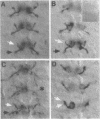
Abstract
Formation of neural precursors in Drosophila is determined by proneural genes. The distinctive pattern of expression of some genes of the achaete-scute complex in the embryonic neuroectoderm has prompted the speculation that they could also function in the specification of neural precursor identity in the CNS. To test this hypothesis, we have analysed the capacity of different proneural proteins to promote the development of a particular CNS precursor, the MP2 precursor. Our results indicate that: (i) all known proneural proteins are similarly able to support the formation of a neural precursor at the position of MP2; (ii) different proneural proteins promote the expression of different characteristics of MP2; and (iii) a totally normal specification of the MP2 fate can only be attained by the proneural genes achaete or scute.
Full text
PDF

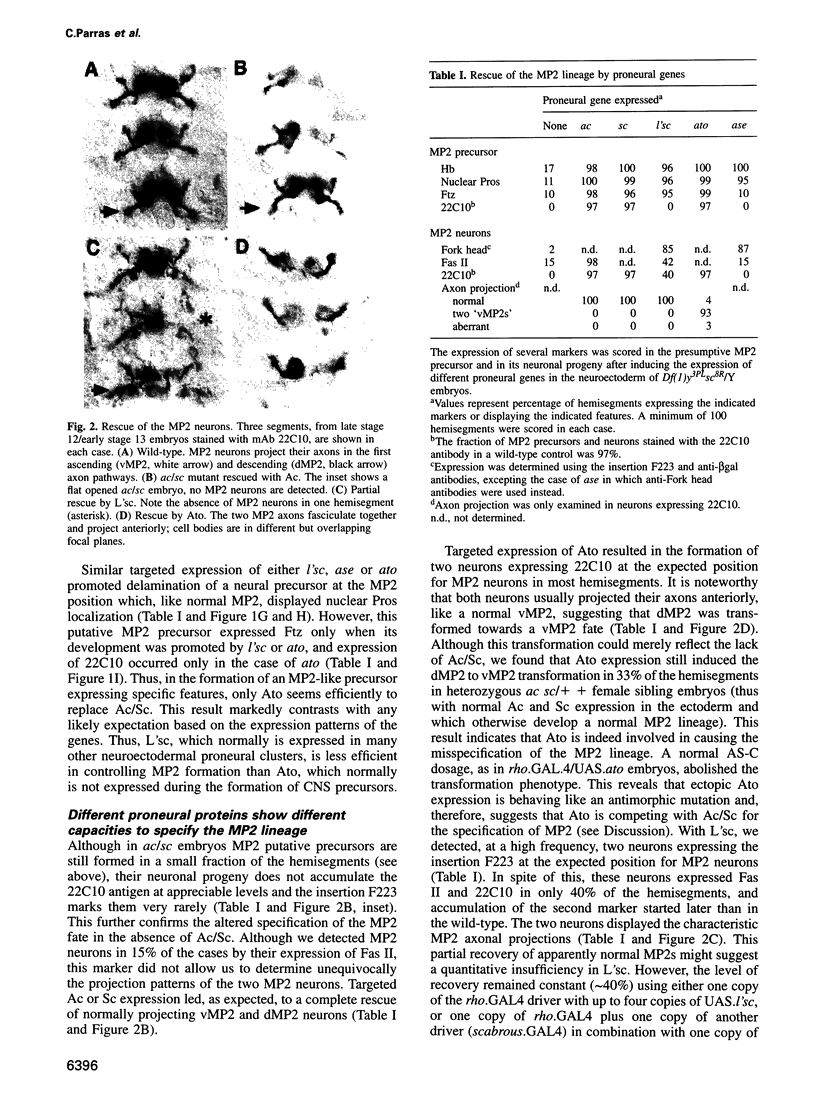



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993 Jun;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brand M., Jarman A. P., Jan L. Y., Jan Y. N. asense is a Drosophila neural precursor gene and is capable of initiating sense organ formation. Development. 1993 Sep;119(1):1–17. doi: 10.1242/dev.119.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Martinez-Arias A., Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987 Jul 31;50(3):425–433. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Carramolino L., Cabrera C. V., Ruíz-Gómez M., Villares R., Boronat A., Modolell J. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985 Feb;40(2):327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- Carmena A., Bate M., Jiménez F. Lethal of scute, a proneural gene, participates in the specification of muscle progenitors during Drosophila embryogenesis. Genes Dev. 1995 Oct 1;9(19):2373–2383. doi: 10.1101/gad.9.19.2373. [DOI] [PubMed] [Google Scholar]
- Cubas P., de Celis J. F., Campuzano S., Modolell J. Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Genes Dev. 1991 Jun;5(6):996–1008. doi: 10.1101/gad.5.6.996. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Hiromi Y., Gehring W. J., Goodman C. S. Expression and function of the segmentation gene fushi tarazu during Drosophila neurogenesis. Science. 1988 Jan 8;239(4836):170–175. doi: 10.1126/science.2892267. [DOI] [PubMed] [Google Scholar]
- Domínguez M., Campuzano S. asense, a member of the Drosophila achaete-scute complex, is a proneural and neural differentiation gene. EMBO J. 1993 May;12(5):2049–2060. doi: 10.1002/j.1460-2075.1993.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G., Rehm E. J., Goodman C. S. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell. 1991 Oct 4;67(1):45–57. doi: 10.1016/0092-8674(91)90571-f. [DOI] [PubMed] [Google Scholar]
- Grens A., Mason E., Marsh J. L., Bode H. R. Evolutionary conservation of a cell fate specification gene: the Hydra achaete-scute homolog has proneural activity in Drosophila. Development. 1995 Dec;121(12):4027–4035. doi: 10.1242/dev.121.12.4027. [DOI] [PubMed] [Google Scholar]
- Gómez-Skarmeta J. L., Rodríguez I., Martínez C., Culí J., Ferrés-Marcó D., Beamonte D., Modolell J. Cis-regulation of achaete and scute: shared enhancer-like elements drive their coexpression in proneural clusters of the imaginal discs. Genes Dev. 1995 Aug 1;9(15):1869–1882. doi: 10.1101/gad.9.15.1869. [DOI] [PubMed] [Google Scholar]
- Hinz U., Giebel B., Campos-Ortega J. A. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994 Jan 14;76(1):77–87. doi: 10.1016/0092-8674(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. Genetic control of cell fate specification in Drosophila peripheral nervous system. Annu Rev Genet. 1994;28:373–393. doi: 10.1146/annurev.ge.28.120194.002105. [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Grau Y., Jan L. Y., Jan Y. N. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993 Jul 2;73(7):1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Jiménez F., Campos-Ortega J. A. Defective neuroblast commitment in mutants of the achaete-scute complex and adjacent genes of D. melanogaster. Neuron. 1990 Jul;5(1):81–89. doi: 10.1016/0896-6273(90)90036-f. [DOI] [PubMed] [Google Scholar]
- Martín-Bermudo M. D., Martínez C., Rodríguez A., Jiménez F. Distribution and function of the lethal of scute gene product during early neurogenesis in Drosophila. Development. 1991 Oct;113(2):445–454. doi: 10.1242/dev.113.2.445. [DOI] [PubMed] [Google Scholar]
- Rodríguez I., Hernández R., Modolell J., Ruiz-Gómez M. Competence to develop sensory organs is temporally and spatially regulated in Drosophila epidermal primordia. EMBO J. 1990 Nov;9(11):3583–3592. doi: 10.1002/j.1460-2075.1990.tb07569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Macagno E. R., Modolell J. Expression of achaete and scute genes in Drosophila imaginal discs and their function in sensory organ development. Genes Dev. 1989 Jul;3(7):997–1007. doi: 10.1101/gad.3.7.997. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991 Jun;5(6):984–995. doi: 10.1101/gad.5.6.984. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development. 1992 Apr;114(4):939–946. doi: 10.1242/dev.114.4.939. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Panganiban G. F., Carroll S. B. The ventral nervous system defective gene controls proneural gene expression at two distinct steps during neuroblast formation in Drosophila. Development. 1994 Jun;120(6):1517–1524. doi: 10.1242/dev.120.6.1517. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Panganiban G., Selegue J., Carroll S. B. Gene regulation in two dimensions: the proneural achaete and scute genes are controlled by combinations of axis-patterning genes through a common intergenic control region. Genes Dev. 1992 Dec;6(12B):2606–2619. doi: 10.1101/gad.6.12b.2606. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Zhang Y., Holmgren R., Carroll S. B., Doe C. Q. Specification of neuroblast identity in the Drosophila embryonic central nervous system by gooseberry-distal. Nature. 1995 Aug 3;376(6539):427–430. doi: 10.1038/376427a0. [DOI] [PubMed] [Google Scholar]
- Spana E. P., Doe C. Q. The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development. 1995 Oct;121(10):3187–3195. doi: 10.1242/dev.121.10.3187. [DOI] [PubMed] [Google Scholar]