Abstract
Purpose
To determine how age at the time of left middle cerebral artery stroke affects language lateralization in a combined sample of subjects with perinatal, childhood, and adult stroke.
Methods
19 participants who had perinatal stroke (<1 month of age), 32 with later stroke, and 51 sex-/age-matched healthy controls (HCs) received fMRI of language using verb generation task (VGT).
Results
Percent lesion volumes were not different between groups (perinatal vs. late stroke) when taking brain volume into account (p = 0.084). Perinatal stroke group showed bilateral signal increases compared to more left-lateralized signals in matched HCs; late stroke group and HCs both showed left-hemispheric signal increases. LIs in the stroke groups were consistently more bilateral than in HCs (all p < 0.008) except for the late group’s posterior LI (p = 0.080). There was greater proportion of leftward language lateralization in HCs compared to their respective stroke groups (78.9% vs. 31.6% in perinatal; 87.5% vs. 59.4% in late stroke; p = 0.004) and a larger proportion of leftward lateralization in late compared to perinatal stroke (p = 0.039). The age of stroke occurrence showed significant positive associations with global and frontal LI (both p ≤ 0.007).
Conclusion
As expected, the age of stroke occurrence affects subsequent verb generation lateralization. Greater cortical plasticity is observed in earlier stroke while later stroke is associated with reliance on the repair of the previously damaged left-hemispheric networks.
Keywords: fMRI, language, aphasia, stroke, child, adult, language lateralization
1. Introduction
Deficits in communication abilities are usually the most dramatic short- and long-term sequelae of a left middle cerebral artery (LMCA) stroke. In recent years, great strides have been made in our knowledge of post-stroke language recovery. The degree of final post-stroke language recovery may depend on multiple factors including e.g., the age at the time of stroke (Chilosi et al., 2008). Further, studies have shown that acute and subacute language recovery may be dependent on different neural substrates, with early contributions from the right hemisphere and later language abilities dependent on the left hemispheric peristroke regions (Saur et al., 2006). This issue – timing of the insult – is very important as age-dependent changes in language localization and lateralization have been observed in various cross-sectional and longitudinal studies of healthy children and adults (Springer et al., 1999; Szaflarski et al., 2002, 2006a, 2012a, 2012b). Thus, an acute lesion may have a different effect on function recovery across ages. For example, in one study, recovery of function was best when the insult occurred before the age of 12 months with the severity of language deficits increasing with age at the time of the insult (Satz et al., 1994).
With adult injury, the patterns of recovery usually follow the previously proposed hierarchy. The best recovery is observed in adults who have restored their pre-stroke language circuits (usually patients with small lesions in the LMCA distribution) and the worst recovery occurs in adults with large LMCA lesions who re-mapped their language centers to the non-dominant homotopic areas (Heiss and Thiel, 2006). However, the hierarchy of post-stroke language recovery proposed by Heiss and Thiel may not apply to children as even large prenatal strokes are infrequently associated with frank language deficits and language production in children with perinatal strokes is usually associated with right more than left cortical activation in fMRI studies (Stiles et al., 2005; Tillema et al., 2008). The relationship between the age at the time of the stroke and subsequent recovery appears to be a controversial issue (Anderson et al., 2011; Raja Beharelle et al., 2010). However, the effects of age at the time of stroke on post-stroke language lateralization have not been examined in large cross-sectional studies that include patients with perinatal, childhood, and adult LMCA strokes. Thus, the main aim of this study was to further elucidate how the age of stroke occurrence affects language lateralization and cortical activation patterns in a large sample of children and adults using a previously well characterized verb generation fMRI task (Szaflarski et al., 2006a; Wise et al., 1991). Our hypothesis was that, with increasing age, reorganization after LMCA stroke would be increasingly dependent on left-lateralized mechanisms. I.e., we hypothesized that in subjects with perinatal stroke language centers as visualized with fMRI and verb generation task will be lateralized to the unaffected hemisphere while in participants with adult LMCA stroke the dependency on the right hemisphere for verb generation would decrease.
2. Methods
2.1. Subjects
Subjects for this study were identified from several previous or ongoing studies of language localization and/or post-stroke aphasia treatment after left middle cerebral artery stroke (HD38578, NS01467, NS048281, and HD068488). Demographic and clinical data of the participants are provided in Table 1. After providing verbal approval and signing written consent (assent for children less than 16 years of age), 54 LMCA stroke subjects received fMRI while performing a covert version of the verb generation task (VGT) (Allendorfer et al., 2012a; Wise et al., 1991). Subsequently, three subjects were excluded. For one subject, the age of the stroke was uncertain; another demonstrated excessive motion during the fMRI task, and the third subject was excluded due to the presence of bilateral and symmetric colpocephaly. The remaining stroke subjects were scanned at least ≥6 months after the stroke, with 19 having suffered from perinatal stroke (<1 month of age) and 32 from late stroke (>1 month of age). Additionally, 51 healthy controls (HCs) matched by sex and age at the time of scanning to the stroke subjects underwent the same fMRI procedures. All perinatal stroke subjects gained language competency at the time of scanning and all late stroke patients enrolled in this study showed improvement in language functions (this was not tested in perinatal stroke group) between the immediate post stroke testing and the time of study participation but the linguistic tests used in the groups and between the studies were not amenable to comparisons or were not available (Jacola et al., 2006; Szaflarski et al., 2013; Tillema et al., 2008). All study-related activities were approved by the Institutional Review Boards of the corresponding institutions (Cincinnati Children’s Hospital Medical Center, University of Cincinnati Academic Health Center and University of Alabama at Birmingham).
Table 1.
Demographic and clinical variables for patients who experienced perinatal (<1 month of age) or late (>1 month of age) stroke
| Perinatal stroke | Late stroke | p-value | |
|---|---|---|---|
| N | 19 | 32 | |
| % female | 52.6 | 43.8 | 0.54 |
| Age at stroke (years) | 0 (0) | 48.5 (15.0) | <0.001 |
| Age at scan (years) | 9.6 (3.5) | 51.8 (15.1) | <0.001 |
| Time since stroke (years) | 9.6 (3.5) | 3.2 (3.1) | <0.001 |
| Stroke volume (cm3) | 30.3 (31.8) | 60.1 (57.5) | 0.021 |
| Brain volume (cm3) | 1222.3 (174.4) | 1294.7 (137.4) | 0.13 |
| % Lesion volume | 2.7 (3.2) | 4.6 (4.3) | 0.084 |
Note: Data are reported as mean (SD) unless indicated otherwise.
2.2. Functional MRI task
The VGT consisted of 11 alternating 30-second blocks of finger-tapping in response to a modulated tone heard every 5 seconds (control condition) and covert verb generation in response to a noun presented every 5 seconds (active condition) starting with the control condition (Karunanayaka et al., 2011). This fMRI task has been widely used to examine hemispheric language dominance in studies of children and adults and has been described in detail in previous publications (Szaflarski et al., 2006a, 2013). Briefly, in the active condition subjects were presented with a series of nouns every 5 seconds, via MRI compatible headphones. They were required to silently (covertly) generate as many verbs as possible associated with the noun. For example, for the presented noun “soup” the participant might have generated such verbs as “eat” or “cook.” In the control condition, the subjects were explicitly instructed to touch each finger of each hand sequentially to the thumb of the same hand each time they heard a high-pitch modulated tone (presented every 5 seconds). This condition served to: (1) control for the auditory stimulation present in the active condition, (2) distract subjects from continuing to generate verbs during the control condition, and (3) create a motor cortex reference area as a method of validating compliance with the task (data not shown). The activation pattern related to finger tapping is unlikely to overlap with those known to occur with verb generation tasks. Intra-scanner behavioral data are not collected with this covert verb generation task. However, the finger tapping condition allows monitoring of task synchronization performance. Both conditions were rehearsed prior to scanning to ensure understanding of the task, particularly for children; subjects were not admitted to the scanner unless they clearly understood the task. In children, consistent age-independent recall of presented nouns was observed with this task on post-scanning testing (Chiu et al., 2006).
2.3. MRI scanners
Forty subjects were scanned on a 3T Bruker scanner between the ages of 5 and 16; 20 subjects had suffered a stroke (19 perinatal stroke, 1 late stroke) and 20 were matched HCs. Sixty-two adult subjects (31 with late stroke and 31 matched HCs) were scanned on a 3T Philips Achieva MRI system using dual Quasar gradients. Some, but not all of these subjects were included in our previous studies of post-stroke language recovery (Allendorfer et al., 2012a; Jacola et al., 2006; Tillema et al., 2008). All subjects were fitted with non-ferromagnetic headphones (Avotec, Inc.) for auditory stimuli presentation, and given a button to push in the left hand to record responses. We used the following parameters for the 3T Bruker T1-weighted 3D whole brain scans (acquired in the sagittal plane): TR/TE = 15/4.3 ms, FOV 256 × 192 × 162 mm, flip angle 20°, slice thickness=1 mm isotropic. The Bruker functional scans were obtained using the following parameters: TR/TE = 3000/38 ms, matrix = 64 × 64, FOV = 256 × 256 mm, slice thickness = 5 mm, 35 axial slices (110 scans with a total duration of 5 minutes and 30 seconds). We acquired the 3T Philips high-resolution T1-weighted anatomical scan (acquired in the sagittal plane) with the following parameters: TR/TE 8.1/3.7 ms, FOV 250 × 211 × 180 mm, flip angle 8°, slice thickness=1 mm isotropic. The Phillips functional scans were performed using a gradient-echo echo planar imaging sequence: TR/TE 2000/38 ms, FOV 240 × 240 mm, matrix 64 × 64, slice thickness = 4 mm, 32 axial slices (165 scans with a duration of 5 minutes and 30 seconds).
2.4. MRI data processing
All MRI data analyses including image reconstruction, motion correction, spatial smoothing, normalization into stereotactic (i.e., Talairach coordinate) space and generation of statistical parametric maps, were performed using CCHIPS (Cincinnati Children’s Hospital Image Processing Software) (Szaflarski and Allendorfer, 2012). The general linear model (GLM) was used to determine single-subject activation related to verb generation by contrasting active > control conditions (Friston et al., 1995). Group activation maps were then generated separately for the perinatal and adult stroke groups. Language lateralization indices (LIs) were computed using 3 previously defined anatomical regions of interest (frontal, posterior, and global ROIs) to quantify differences between groups in left-right hemisphere language activation (Szaflarski et al., 2006a). To avoid subjective selection of a particular threshold, LIs were determined using the individual Z score maps with only voxels with Z scores greater than or equal to the median Z score within an ROI (left or right hemispheric) for each individual subject counted in the calculation of LIs (Szaflarski et al., 2006a, 2006b). This approach provides the least variability and most robust LIs with immunity to outlying voxels with high statistical values (Holland et al., 2007). Pixels above this Z score threshold were counted and LI was defined as the difference in the number of activated voxels, summed independently for the left and right regions of interest, divided by the summed total of active voxels in the left and right regions of interest. Because the LI is a ratio of the difference of active voxels between the hemispheres divided by the total number of super-threshold voxels for an individual subject’s statistical parametric map, it is largely independent of scan parameters and/or field strength. Previous studies have used the LI to facilitate comparison of fMRI language lateralization among subjects scanned on scanners from different manufacturers and field strengths of 3.0 and 4.0 Tesla (Szaflarski et al., 2006a). Anatomically defined frontal and posterior language areas were used as regions of interest (ROIs) for LI analysis (Allendorfer et al., 2012a). Briefly, the frontal ROI comprised of anterior language areas in both hemispheres: inferior and middle frontal gyri and insulae (Brodmann areas 13, 44–47); the posterior ROI comprised of superior temporal, marginal and angular gyri (Brodmann areas 20, 21, 37–39 and 42). Values between −0.1< LI ≤ 0.1 define symmetric language distribution, while LI > 0.1 is categorized as left-dominant and LI <−0.1 is categorized as right-dominant for language (Szaflarski et al., 2006a). Chi-square analysis was used to assess group differences in language lateralization. The relationship between age of stroke occurrence and LIs was examined using Pearson’s correlation coefficient. All statistical analyses were performed using SAS 9.3.
Stroke lesion masks were traced manually on each subject’s anatomical MRI data by a trained neuroanatomist using AFNI and the number of voxels within each lesion mask was used to calculate the lesion’s residual volume (cm3) (Cox, 1996; Szaflarski et al., 2013). Brain volume (cm3) and corresponding percent lesion volume were also computed. To visualize the percent overlap of lesions within each stroke group, the lesion masks were applied to the anatomical MRI scans prior to performing the spatial normalization algorithm as previously described (Szaflarski et al., 2013). Spatial normalization of the lesion masks were then performed using the same transformation matrix used for the anatomical MRI and visually inspected by the neuroanatomist for correctness.
3. Results
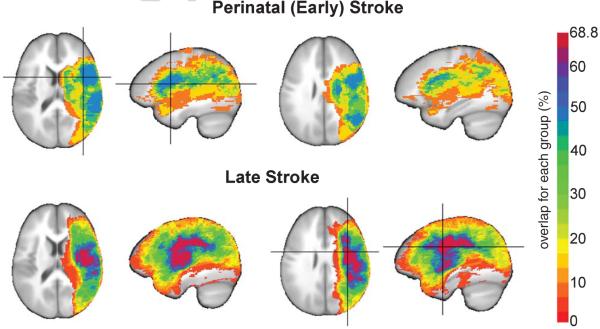
Perinatal and late stroke patients were matched 1 : 1 to HCs by age (p = 1.0 and p = 0.99, respectively) and sex (both p = 1.0). Demographic data are presented in Table 1. Close to half (47.4%) of the perinatal stroke group and 68.8% of the late stroke group exhibited lesion overlap (Fig. 1). Stroke lesion volumes were significantly larger in late stroke than in perinatal stroke group (p = 0.021). However, when taking brain volume into account (Table 1), which was somewhat smaller in the perinatal than in the late group (p = 0.13), percent lesion volumes did not significantly differ between groups (p = 0.084).
Fig. 1.
Lesion overlap maps in patients who suffered perinatal (early; n = 19; top) or late (n = 32; bottom) LMCA stroke. Stroke lesions are represented as the percentage of subjects who showed overlap in a region for each group and overlaid on to the ICB452 anatomical image. Crosshairs indicating the region of maximum overlap in perinatal stroke subjects (47.4%) was located in the left insula and in late stroke subjects (68.8%) was located in the left precentral gyrus.
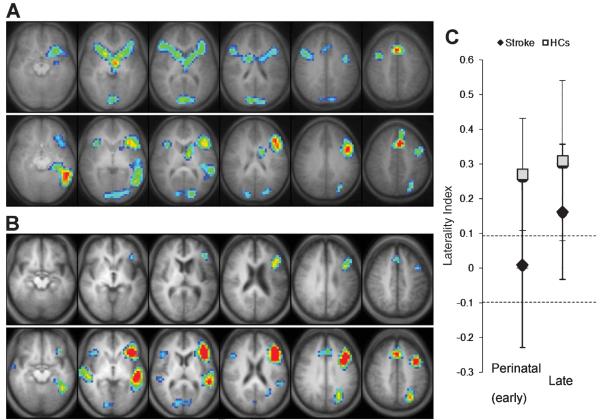
When compared to their respective age- and sex-matched HCs, the stroke groups exhibited distinct cortical activation patterns for the VGT (Fig. 2A, B). The perinatal stroke group showed bilateral activation increases in frontal (inferior, middle and superior frontal gyrus), temporal (superior temporal gyrus), visual and striatal regions, while their HCs exhibited overall left hemisphere activation in frontal, temporal and parietal cortex. The adult stroke group also showed left-hemisphere activation increases in the inferior/middle frontal gyri similar to their HCs, which exhibited predominately left > right hemisphere activation in frontal and temporal regions, as well as in left parietal and bilateral visual cortex. LIs of the stroke groups were significantly smaller than their respective HCs (all p < 0.008) except for the posterior region LI in the late stroke group (p = 0.080), though there is a trend toward significance even in this region for this group. In particular, the global LI that is used to determine language laterality was significantly smaller in stroke groups (Fig. 2C; Table 2). Chi-square analysis revealed a significant difference in the distribution of language lateralization between HCs and their respective stroke groups (p = 0.004 for perinatal stroke and p = 0.039 for late stroke), with a greater proportion of symmetric and right-lateralized fMRI signal distribution in perinatal stroke (68.4% vs. 21.1%) compared to HCs and in late stroke (40.6% vs. 12.5%) compared to HCs. Distribution of language lateralization was also significantly different between stroke groups, with a larger proportion of leftward lateralization in late stroke (p = 0.039). Finally, the age of stroke occurrence showed significant positive associations with frontal (r = 0.39; p = 0.005) and global (r = 0.37; p = 0.007). LIs and a trend towards a positive association with posterior LI (r = 0.25; p = 0.072; Fig. 3).
Fig. 2.
Statistical activation maps (corrected p < 0.05) for the verb generation task performed during fMRI in each of the groups (A, B) and corresponding global laterality index (LI) values (C). Activation clusters are overlaid onto axial slices of an averaged anatomical image of all subjects in each group, spanning z = −10 (left) to z = +40 (right). All images are shown in radiological convention (left in image is right in the brain). (A) Patients who suffered perinatal stroke (top row) show more symmetrical activation than their sex-/age-matched healthy controls (HCs; bottom row). (B) Patients who suffered a late stroke (top row) show less activation overall but similar left-lateralized pattern compared to their HCs (bottom row). (C) Global LI (mean ± SD) used to determine language laterality (i.e., dashed lines with LI > 0.1 as leftward, LI <− 0.1 as rightward, and −0.1 ≤ LI ≤ 0.1 as symmetric) is significantly higher in both HCs compared to their respective stroke groups.
Table 2.
LI scores and lateralization for verb generation task in perinatal and late stroke patients and their respective sex- and age-matched healthy controls (HCs)
| Perinatal stroke N=19 |
Perinatal stroke HCs N=19 |
Late stroke N=32 |
Late stroke HCs N=32 |
||
|---|---|---|---|---|---|
| LI scores | Frontal | 0.008 (0.30) | 0.28 (0.21) | 0.19 (0.22) | 0.37 (0.26) |
| Posterior | 0.026 (0.26) | 0.26 (0.15) | 0.13 (0.21) | 0.24 (0.25) | |
| Global | 0.009 (0.24) | 0.27 (0.16) | 0.16 (0.19) | 0.31 (0.23) | |
| Lateralizationa | Left | 6 (31.6) | 15 (78.9) | 19 (59.4) | 28 (87.5) |
| Right | 7 (36.8) | 0 (0.0) | 3 (9.4) | 1 (3.1) | |
| Symmetric | 6 (31.6) | 4 (21.1) | 10 (31.3) | 3 (9.4) |
Note. Data are reported as mean (SD) for LI scores and as frequency (proportions) for lateralization.
Lateralization was determined from the global laterality index with LI > 0.1 as leftward, LI <−0.1 as rightward and −0.1 ≤ LI ≤ 0.1 as symmetric. The mean LI is significantly smaller in the stroke groups than in their respective HCs (all p < 0.008) except for posterior LI in the late stroke group and their HCs (p = 0.080). The distribution of language lateralization is also significantly different between the stroke groups and their respective HCs (p = 0.004 for perinatal stroke and p = 0.039 for late stroke) as determined by Chi-square analysis, with HCs being more left-lateralized.
Fig. 3.
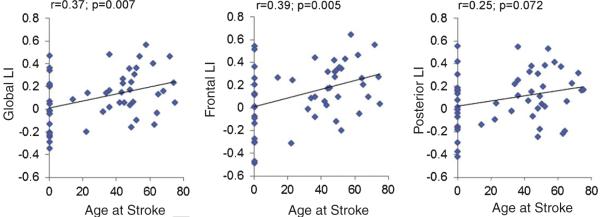
Results of correlation analysis between age at the time of stroke and LIs calculated from global, frontal and posterior ROIs. Age of stroke occurrence showed significant positive associations with global and frontal LIs, and a positive trend for posterior LI. Age ranges at the time of stroke are from 0 (perinatal or prenatal stroke) to 75 years; ages at the time of MRI are 6 to 78.
4. Discussion
As expected, the analyzes conducted in a large sample of participants with history of perinatal, postnatal, and adult stroke provide evidence that the age of stroke occurrence significantly affects the lateralization of language functions, as tested with a verb generation fMRI task. In agreement with the initial hypothesis, children exhibited more symmetric language lateralization when compared to the matched healthy controls and to the adult stroke group. When older individuals suffered a LMCA stroke, they were more likely to exhibit language activation patterns that were lateralized to the left hemisphere compared to the younger individuals with perinatal stroke. This difference is likely due to greater brain plasticity observed during early human development, such that reorganization of language function to the contralateral hemisphere is more pronounced following perinatal stroke while there is greater reliance on the peristroke areas for language production after adult stroke. This relationship was not affected by stroke size; in fact, it held despite the fact that stroke size was significantly smaller in the perinatal group. Therefore, our findings support the notion that damage to the left fronto-temporal brain regions induces different patterns of language reorganization depending on age at the time of insult and that the underlying mechanism of this process in children is different from the mechanism of post-stroke language recovery in adults.
4.1. Language lateralization after prenatal/perinatal stroke
The studies in children who have suffered from a perinatal or early postnatal injury indicate that these children usually recover language and other cognitive functions completely or almost completely. For children, the degree of recovery and the presence of specific deficits may be related to the location of the lesion and the age at the time of testing, but not the size of the lesion (Ballantyne et al., 2008; Stiles et al., 2005; Thal et al., 1991; Vicari et al., 2000). One of the early studies, of adults who suffered a perinatal or early postnatal stroke (<12 months), revealed similar language performance when compared to children who had a stroke later in development (>12 months of age). At the same time, the children with later stroke demonstrated more significant impairment of non-verbal functions that are typically located in the non-dominant hemisphere. This finding suggests that language recovery after a late but not early childhood stroke (due to a lesion >12 months after birth) may lead to crowding out of some of the right-hemispheric functions (crowding hypothesis) if the shift to the previously non-dominant hemisphere is to underlie language improvement (Satz et al., 1994). In fact, in children who have even the entire dominant hemisphere removed early in life, e.g., hemispherectomy for seizure control, relatively normal language functions may be expected after a period of recovery (Liegeois et al., 2008). But again, this relationship and excellent recovery may not be observed after later childhood left hemispheric injury (Loddenkemper et al., 2004). In the context of early injury, it is expected that cortical plasticity is beneficial and that it leads to better language functions due to their relocation to the right hemispheric homologues as an adaptive process. However, as indicated above, the typically non-dominant hemisphere functions may be impaired due to this interhemispheric transfer and crowding out of the less vital functions.
The initial neuroimaging studies from children with early brain injury have indicated that language recovery may be related to neuroplasticity with shifts of language functions to brain regions not typically involved in language production, including right hemispheric homologues (Jacola et al., 2006; Staudt et al., 2001). This notion was recently questioned in a study that revealed better receptive and expressive language skills in a large cohort of children with history of perinatal stroke who demonstrated fMRI activation patterns similar to those of their healthy siblings who showed bilateral involvement of the superior temporal gyri (STG) and the left inferior frontal gyrus (IFG) (Raja Beharelle et al., 2010). In a review, Anderson et al. argued that the brain’s capacity for plasticity during development may also reflect its vulnerability. More specifically, when the normal and predetermined process of function development is disrupted during development, substitution of other areas for the support of important functions may derail the development of other cognitive abilities. Such events could then lead to incomplete or abnormal function development for non-language functions (Anderson et al., 2011; Rothi and Horner, 1983; Satz et al., 1994).
4.2. Adult stroke and language recovery
In adults, post-stroke language recovery may rely on one of several neurological processes that typically occur with recovery of function: (1) repair of damaged networks, (2) activation of compensatory areas, or (3) activation of previously inactive pathways (Lee and van Donkelaar, 1995). This process is likely dependent on the regional hierarchy for recovery (Heiss and Thiel, 2006). In other words, better language function is observed in subjects who recover the dominant language network via repair rather than via compensatory mechanisms of increased involvement of the contralateral homologues in speech production as we have shown previously in this population (Rothi and Horner, 1983). The lesions that occur as sequelae of LMCA strokes in adults are regarded as different from similar lesions that occur in childhood and, therefore, the process of recovery of function may be dependent on different factors including lesion size (Allendorfer et al., 2012a; Kertesz et al., 1979; Knopman et al., 1983; Naeser et al., 1981). In general, the patterns of recovery described in adults include varying degrees of left- or right-hemispheric involvement but overall, as observed here with the verb generation task, are similar to the language activation patterns seen in healthy controls. In one of the early studies, recovery from post-stroke stroke was related to right-hemispheric language shift (Thulborn et al., 1999). However, subsequent studies postulated that right-hemispheric activation is detrimental for post-stroke language recovery (Rosen et al., 2000) and that the compensatory potential of the right IFG is lower than that of the recovery of the left IFG for subsequent language abilities (Winhuisen et al., 2005; Winhuisen et al., 2007). Others have shown that stimulation of the residual language areas is beneficial for subsequent recovery (Baker et al., 2010; Szaflarski et al., 2011), and that recovery may be associated with plasticity in the gray and white matter (Allendorfer et al., 2012b).
In summary, this study focuses on cortical language activation patterns that are associated with the performance of VGT in children and adults who have suffered from early or late stroke. While we are aware of complete or almost complete function recovery in all or almost all children and a substantial portion of the included adults, the neuropsychological correlates of the post-stroke language recovery are not specifically examined here due to the lack of compatibility between the language tests performed in the four studies from which the participants were drawn for this analysis (Szaflarski et al., 2013; Tillema et al., 2008). The observed differences in post-stroke language fMRI patterns between children and adults likely result from different recovery mechanisms. Whereas the process of activation of the preexisting (and likely previously non-dominant) pathways and locations may be an efficient and preferred mechanism of recovery of function in pre-/perinatal stroke, this mechanism is unlikely to contribute to the language recovery in adult LMCA stroke. In contrast, recovery from aphasia in adults following stroke appears to rely more heavily on reactivation of the previously established peri-lesional language areas, rather than a shift to contralateral brain regions not previously used for language function.
Acknowledgments
This study was supported by HD38578 (SKH), NS048281 (JPS), HD/NS01467 (AWB), and HD068488 (JPS). This study was presented, in part, at the Meeting of the Organization for Human Brain Mapping, Seattle, WA 6/2013. The authors recognize the help of the following individuals in subject recruitment and data collection: Wendy Bommer, RN, Jennifer Ret, BS, and Christi Banks, CCRC.
Footnotes
Author contributions
JPS – designed and developed the study concept, analyzed and interpreted the data, and drafted/revised the manuscript for content and approved the final version
JBA – developed the study concept, analyzed and interpreted the data, and drafted/revised the manuscript for content and approved the final version
JV – developed the study concept, drafted/revised the manuscript for content and approved the final version
AWB – developed the study concept, drafted/revised the manuscript for content and approved the final version
AD – developed the study concept, drafted/revised the manuscript for content and approved the final version
KH – developed the study concept, analyzed the data and drafted/revised the manuscript for content and approved the final version
SKH – designed and developed the study concept, interpreted the data, and drafted/revised the manuscript for content and approved the final version
References
- Allendorfer JB, Kissela BM, Holland SK, et al. Different patterns of language activation in post-stroke aphasia are detected by overt and covert versions of the verb generation fMRI task. Med Sci Monit. 2012a;18:CR135–CR147. doi: 10.12659/MSM.882518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorfer JB, Storrs JM, Szaflarski JP. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci. 2012b;30:103–113. doi: 10.3233/RNN-2011-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134:2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne AO, Spilkin AM, Hesselink J, et al. Plasticity in the developing brain: Intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain. 2008;131:2975–2985. doi: 10.1093/brain/awn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi AM, Cipriani P, Pecini C, et al. Acquired focal brain lesions in childhood: Effects on development and reorganization of language. Brain Lang. 2008;106:211–225. doi: 10.1016/j.bandl.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Chiu CY, Schmithorst VJ, Brown RD, et al. Making memories: A cross-sectional investigation of episodic memory encoding in childhood using FMRI. Dev Neuropsychol. 2006;29:321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46:533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacola LM, Schapiro MB, Schmithorst VJ, et al. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke1. Neuropediatrics. 2006;37:46–52. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanayaka P, Schmithorst VJ, Vannest J, et al. A linear structural equation model for covert verb generation based on independent component analysis of FMRI data from children and adolescents. Front Syst Neurosci. 2011;5:29. doi: 10.3389/fnsys.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain Lang. 1979;8:34–50. doi: 10.1016/0093-934x(79)90038-5. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Selnes OA, Niccum N, et al. A longitudinal study of speech fluency in aphasia: CT correlates of recovery and persistent nonfluency. Neurology. 1983;33:1170–1178. doi: 10.1212/wnl.33.9.1170. [DOI] [PubMed] [Google Scholar]
- Lee RG, van Donkelaar P. Mechanisms underlying functional recovery following stroke. Can J Neurol Sci. 1995;22:257–263. doi: 10.1017/s0317167100039445. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Baldeweg T, et al. Speaking with a single cerebral hemisphere: fMRI language organization after hemispherectomy in childhood. Brain Lang. 2008;106:195–203. doi: 10.1016/j.bandl.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Dinner DS, Kubu C, et al. Aphasia after hemispherectomy in an adult with early onset epilepsy and hemiplegia. J Neurol Neurosurg Psychiatry. 2004;75:149–151. [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Hayward RW, Laughlin SA, et al. Quantitative CT scan studies in aphasia. I. Infarct size and CT numbers. Brain Lang. 1981;12:140–164. doi: 10.1016/0093-934x(81)90010-9. [DOI] [PubMed] [Google Scholar]
- Raja Beharelle A, Dick AS, Josse G, et al. Left hemisphere regions are critical for language in the face of early left focal brain injury. Brain. 2010;133:1707–1716. doi: 10.1093/brain/awq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Horner J. Restitution and substitution: Two theories of recovery with application to neurobehavioral treatment. J Clin Neuropsychol. 1983;5:73–81. doi: 10.1080/01688638308401152. [DOI] [PubMed] [Google Scholar]
- Satz P, Strauss E, Hunter M, et al. Re-examination of the crowding hypothesis: Effects of age of onset. Neuropsychology. 1994;8:255–262. [Google Scholar]
- Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Niemann G, et al. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–125. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly J, Paul B, et al. Cognitive development following early brain injury: Evidence for neural adaptation. Trends Cogn Sci. 2005;9:136–143. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Szaflarski J, Vannest J, Wu S, et al. Excitatory repetitive transcranial magnetic stimulation induces improvements in chronic post-stroke aphasia. Med Sci Monitor. 2011;17:CR132–CR139. doi: 10.12659/MSM.881446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Allendorfer JB. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav. 2012;24(1):74–80. doi: 10.1016/j.yebeh.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Allendorfer JB, Banks C, et al. Recovered vs. not-recovered from post-stroke aphasia: The contributions from the dominant and non-dominant hemispheres. Restor Neurol Neurosci. 2013;31:347–360. doi: 10.3233/RNN-120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Altaye M, Rajagopal A, et al. A 10-year longitudinal fMRI study of narrative comprehension in children and adolescents. Neuroimage. 2012a;63(3):1188–1195. doi: 10.1016/j.neuroimage.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, et al. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006a;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Rajagopal A, Altaye M, et al. Left-handedness and language lateralization in children. Brain Res. 2012b;1433:85–97. doi: 10.1016/j.brainres.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006b;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DJ, Marchman V, Stiles J, et al. Early lexical development in children with focal brain injury. Brain Lang. 1991;40:491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- Tillema JM, Byars AW, Jacola LM, et al. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang. 2008;105:99–111. doi: 10.1016/j.bandl.2007.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Albertoni A, Chilosi AM, et al. Plasticity and reorganization during language development in children with early brain injury. Cortex. 2000;36:31–46. doi: 10.1016/s0010-9452(08)70834-7. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, et al. The right inferior frontal gyrus and poststroke aphasia: A follow-up investigation. Stroke. 2007;38:1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, et al. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991;114:1803–1817. doi: 10.1093/brain/114.4.1803. Pt 4. [DOI] [PubMed] [Google Scholar]