Abstract
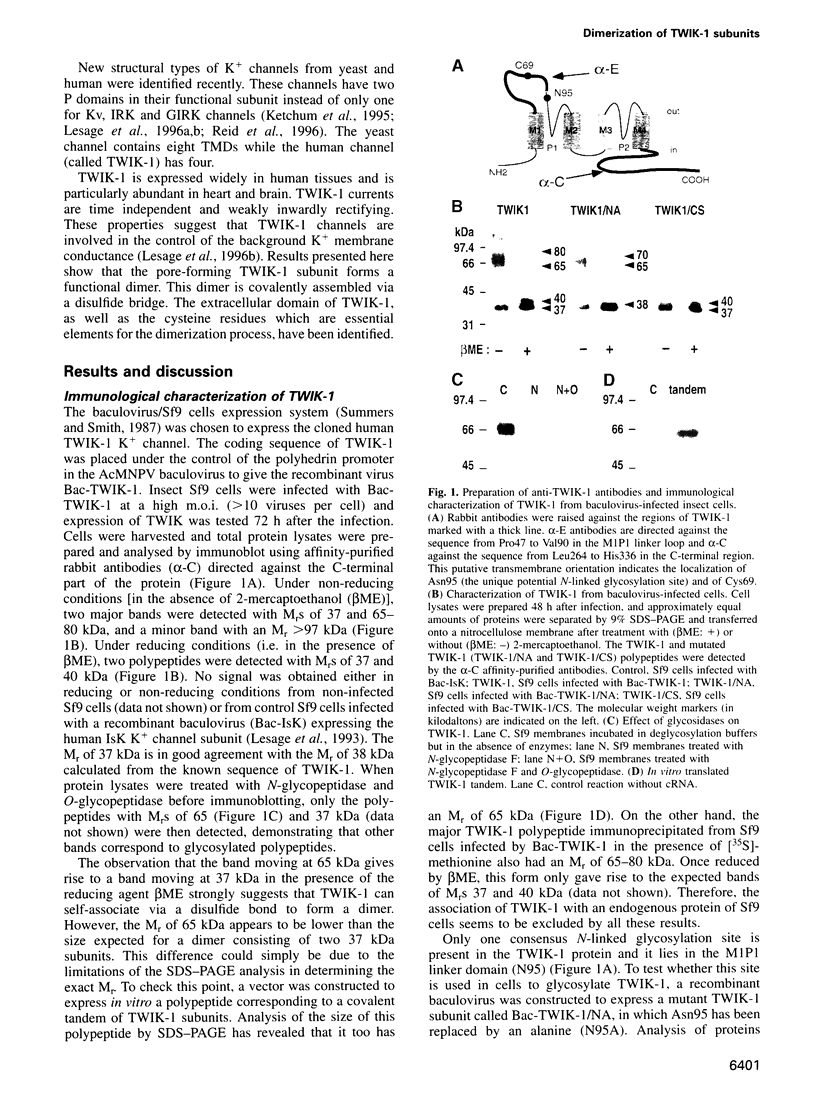
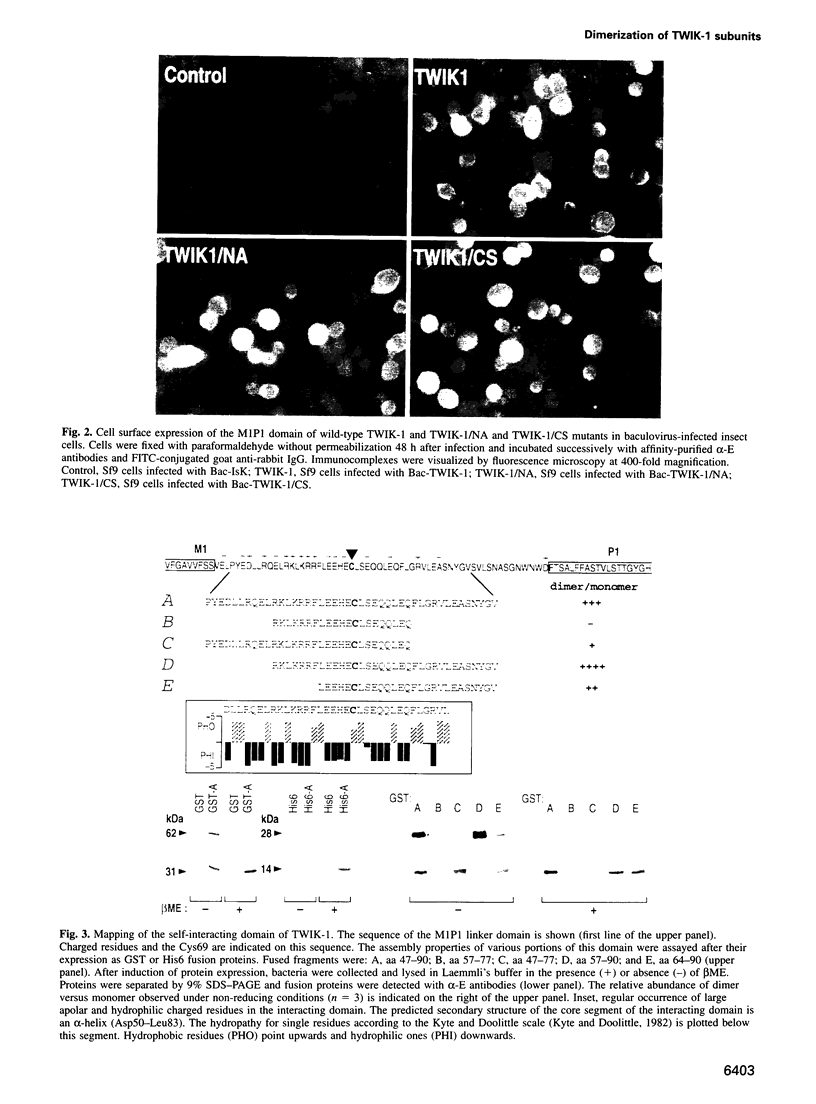
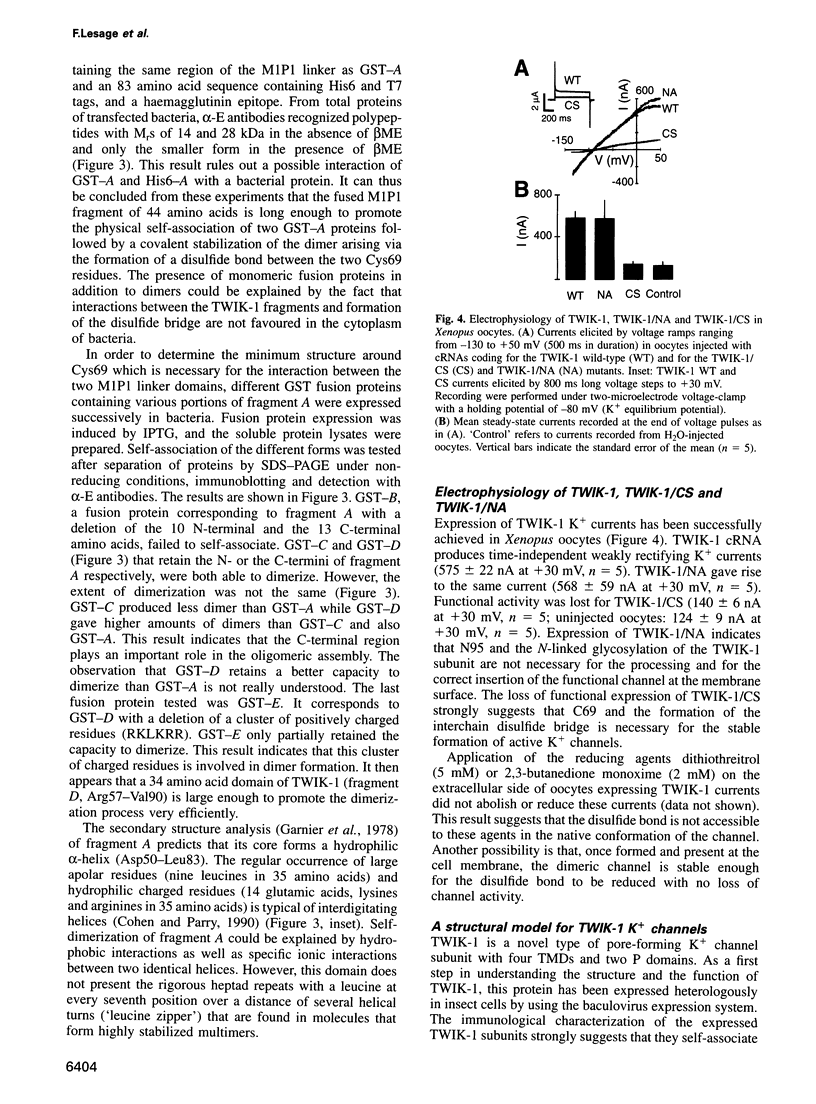
TWIK-1 is a new type of K+ channel with two P domains and is abundantly expressed in human heart and brain. Here we show that TWIK-1 subunits can self-associate to give dimers containing an interchain disulfide bridge. This assembly involves a 34 amino acid domain that is localized to the extracellular M1P1 linker loop. Cysteine 69 which is part of this interacting domain is implicated in the formation of the disulfide bond. Replacing this cysteine with a serine residue results in the loss of functional K+ channel expression. This is the first example of a covalent association of functional subunits in voltage-sensitive channels via a disulfide bridge.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W. Potassium channels. New channel subunits are a turn-off. Curr Biol. 1994 Sep 1;4(9):839–840. doi: 10.1016/s0960-9822(00)00187-1. [DOI] [PubMed] [Google Scholar]
- Attali B., Latter H., Rachamim N., Garty H. A corticosteroid-induced gene expressing an "IsK-like" K+ channel activity in Xenopus oocytes. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6092–6096. doi: 10.1073/pnas.92.13.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babila T., Moscucci A., Wang H., Weaver F. E., Koren G. Assembly of mammalian voltage-gated potassium channels: evidence for an important role of the first transmembrane segment. Neuron. 1994 Mar;12(3):615–626. doi: 10.1016/0896-6273(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990 Apr 17;29(15):3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Perozo E., Stefani E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys J. 1994 Apr;66(4):1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy K. G., Gutman G. A. Nomenclature for mammalian potassium channel genes. Trends Pharmacol Sci. 1993 Dec;14(12):434–434. doi: 10.1016/0165-6147(93)90181-i. [DOI] [PubMed] [Google Scholar]
- Cohen C., Parry D. A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7(1):1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Wei A. A., Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991 Nov;7(5):763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Deal K. K., England S. K., Tamkun M. M. Molecular physiology of cardiac potassium channels. Physiol Rev. 1996 Jan;76(1):49–67. doi: 10.1152/physrev.1996.76.1.49. [DOI] [PubMed] [Google Scholar]
- Doupnik C. A., Davidson N., Lester H. A. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995 Jun;5(3):268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Duprat F., Lesage F., Guillemare E., Fink M., Hugnot J. P., Bigay J., Lazdunski M., Romey G., Barhanin J. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun. 1995 Jul 17;212(2):657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- Fink M., Duprat F., Heurteaux C., Lesage F., Romey G., Barhanin J., Lazdunski M. Dominant negative chimeras provide evidence for homo and heteromultimeric assembly of inward rectifier K+ channel proteins via their N-terminal end. FEBS Lett. 1996 Jan 2;378(1):64–68. doi: 10.1016/0014-5793(95)01388-1. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Guillemare E., Honoré E., Pradier L., Lesage F., Schweitz H., Attali B., Barhanin J., Lazdunski M. Effects of the level of mRNA expression on biophysical properties, sensitivity to neurotoxins, and regulation of the brain delayed-rectifier K+ channels Kv1.2. Biochemistry. 1992 Dec 15;31(49):12463–12468. doi: 10.1021/bi00164a024. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994 Apr;66(4):1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Potassium channels and their evolving gates. Nature. 1994 Sep 8;371(6493):119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Ketchum K. A., Joiner W. J., Sellers A. J., Kaczmarek L. K., Goldstein S. A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995 Aug 24;376(6542):690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- Kofuji P., Davidson N., Lester H. A. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G., Gordon E. A., Wickman K., Velimirović B., Krapivinsky L., Clapham D. E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995 Mar 9;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee T. E., Philipson L. H., Kuznetsov A., Nelson D. J. Structural determinant for assembly of mammalian K+ channels. Biophys J. 1994 Mar;66(3 Pt 1):667–673. doi: 10.1016/s0006-3495(94)80840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Attali B., Lakey J., Honoré E., Romey G., Faurobert E., Lazdunski M., Barhanin J. Are Xenopus oocytes unique in displaying functional IsK channel heterologous expression? Receptors Channels. 1993;1(2):143–152. [PubMed] [Google Scholar]
- Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. A pH-sensitive yeast outward rectifier K+ channel with two pore domains and novel gating properties. J Biol Chem. 1996 Feb 23;271(8):4183–4187. doi: 10.1074/jbc.271.8.4183. [DOI] [PubMed] [Google Scholar]
- Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996 Mar 1;15(5):1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. Potassium and calcium channels in lymphocytes. Annu Rev Immunol. 1995;13:623–653. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- Li M., Jan Y. N., Jan L. Y. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992 Aug 28;257(5074):1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Movahedi S., Satler C., Lindpaintner K., Nadal-Ginard B. Incremental reductions of positive charge within the S4 region of a voltage-gated K+ channel result in corresponding decreases in gating charge. Neuron. 1992 Mar;8(3):531–540. doi: 10.1016/0896-6273(92)90281-h. [DOI] [PubMed] [Google Scholar]
- Lu Z., MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994 Sep 15;371(6494):243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Pore loops: an emerging theme in ion channel structure. Neuron. 1995 May;14(5):889–892. doi: 10.1016/0896-6273(95)90327-5. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Makhina E. N., Pearson W. L., Sha Q., Lopatin A. N. Inward rectification and implications for cardiac excitability. Circ Res. 1996 Jan;78(1):1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Pongs O. Structure-function studies on the pore of potassium channels. J Membr Biol. 1993 Oct;136(1):1–8. doi: 10.1007/BF00241484. [DOI] [PubMed] [Google Scholar]
- Rehm H., Lazdunski M. Purification and subunit structure of a putative K+-channel protein identified by its binding properties for dendrotoxin I. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4919–4923. doi: 10.1073/pnas.85.13.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. D., Lukas W., Shafaatian R., Bertl A., Scheurmann-Kettner C., Guy H. R., North R. A. The S. cerevisiae outwardly-rectifying potassium channel (DUK1) identifies a new family of channels with duplicated pore domains. Receptors Channels. 1996;4(1):51–62. [PubMed] [Google Scholar]
- Rhodes K. J., Keilbaugh S. A., Barrezueta N. X., Lopez K. L., Trimmer J. S. Association and colocalization of K+ channel alpha- and beta-subunit polypeptides in rat brain. J Neurosci. 1995 Jul;15(7 Pt 2):5360–5371. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Baker K., Butler A., Covarrubias M., Pak M. D., Wei A. An essential 'set' of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992 May;15(5):161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- Shen N. V., Pfaffinger P. J. Molecular recognition and assembly sequences involved in the subfamily-specific assembly of voltage-gated K+ channel subunit proteins. Neuron. 1995 Mar;14(3):625–633. doi: 10.1016/0896-6273(95)90319-4. [DOI] [PubMed] [Google Scholar]
- Sheng M., Liao Y. J., Jan Y. N., Jan L. Y. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993 Sep 2;365(6441):72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- Wang H., Kunkel D. D., Martin T. M., Schwartzkroin P. A., Tempel B. L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993 Sep 2;365(6441):75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Xu J., Yu W., Jan Y. N., Jan L. Y., Li M. Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits. J Biol Chem. 1995 Oct 20;270(42):24761–24768. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995 Dec;15(6):1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]