–1449. doi: 10.1128/jvi.65.3.1440-1449.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle K. J., Peck J. W., Higashikubo R. Protection against heat-induced cell killing by polyols in vitro. Cancer Res. 1983 Apr;43(4):1624–1627. [PubMed] [Google Scholar]
- Hooper N. M., Bashir A. Glycosyl-phosphatidylinositol-anchored membrane proteins can be distinguished from transmembrane polypeptide-anchored proteins by differential solubilization and temperature-induced phase separation in Triton X-114. Biochem J. 1991 Dec 15;280(Pt 3):745–751. doi: 10.1042/bj2800745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K. K., Groth D., Scott M., Yang S. L., Serban H., Rapp D., Foster D., Torchia M., Dearmond S. J., Prusiner S. B. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9126–9130. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Gabriel J. M., Baldwin M. A., Fletterick R. J., Prusiner S. B., Cohen F. E. Proposed three-dimensional structure for the cellular prion protein. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7139–7143. doi: 10.1073/pnas.91.15.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
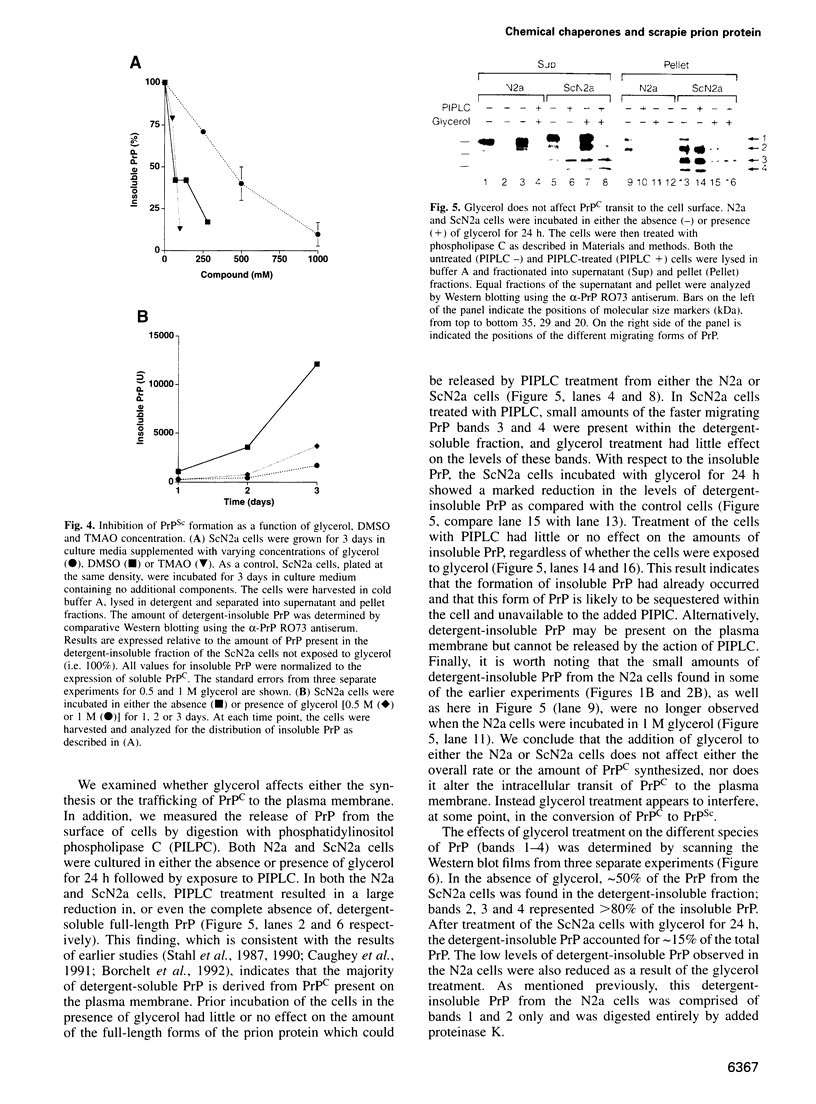
- Kocisko D. A., Come J. H., Priola S. A., Chesebro B., Raymond G. J., Lansbury P. T., Caughey B. Cell-free formation of protease-resistant prion protein. Nature. 1994 Aug 11;370(6489):471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin P. S., Kwock L., Hefter K. Protection of heat induced cytoxicity by glycerol. J Cell Physiol. 1981 Sep;108(3):439–443. doi: 10.1002/jcp.1041080318. [DOI] [PubMed] [Google Scholar]
- Ma J., Yee A., Brewer H. B., Jr, Das S., Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994 Nov 3;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Taraboulos A., Kenaga L., Serban D., Stieber A., DeArmond S. J., Prusiner S. B., Gonatas N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Invest. 1991 Dec;65(6):622–630. [PubMed] [Google Scholar]
- Medori R., Tritschler H. J., LeBlanc A., Villare F., Manetto V., Chen H. Y., Xue R., Leal S., Montagna P., Cortelli P. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med. 1992 Feb 13;326(7):444–449. doi: 10.1056/NEJM199202133260704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Füzi M., Scott M., Serban D., Serban H., Taraboulos A., Gabriel J. M., Wells G. A., Wilesmith J. W., Bradley R. Immunologic and molecular biologic studies of prion proteins in bovine spongiform encephalopathy. J Infect Dis. 1993 Mar;167(3):602–613. doi: 10.1093/infdis/167.3.602. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Scrapie prions. Annu Rev Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- Riek R., Hornemann S., Wider G., Billeter M., Glockshuber R., Wüthrich K. NMR structure of the mouse prion protein domain PrP(121-231). Nature. 1996 Jul 11;382(6587):180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- Safar J., Roller P. P., Gajdusek D. C., Gibbs C. J., Jr Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem. 1993 Sep 25;268(27):20276–20284. [PubMed] [Google Scholar]
- Safar J., Roller P. P., Gajdusek D. C., Gibbs C. J., Jr Scrapie amyloid (prion) protein has the conformational characteristics of an aggregated molten globule folding intermediate. Biochemistry. 1994 Jul 12;33(27):8375–8383. doi: 10.1021/bi00193a027. [DOI] [PubMed] [Google Scholar]
- Safar J., Roller P. P., Gajdusek D. C., Gibbs C. J., Jr Thermal stability and conformational transitions of scrapie amyloid (prion) protein correlate with infectivity. Protein Sci. 1993 Dec;2(12):2206–2216. doi: 10.1002/pro.5560021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar J., Wang W., Padgett M. P., Ceroni M., Piccardo P., Zopf D., Gajdusek D. C., Gibbs C. J., Jr Molecular mass, biochemical composition, and physicochemical behavior of the infectious form of the scrapie precursor protein monomer. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6373–6377. doi: 10.1073/pnas.87.16.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Ward C. L., Krouse M. E., Wine J. J., Kopito R. R. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem. 1996 Jan 12;271(2):635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- Schein C. H. Solubility as a function of protein structure and solvent components. Biotechnology (N Y) 1990 Apr;8(4):308–317. doi: 10.1038/nbt0490-308. [DOI] [PubMed] [Google Scholar]
- Serban D., Taraboulos A., DeArmond S. J., Prusiner S. B. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology. 1990 Jan;40(1):110–117. doi: 10.1212/wnl.40.1.110. [DOI] [PubMed] [Google Scholar]
- Somero G. N. Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol. 1986 Aug;251(2 Pt 2):R197–R213. doi: 10.1152/ajpregu.1986.251.2.R197. [DOI] [PubMed] [Google Scholar]
- Stahl N., Baldwin M. A., Hecker R., Pan K. M., Burlingame A. L., Prusiner S. B. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry. 1992 Jun 2;31(21):5043–5053. doi: 10.1021/bi00136a600. [DOI] [PubMed] [Google Scholar]
- Stahl N., Baldwin M. A., Teplow D. B., Hood L., Gibson B. W., Burlingame A. L., Prusiner S. B. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry. 1993 Mar 2;32(8):1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Prusiner S. B. Differential release of cellular and scrapie prion proteins from cellular membranes by phosphatidylinositol-specific phospholipase C. Biochemistry. 1990 Jun 5;29(22):5405–5412. doi: 10.1021/bi00474a028. [DOI] [PubMed] [Google Scholar]
- Taraboulos A., Raeber A. J., Borchelt D. R., Serban D., Prusiner S. B. Synthesis and trafficking of prion proteins in cultured cells. Mol Biol Cell. 1992 Aug;3(8):851–863. doi: 10.1091/mbc.3.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboulos A., Scott M., Semenov A., Avrahami D., Laszlo L., Prusiner S. B., Avraham D. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J Cell Biol. 1995 Apr;129(1):121–132. doi: 10.1083/jcb.129.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatzelt J., Zuo J., Voellmy R., Scott M., Hartl U., Prusiner S. B., Welch W. J. Scrapie prions selectively modify the stress response in neuroblastoma cells. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2944–2948. doi: 10.1073/pnas.92.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling G. C., Haga T., Torchia M., Tremblay P., DeArmond S. J., Prusiner S. B. Interactions between wild-type and mutant prion proteins modulate neurodegeneration in transgenic mice. Genes Dev. 1996 Jul 15;10(14):1736–1750. doi: 10.1101/gad.10.14.1736. [DOI] [PubMed] [Google Scholar]
- Telling G. C., Scott M., Mastrianni J., Gabizon R., Torchia M., Cohen F. E., DeArmond S. J., Prusiner S. B. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell. 1995 Oct 6;83(1):79–90. doi: 10.1016/0092-8674(95)90236-8. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. A., Scott A. C., Johnson C. T., Gunning R. F., Hancock R. D., Jeffrey M., Dawson M., Bradley R. A novel progressive spongiform encephalopathy in cattle. Vet Rec. 1987 Oct 31;121(18):419–420. doi: 10.1136/vr.121.18.419. [DOI] [PubMed] [Google Scholar]
- Williamson R. A., Peretz D., Smorodinsky N., Bastidas R., Serban H., Mehlhorn I., DeArmond S. J., Prusiner S. B., Burton D. R. Circumventing tolerance to generate autologous monoclonal antibodies to the prion protein. Proc Natl Acad Sci U S A. 1996 Jul 9;93(14):7279–7282. doi: 10.1073/pnas.93.14.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kaneko K., Nguyen J. T., Livshits T. L., Baldwin M. A., Cohen F. E., James T. L., Prusiner S. B. Conformational transitions in peptides containing two putative alpha-helices of the prion protein. J Mol Biol. 1995 Jul 21;250(4):514–526. doi: 10.1006/jmbi.1995.0395. [DOI] [PubMed] [Google Scholar]