Abstract
The contribution of hyaluronan-dependent pericellular matrix to TGF-β1-driven induction and maintenance of myofibroblasts is not understood. Hyaluronan is an extracellular matrix (ECM) glycosaminoglycan important in cell adhesion, proliferation and migration, and is implicated in myofibroblast formation and maintenance. Reduced turnover of hyaluronan has been linked to differentiation of myofibroblasts and potentiation of lung fibrosis. Fibronectin is a fibril forming adhesive glycoprotein that is also upregulated following induction with TGF-β1. Although they are known to bind each other, the interplay between hyaluronan and fibronectin in the pericellular matrix during myofibroblast induction and matrix assembly is not clear. This study addresses the role of hyaluronan and its interaction with fibrillar matrix components during myofibroblast formation. Hyaluronan and fibronectin were increased and co-localized in the ECM following myofibroblast induction by TGF-β1. Inhibition of hyaluronan synthesis in TGF-β1-induced lung myofibroblasts over a 4 day period with 4-methyl umbelliferone (4-MU) further enhanced myofibroblast morphology, caused increased deposition of fibronectin and type I collagen in the ECM, and increased expression of alpha-smooth muscle actin and hyaluronan synthase 2 (HAS2) mRNA. Hyaluronan oligosaccarides or hyaluronidase treatment, which more effectively disrupted the pericellular matrix, had similar effects. CD44 and β1 integrins co-localized in the cell membrane and along some stress fibers. However, CD44 and hyaluronan were specifically excluded from focal adhesions, and associated primarily with cortical actin. Time-lapse imaging of the immediate effects of hyaluronidase digestion showed that hyaluronan matrix primarily mediates attachment of membrane and cortical actin between focal contacts, suggesting that surface adhesion through hyaluronan and CD44 is distinct from focal adhesion through β1 integrins and fibronectin. Fluorescein-labeled hyaluronan bound regularly along fibronectin fibers and co-localized more with β1 integrin and less with CD44. Therefore, the hyaluronan matrix can interfere with the assembly of fibrillar ECM components, and this interplay regulates the degree of myofibroblast formation. These data also suggest that adhesion through hyaluronan matrix impacts cytoskeletal organization, and is potentially part of a clutch mechanism that regulates stick and slip of myofibroblasts by affecting the adhesion to and organization of fibronectin and collagen.
Keywords: myofibroblast, hyaluronan, fibronectin, collagen, TGF-β1, 4-methyl umbelliferone
Introduction
Differentiation of fibroblasts and other cells into the myofibroblast functional state is controlled by transforming growth factor-beta 1 (TGF-β1), a well-known driver of fibrosis in lungs and other tissues [1]. Expression of alpha smooth muscle actin (αSMA), which contributes to cellular contractility, is a characteristic feature of myofibroblasts, as is increased mechanical coupling [2], and production of extracellular matrix (ECM) components. Tissue or substrate stiffness and tensile mechanical loading also drive the formation of myofibroblasts as part of a detrimental feed forward loop in which cellular contraction serves to mechanically activate latent TGF-β1 via integrins that are implicated in myofibroblast mechanoperception and transduction [1]. However, how and whether specific ECM components control the differentiation and stability of the myofibroblasts is not clear. Matrix adhesion structures may be promising targets to modulate myofibroblast differentiation and activity and diminish fibrosis, and clarifying the interactions among matrix components and their cell surface receptors will be vital for developing the most effective strategies.
Hyaluronan is an extracellular glycosaminoglycan important in cell proliferation, migration, wound healing, and inflammation, but its role in fibrosis is only beginning to be studied. Reduced turnover of hyaluronan via knockout of the hyaluronan receptor, CD44, has been linked to potentiation of inflammation and lung fibrosis [3]. Hyaluronan appears to play a direct role in the differentiation of myofibroblasts [4], and previous studies have also suggested a role for pericellular hyaluronan in generating and maintaining the myofibroblast phenotype by modulating TGF-β1 signaling pathways [5, 6]. As a surface coating, hyaluronan is primarily anti-adhesive for cells that already have it on their surface [7] and its effects on collagen gel contraction by fibroblasts in vitro are variable [8, 9]. High hyaluronan production has also been linked to detachment of cells [10, 11]. Therefore, the question of how hyaluronan controls myofibroblast adhesion, differentiation, and matrix assembly remains unclear.
Increased production of fibrillar ECM, particularly collagen and fibronectin, is a hallmark of myofibroblasts and the resulting fibrosis ultimately interferes with tissue function. However, the questions of how these fibrillar ECM components interact with hyaluronan, what controls their interactions, and how important these interactions are to myofibroblast formation and maintenance need to be addressed.
Hyaluronan, in part, plays a space filling role and was shown to affect collagen fibril spacing in synovial tissue [12]. Fibronectin is also deposited by fibroblasts during wound healing and requires β1 integrins to be organized into fibrils [13], but the effects of hyaluronan on fibronectin fiber formation are not known. Earlier studies suggested that hyaluronan binds to cellular extra domain A (EDA)-containing fibronectin [14, 15]. Other data suggests there is cross talk between CD44 and β1 integrin receptors and cooperative binding of these receptors to fibronectin [16, 17]. However, the physical relationship between these two matrix components and their receptors in myofibroblasts is not clear.
In this study, we test whether hyaluronan affects the assembly of fibrillar matrix components during myofibroblast induction by TGF-β1, as well as determine the relationships between hyaluronan, fibronectin, CD44, β1 integrins, and the cytoskeleton by immunocytochemistry. We wanted to know if inhibition of hyaluronan synthesis or disruption of pericellular matrix integrity during induction by TGF-β1 would affect deposition of fibrillar matrix components in human lung fibroblasts (HLFs) and influence myofibroblast differentiation.
Results
Hyaluronan and fibronectin are closely interwoven in the ECM
The spatial relationship of hyaluronan to fibrillar matrix components has not been extensively studied in myofibroblasts. Therefore, immunocytochemistry was used to compare the distribution of hyaluronan with fibronectin in the ECM of control fibroblasts and TGF-β1-induced myofibroblasts. Compared to non-induced fibroblasts (Fig. 1a), stronger staining for both hyaluronan and fibronectin was seen in the myofibroblasts (Fig. 1b), where the hyaluronan was present in the form of cable-like structures. The molecules co-localized on the substrate and in the matrix above the myofibroblasts. Control fibroblasts tended to have less pericellular hyaluronan, less fibronectin, and consequently less colocalization. However, higher magnification revealed that in both control fibroblasts and TGF-β1-treated cells, the processes of fibronectin fibril formation and hyaluronan pericellular matrix formation are closely juxtaposed along the membrane, controlled via the same microspikes and filopodia, indicating that these matrix components are spatially positioned to interact with each other directly upon secretion (Fig. 1c and d). As was previously described [13], cells use tractional forces to pull globular fibronectin that was deposited on the substrate into thick mature fibrils, and our results showed that the same filopodia and finer protrusions that participated in this process were also hyaluronan-positive. Other images clearly indicated that cells closely interweave the hyaluronan cables and fibronectin fibers as the matrix is laid down (Fig. 1e). This suggests that hyaluronan may coat the fibronectin fibrils to varying extents. Very little collagen was detected in control cells and only occasional colocalization of collagen with endogenous hyaluronan was seen in myofibroblasts (data not shown).
Figure 1.
Hyaluronan associates with fibronectin in the ECM of fibroblast and myofibroblasts. (a) Control HLFs, and (b) TGF-β1-induced myofibroblasts were stained for endogenous hyaluronan (green) and cellular fibronectin (red). Nuclei (blue) were counterstained with DAPI. (c) Higher magnification of the boxed area in (a), showing the close juxtaposition of fine hyaluronan-positive microvillous protrusions (arrows) also involved in fibronectin fiber formation. (d) HLFs plated in medium with 0.1% serum showing colocalization of hyaluronan and fibronectin along two large filopodia (arrows). Asterisk indicates amorphous fibronectin on the coverslip that gets pulled into fibers. (e) Image shows a TGF-β1-induced myofibroblast with arrows indicating how hyaluronan and fibronectin are closely interwoven in the ECM. Nuclei were stained with DAPI. Bar equals approximately 50 μm.
Exogenous hyaluronan binds fibronectin fibers
Previous studies have shown that hyaluronan binds cellular fibronectin [14, 15], and others have shown a relationship between myofibroblast differentiation and reduced hyaluronan turnover [4]. We tested whether the interaction of hyaluronan and fibronectin would influence hyaluronan binding and uptake in myofibroblasts. Initial studies determined the degree of binding of exogenous fluorescein-labeled hyaluronan to control fibroblasts and TGF-β1-induced myofibroblasts during a brief, 30-min incubation. Myofibroblasts clearly bound more fluorescein-hyaluronan than control fibroblasts (Fig. 2a and b). Surprisingly, the exogenous hyaluronan bound primarily to fibrous elements in the ECM and rarely co-localized with CD44 (not shown). Co-staining for cellular fibronectin revealed that the fluorescein-hyaluronan was associated and co-localized primarily with organized fibronectin fibers, in both control (Fig. 2c) and TGF-β1-induced myofibroblasts (Fig. 2d). The labeled hyaluronan bound in a regular array along the fibronectin fibers (Fig. 2e). There was only occasional colocalization of the fluorescein-hyaluronan with type I collagen fibers (data not shown).
Figure 2.
Fluorescein-hyaluronan binds to fibronectin and co-aligns with β1 integrins. Fluorescein-hyaluronan (green) was incubated with control (a and c), and TGF-β1-induced (b, d, e, and f) myofibroblasts for 30 min. (c and d) co-staining showed that the labeled hyaluronan primarily bound to cellular fibronectin (red). (e) Higher magnification of a control cell showing a regular array of f-hyaluronan deposits (arrows) lined up along the fibronectin fibers. (f) Myofibroblast stained for β1 integrin (red) with f-hyaluronan co-aligned along stress fibers. Nuclei were stained with DAPI. Bar equals 50 μm.
The exogenous fluorescein-hyaluronan also co-aligned with the β1 integrins, which are the main receptors used for fibronectin organization (Fig. 2f). This indicates that the integrins could participate indirectly in remodeling hyaluronan through its interaction with fibronectin. We used an RGD peptide to disrupt interactions of integrins with fibronectin to examine the effect on hyaluronan uptake in myofibroblasts. Although disruption of β1 integrin interactions reduced the overall binding of fluorescein-hyaluronan to the fibers in the ECM (likely due to less fibronectin fiber formation), the RGD peptide did not have a significant effect on the uptake of fluorescein-hyaluronan to the intracellular compartment, compared to cells treated with RGE control peptide, as measured morphometrically or by flow cytometry (Supplemental Figure 1).
CD44 and hyaluronan associate with the cortical actin network
Myofibroblasts generate contractile force primarily through the association of stress fibers with integrins and their connection to ECM fibers through focal adhesions [1], while previous studies have suggested that hyaluronan mediates cell detachment through steric exclusion [10, 18]. This suggests that one role of hyaluronan may be to counterbalance integrin/focal adhesion-mediated force generation with the degree of overall adhesion. We used immunocytochemistry to determine whether hyaluronan and CD44 were positioned to take part in the focal adhesion complexes of myofibroblasts. Hyaluronan and CD44 were distributed essentially over the entire cell surface in both control (not shown) and TGF-β1-treated cells (Fig. 3a). Long cables of hyaluronan also extended many microns into the pericellular space in TGF-β1-treated cells, while the cables were seldom seen in control cells (not shown). Unexpectedly for a ligand and its receptor, there was very little yellow overlap between hyaluronan and CD44, and this tended to occur only where several strands of hyaluronan were concentrated along a ruffling cell edge or in the perinuclear area. This was true in both control cells (not shown) and myofibroblasts. The general lack of a yellow signal for hyaluronan and CD44 could be due to the extension of most of the hyaluronan well away from the focal plane of the cell surface.
Figure 3.
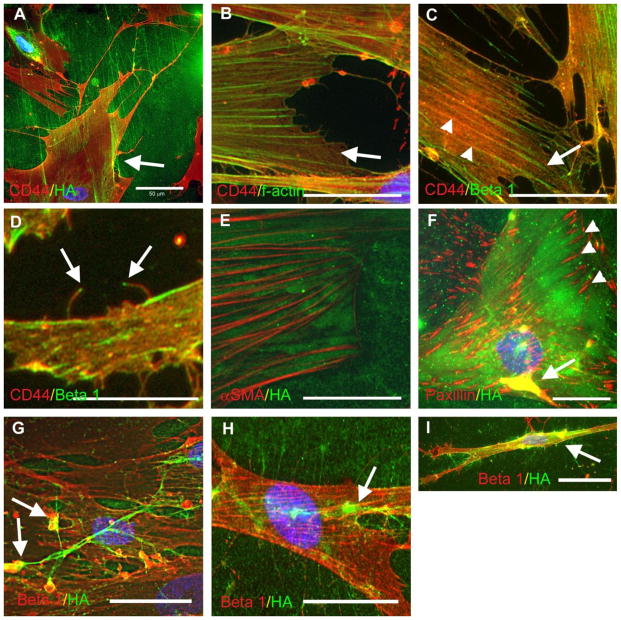
Hyaluronan and CD44 are not localized to focal adhesions of myofibroblasts. TGF-β1-induced myofibroblasts were double stained with various combinations of antibodies as indicated. (a) CD44 (red) and hyaluronan (green). Arrow indicates small region of membrane ruffling where a yellow colocalization signal is visible. (b) CD44 (red) and f-actin (green). Arrow indicates where CD44 mostly localizes with the cortical actin newtork between stress fibers. (c and d) CD44 (red) and β1 integrin (green). Arrow in (c) points to the termini of the stress fibers where β1 integrins cluster and CD44 is excluded. The arrowheads point to where they co-localize along the proximal part of the stress fiber. The arrows in (d) point to the integrin, often seen at the tips of some of the fine filopdia/microvilli. (e) This image depicts subcellular hyaluronan (green) between αSMA-positive stress fibers (red). (f) Myofibroblasts stained for paxillin (red) and hyaluronan (green). The focal adhesions are indicated with arrowheads. Colocalization of paxillin and hyaluronan on the upper cell surface is indicated with an arrow. Note the exclusion of hyaluronan from the focal adhesions. (g, h, and i) β1 integrin (red) and hyaluronan (green). The arrows in (g) and (h) indicate where β1 integrin co-localizes with remodeling hyaluronan cables. (i) Cells with a migratory morphology showed strong colocalization of hyaluronan and β1 integrin along the flanks of the cell (arrow), where focal adhesion turnover is high. Nuclei were stained with DAPI. Bar equals approximately 50 μm.
Co-staining for CD44 and f-actin showed that some of the CD44 co-aligned with stress fibers, while the majority of CD44 was associated with the cortical actin network between stress fibers (Fig. 3b). This pattern was also similar between control cells (data not shown) and TGF-β1-induced myofibroblasts (Fig. 3b). The cortical actin network gives rise to lamellipodia and filopodia, suggesting that adhesion via CD44 and hyaluronan might be a precursor to the formation of these structures in fibroblasts and myofibroblasts.
When the distributions of CD44 and β1 integrin were compared, strong yellow staining indicated they co-localized and were closely juxtaposed within the membrane. The two receptors occasionally co-localized along the proximal parts of stress fibers in both control (not shown) and TGF-β1-induced myofibroblasts (Fig. 3c, arrowheads). However, CD44 staining was generally less aligned with stress fibers, whereas the linear staining for β1 integrins was more obviously associated with stress fibers. Also, the β1 integrins tended to cluster at the termini of the stress fibers, i.e., focal adhesions, while CD44 was distinctly segregated from the stress fiber termini (Fig. 3c, arrow). This location suggests that CD44 is probably not involved in major contractile force generation which occurs through focal adhesions.
Earlier studies showed that hyaluronan pericellular matrix forms around the microspikes or filopodia of spreading cells, and others have shown that hyaluronan synthesis via overexpression of hyaluronan synthase can drive the formation of microvillous protrusions in various cell types, including fibroblasts [19, 20]. The microspikes and filopodial protrusions in HLFs and TGF-β1-induced myofibroblasts were positive both for CD44 and β1 integrin, as well as f-actin (data not shown, see [21]). The integrin staining was often concentrated at the very tip of the finest protrusions (Fig. 3d). However, the significance of this localization is not clear.
In co-stained myofibroblasts, hyaluronan (specifically the hyaluronan that was underneath the cells) was located mostly between αSMA-positive stress fibers (Fig. 3e). Co-staining of myofibroblasts for paxillin and hyaluronan showed that, like CD44, the hyaluronan under the cell was also excluded from focal adhesions (Fig. 3f, arrowheads), consistent with a previous report in embryonic fibroblasts [18]. On the upper surface of the cells, staining for paxillin and hyaluronan co-localized in areas of membrane ruffling and matrix remodeling (Fig. 3f, arrow).
Hyaluronan and β1 integrins tended to partially co-localize, primarily where hyaluronan cables were being reorganized on the upper cell surface, and along the flanks of migratory cells, where focal adhesion turnover is high (Fig. 3g, h, and i). Hyaluronan was also distributed between cells along membrane protrusions that connected two cells laterally. This pattern was similar between control cells (data not shown) and TGF-β1-induced myofibroblasts, and indicates that integrins may participate in remodeling hyaluronan cables.
The lack of strong association with stress fibers suggests that CD44 and hyaluronan are not effectively positioned to participate in the major contractile force generation that occurs through focal adhesions in myofibroblasts, but instead may modulate the local viscoelastic properties, overall adhesion, or relative access of integrins to fibronectin fibers.
Hyaluronan supports adhesion of the membrane between stress fibers
We previously showed that a brief treatment with Streptomyces hyaluronidase dramatically reversed the spread morphology in myofibroblastic synoviocytes that had been induced by the viral mimic, poly I:C [21]. To determine the role of hyaluronan in lung myofibroblast adhesion in more detail, hyaluronidase was used to remove it from the pericellular matrix. As shown in Fig. 4a, a brief 30-min treatment of both control lung fibroblasts and TGF-β1-induced myofibroblasts with hyaluronidase caused partial retraction of the cell margins and cytoplasm and revealed significantly more area of the plastic culture surface. As a result of the retraction, CD44 on the cell surface was pulled into more concentrated deposits. However, the cells remained adherent via the focal adhesions and large stress fibers and did not detach completely (data not shown). This indicates that hyaluronan is adhesive in this context, but supports only a small portion of myofibroblast adhesion.
Figure 4.
Hyaluronan mediates partial adhesion of myofibroblasts and stabilizes some fibronectin. (a) Quantitation of the bare plastic area revealed by 30-min treatment with Streptomyces hyaluronidase as a measure of cell retraction, compared to untreated cells. Images on the right show representative images of myofibroblasts without or with hyaluronidase. Cells were stained for CD44, which labels the entire cell surface. n = 20 fields per condition. (b) Quantitation of fibronectin staining in the matrix in control cells and TGF-β1-treated myofibroblasts without and with 30-min treatment with hyaluronidase. n = 20 fields per condition. * P < 0.05 compared to TGF-β1 alone. (c) Time-lapse microscopy of myofibroblasts after addition of hyaluronidase. Following induction with TGF-β1 for 4 days, sequential images were taken before (0 min.) and after digestion with Streptomyces hyaluronidase. In the upper panels (a–c), note how the membrane between stress fibers (arrow) was selectively retracted by 15 and 30 min. The cell in the lower right corner retracted more fully. In the lower panels (d–f), matrix disruption caused membrane and cytoplasmic components (arrows) to retract toward the nucleus, and the nucleus itself rolled out of the focal plane.
We next wanted to determine whether the removal of hyaluronan from the matrix would affect the amount of fibronectin in the matrix. The brief 30-min hyaluronidase treatment associated with partial cell retraction also partially reduced the amount of fibronectin staining in the ECM of both control cells and myofibroblasts (Fig. 4b). The effect was more pronounced in control cells (~ 40 % reduction) than in the myofibroblasts (~ 20 % reduction), suggesting either greater stability of the formed fibronectin in myofibroblasts, or a smaller proportion actually associated with hyaluronan. This also suggests that hyaluronan-fibronectin interactions may be important for part of the adhesion of these cells.
The dynamics of the immediate effects of hyaluronidase digestion on cell morphology and adhesion was monitored by time-lapse microscopy (Fig. 4c). These results showed that the focal contacts themselves tended to remain attached, and it was primarily the membrane and cytoplasm between the focal adhesions/stress fibers that preferentially retracted when the matrix was degraded (Fig. 4c, upper panels). The corresponding videos are presented in Supplemental Materials.
These sequences also clearly indicated that the rapid loss of hyaluronan matrix integrity was accompanied by pronounced centripetal flow of membrane, together with cytoplasmic material, toward the nucleus, as well as nuclear displacements and subtle nuclear shape changes without complete cell detachment (Fig. 4c, lower panels). Collectively, these observations suggest that hyaluronan-dependent matrix and CD44 contribute positively to fibroblast adhesion through the cortical actin network, rather than through focal adhesions, and these interactions are important for maintaining cell volume and cell-cell contact. The rapid partial retraction of cells also suggests that degradation of the hyaluronan releases tension held by the matrix, causing membrane flow and reorganization of CD44 and the cytoskeleton, further suggesting that matrix integrity potentially influences mechanosensing/signaling capabilities.
Hyaluronan disruption promotes fibronectin and collagen fiber deposition
Given the close juxtapositioning of fibronectin, hyaluronan and their receptors, we next wanted to examine the effect of disruption of hyaluronan matrix integrity using hyaluronan oligosaccharides or hyaluronidase digestion on the deposition of fibronectin over a longer 4-day induction period. We also examined the effect of inhibition of hyaluronan synthesis with 4-methyl umbelliferone (4-MU) over the 4-day period.
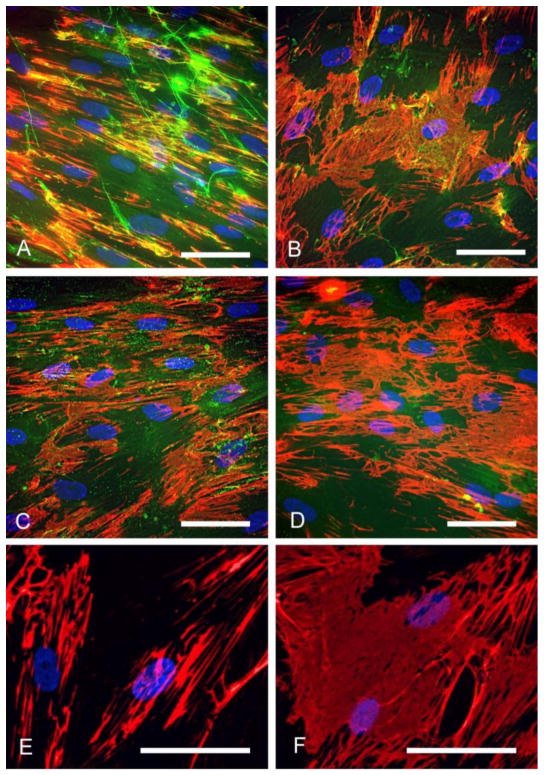
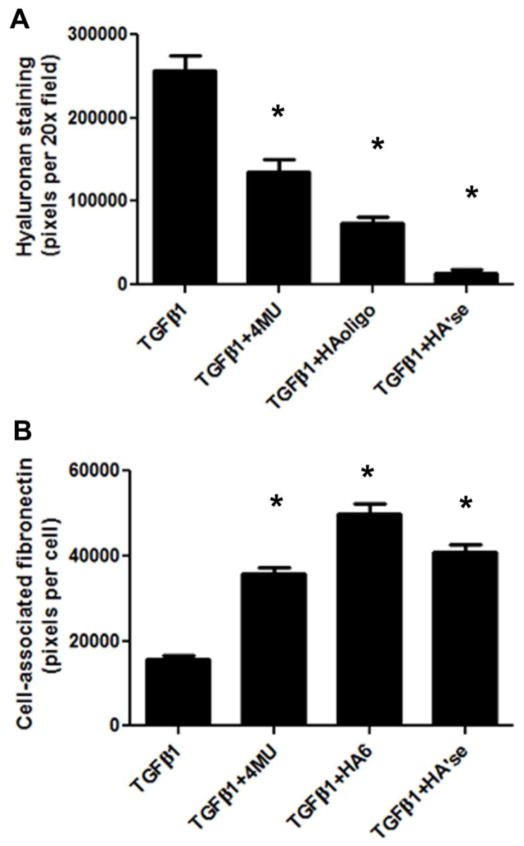
All three treatments diminished the amount of hyaluronan in the cell layer of the induced myofibroblasts (Fig. 5 and Fig. 6a). The quality of the hyaluronan matrix was also notably affected by each of these treatments. TGF-β1 alone stimulated the production of hyaluronan in the form of cable structures (Fig. 5a). Inhibition of hyaluronan synthesis with 4-MU abolished most of the cables (Fig. 5b), and there was a 50% reduction in deposited hyaluronan (Fig. 6a). Hyaluronan oligosaccharides completely abolished the cables (Fig. 5c), and caused about a 70 % reduction in hyaluronan staining. The remaining pericellular hyaluronan was mostly punctate in form. Hyaluronidase was the most effective at removing hyaluronan from the matrix (Fig. 5d), with only about 5% or less of the staining intensity remaining after 4 days, compared to controls (Fig. 6a).
Figure 5.
Hyaluronan matrix disruption over a longer time frame promoted fibronectin deposition. (a) TGF-β1 treated myofibroblasts were left untreated, or (b) treated with 4-MU, (c) hyaluronan oligosaccarides, or (d), Streptomyces hyaluronidase for 4 days, and then stained for hyaluronan (green) and fibronectin (red). (e) Control myofibroblasts and (f) 4-MU-treated myofibroblasts, stained for fibronectin only (red) and viewed at higher magnification. Note how the fibronectin in the cells treated with 4-MU is organized into a dense mat. Nuclei were stained blue with DAPI. Bar equals 50 μm.
Figure 6.
Hyaluronan matrix disruption promoted fibronectin deposition. Quantitiation of (a) hyaluronan and (b) fibronectin staining in the matrix of myofibroblasts left untreated, or induced in the presence of 4-MU, HA oligo, or hyaluronidase. n = 20 fields per condition. * P < 0.05 compared to TGF-β1 alone. Data are representative of three independent experiments.
All three hyaluronan-disrupting treatments enhanced the deposition of fibronectin by the myofibroblasts after 4 days of treatment compared to TGF-β1 alone (Fig. 5, 6b). In contrast, no effect of 4-MU or oligosaccharides on fibronectin formation was seen in control cells, nor in cells freshly plated and treated with the reagents for 24 h in 0.1% serum (data not shown). Notably, in the myofibroblasts induced in the presence of either 4-MU, hyaluronan oligosaccharides, or hyaluronidase, the fibronectin was frequently in the form of a densely woven mat underneath the cells. A example of the fibronectin mats from 4-MU treated cells is shown at higher magnification in Fig. 5f. This was not apparent in cells treated with TGF-β1 alone, where larger fibers dominated (Fig. 5e).
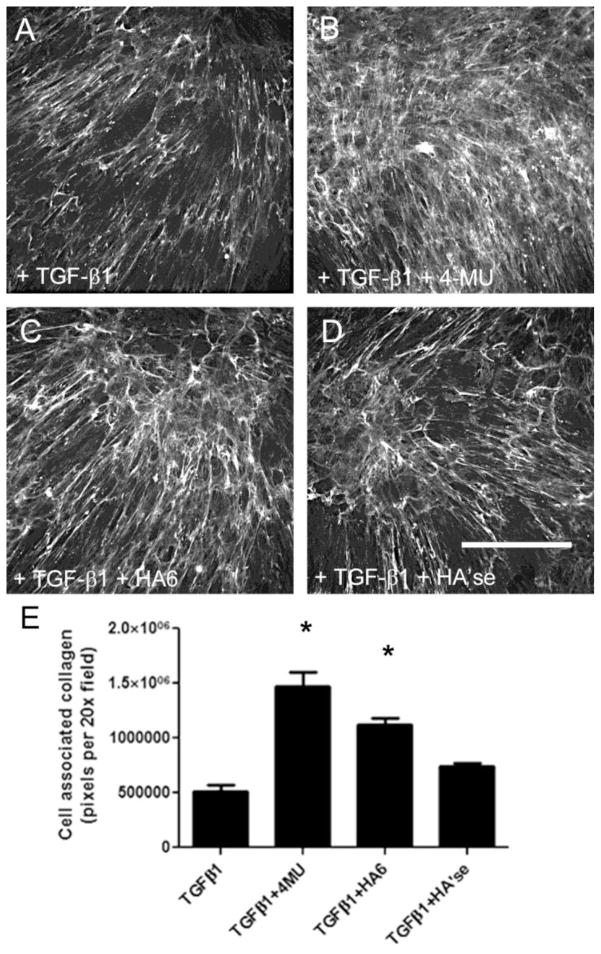
Collagen I deposition in the ECM was also significantly increased in myofibroblasts induced in the presence of 4-MU and hyaluronan oligosaccharides compared to untreated myofibroblasts. Collagen staining was also elevated, but not significantly, with hyaluronidase treatment (Fig. 7). Very little collagen I was detected in control HLFs (data not shown).
Figure 7.
Hyaluronan matrix disruption promoted collagen I deposition. Collagen I staining in (a) untreated myofibroblasts; (b) 4-MU treated cells; (c) cells treated with hyaluronan oligosaccharides; or (d) hyaluronidase during the 4-day induction period. (e) Quantitiation of collagen staining in the matrix. Bar equals 200 μm. Data are from a single experiment with n = 20 fields per condition. * P < 0.05 compared to TGF-β1 alone.
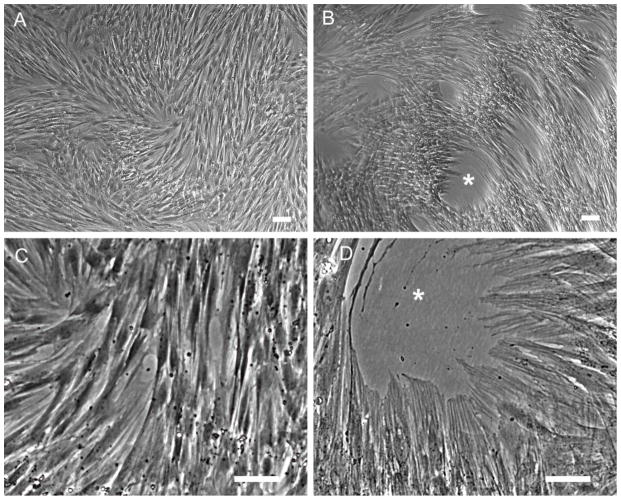
The morphological changes induced by longer term inhibition of hyaluronan synthesis were dramatic. By phase contrast microscopy, compared to cells treated with TGF-β1 alone (Fig. 8a and c), the myofibroblasts induced in the presence of 4-MU for 4 days (Fig. 8b and d) organized themeselves into denser aggregations and pulled holes into the monolayer. They also had extremely pronounced stress fibers and looked more contractile. This morphology was also apparent, but less pronounced, in myofibroblasts treated with the oligosaccharides or hyaluronidase (not shown).
Figure 8.
Myofibroblast morphology is enhanced by 4-MU treatment. Phase contrast images of myofibroblasts induced with TGF-β1 alone for 4 days (a and c), or induced in the presence of 4-MU (b and d). Note the holes pulled into the monolayer and the extremely pronounced stress fibers in cells induced in the presence of 4-MU. These are the same cells used for the RNA analysis in shown Fig. 9b. Bar equals 50 μm.
Alpha actin, fibronectin and collagen I expression is increased by longer term disruption of hyaluronan
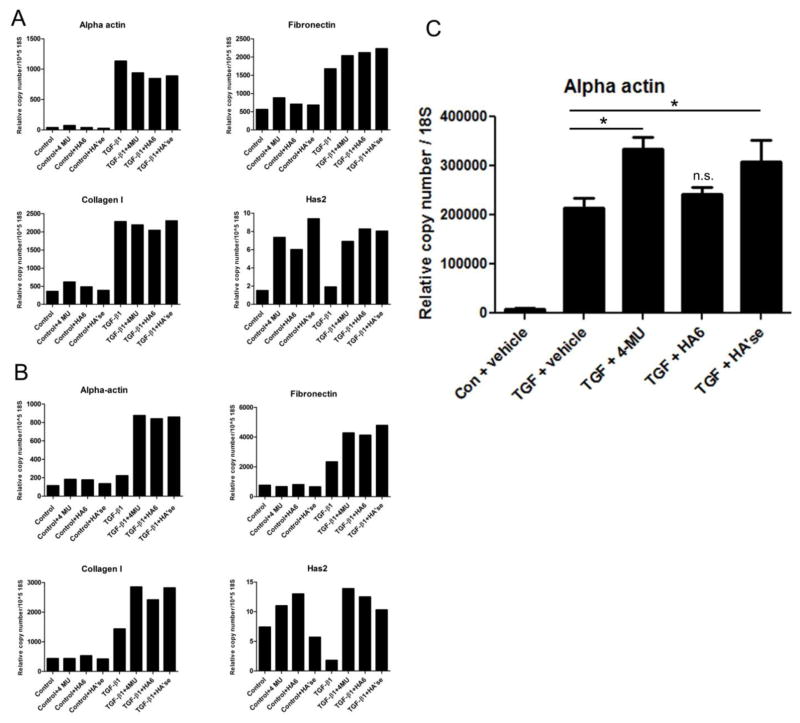
At 24 h after induction by TGF-β1, expression of αSMA was increased several fold over untreated controls (Fig. 9a). In myofibroblasts induced in the presence of 4-MU, hyaluronan oligosaccharides, or Streptomyces hyaluronidase, however, the expression of αSMA was slightly diminished compared to cells treated with TGF-β1 alone. Fibronectin expression was also increased by TGF-β1 and slightly increased by the hyaluronan disrupting treatments, while type I collagen expression was increased by TGF-β1, but unaffected by further hyaluronan disruption. Hyaluronan synthase 2 (HAS2) expression was increased by 4-MU and the other hyaluronan disrupting treatments in both control fibroblasts and TGF-β1-induced myofibroblasts.
Figure 9.
Expression of mRNA in cells treated with hyaluronan disrupting agents. qRTPCR was performed on RNA extracted from cells treated for 24 h (a), or 96 h (b) and expression levels of αSMA (α actin), fibronectin, HAS2, and collagen I were determined. DMSO vehicle (0.1%), 4-MU (400 μM in 0.1% DMSO vehicle), hyaluronan oligosaccharides (50 μg/ml, no DMSO), Streptomyces hyaluronidase (4 U/ml, no DMSO), were added immediately after the TGF-β1, and cells were incubated with TGF-β1 and the reagents together for 24 h or 4 days. Data are the average of duplicates and expressed as relative copy number per 18S RNA. (c) qRTPCR analysis of αSMA in second isolate of HLF at 4 days following treatment. TGF-β1-treated cells all were significantly elevated over vehicle control. Samples were analyzed in triplicate. *P < 0.05 compared to TGF-β1 alone by one way ANOVA with Tukey’s multiple comparison post-test.
At 4 days following induction however, expression of αSMA in myofibroblasts treated with 4-MU, hyaluronan oligosaccharides, or hyaluronidase was considerably elevated over cells treated with TGF-β1 alone (Fig. 9b). Fibronectin and type I collagen expression was also elevated over cells treated with TGF-β1 alone. HAS2 expression was still elevated in both control fibroblasts and TGF-β1-induced myofibroblasts by the matrix-disrupting treatments after 4 days, except for hyaluronidase treatment of the control cells. Repeating in a second isolate of HLF (Fig. 9c), αSMA expression was significantly elevated in cells treated with 4-MU or hyaluronidase over TGF-β1 alone, whereas hyaluronan oligosaccharides did not have a significant effect in this experiment. These data suggest that removal of hyaluronan over the longer term can promote the myofibroblast phenotype, at least in some cases.
Discussion
We have shown here that inhibition of hyaluronan synthesis or disruption of the pericellular matrix during myofibroblast induction by TGF-β1 increased the deposition of fibronectin and collagen and promoted myofibroblast morphology and expression of αSMA and HAS2 mRNA. This highlights the importance of the ECM in controlling myofibroblast formation. The results also show for the first time that hyaluronan and fibronectin interact in the pericellular matrix of myofibroblasts, and are closely interwoven during matrix assembly. The overall colocalization of CD44 and β1 integrins, but local segregation of CD44 from focal adhesions, suggest a mechanism for fine-tuning adhesion and potentially integrating mechanochemical signaling. The present findings also suggest an apparent reciprocal relationship between the amount of hyaluronan in the matrix and the ability of myofibroblasts to deposit fibrillar matrix components.
When 4-MU and the other reagents were applied during a 4-day induction with TGF-β1, there was further increase in αSMA expression, as well as EDA fibronectin and collagen deposition and clear morphological changes that suggest even more developed myofibroblasts. The effect on fibronectin deposition was consistent between two different primary isolates of HLFs. Abundant hyaluronan has been associated with scarless wound healing in fetal and young tissues [22]. In simple terms, the data presented here would seem to fit with this paradigm, in that a lack of hyaluronan further promoted the myofibroblast phenotype and ECM fiber deposition.
Hyaluronan is known to bind to cellular fibronectin, but the significance of this is not known [14, 15]. Although the heparin binding domain of fibronectin has been identified [13], it is not clear from the literature which domain(s) bind hyaluronan. One early study showed that hyaluronan binding can occur in cryptic domains within the fibronectin molecule [23]. We show here that exogenous fluorescein-labeled hyaluronan bound in a regular array along fibronectin fibers in the matrix, and tended to co-localize more with β1 integrins, rather than CD44. In addition, a brief treatment with hyaluronidase removed a higher proportion of the formed fibronectin from control cells compared to myofibroblasts. This suggests that the fibronectin of myofibroblasts was more stable and less susceptible to loss when the hyaluronan matrix was removed. An RGD peptide known to interfere with fibronectin fibrillogenesis [13], diminished binding of hyaluronan to the fibrillar matrix, but did not affect hyaluronan internalization.
Hyaluronan oligosaccharides disrupt most hyaluronan binding interactions, and hyaluronidase completely destroys pericellular matrix integrity. These reagents also affected αSMA expression, as well as HAS2, fibronectin, and collagen mRNA expression, over a 4-day time frame. This suggests that either the amount or structural integrity of the hyaluronan matrix can influence myofibroblast differentiation and the ability of the cells to organize fibrillar matrix components. Interestingly, 4-MU was the least effective of the treatments at reducing the amount of hyaluronan in the matrix, yet caused the most pronounced morphological changes. This suggest there may be effects of 4-MU other than inhibition of hyaluronan synthesis that influence myofibroblasts, such as the promotion of decorin production [24]. In addition, others have shown that 4-MU inhibited expression of HAS2 and HAS3 in cancer cells [25], whereas the present study showed an increase in HAS2 expression by all the hyaluronan disrupting treatments. An earlier study of corneal epithelial cells showed that loss of hyaluronan by hyaluronidase treatment led to the deposition of less fibronectin [26], highlighting the complexity of these interactions among different cell types.
Mechanotransduction via the β1 integrins depends on pericellular fibronectin [27]. Hyaluronan bound to fibronectin could potentially directly block or sterically inhibit integrin binding, or conversely, their receptors could act cooperatively. The β1 integrins did co-localize with attachment sites of reorganizing hyaluronan cables on the upper surface of cells, suggesting that hyaluronan and fibronectin are remodeled together. Fibronectin is extremely extensible, and undergoes force-induced structural unfolding to reveal cryptic binding sites for various ligands [28]. This suggests that stretching of fibronectin, either by cells or external forces, could potentially reveal more hyaluronan binding sites within the cryptic domains [23, 28], and may be one way by which the cellular response to mechanical stress is modulated or that cells use to fine-tune their adhesion. We found that exogenous fluorescein-labeled hyaluronan binds regularly along nascent and mature fibronectin fibrils, which is consistent with this idea. In vitro, hyaluronan-coated surfaces tend to be anti-adhesive, unless they are coated with fibronectin or crosslinked [29, 30] or cells or substrate are first treated with hyaluronidase to remove hyaluronan from one surface [7]. This suggests that fibronectin coated with hyaluronan would be less adhesive. Hyaluronan is known to mediate mechanosensing of shear force in endothelial cells [31], and probably plays some similar mechanotransductive role in fibroblasts. The present data are consistent with hyaluronan being part of a proposed clutch mechanism [21] for adhesion, force generation, or potentially, modulating and integrating mechanical signals. Recent studies showing that hyaluronan alters the integrin-dependent stiffness response of cells in vitro supports this idea [30, 32].
In the present study, in addition to fibronectin, collagen deposition was also increased in myofibroblasts when hyaluronan synthesis was inhibited or the matrix disrupted. Hyaluronan can regulate spacing of collagen fibrils in vivo [12] and has variable effects on collagen gel contraction in vitro [8, 9]. Thus the ratio of fibrillar to non fibrillar matrix components may play a role in driving cell phenotype or contractility, possibly by altering ECM swelling pressure, stiffness or viscosity.
Our results differ from previous studies which implicated hyaluronan in modulating TGF-β1 signaling, and promoting differentiation and maintenance of the myofibroblast phenotype in HLFs [5, 6]. In those studies, αSMA expression was reduced when 4-MU was applied to induced lung myofibroblasts for 3 days. We saw a slight decrease in αSMA expression after 24 h, but there was clear increase in αSMA expression after 4 days with 4-MU. The reason for this discrepancy is not clear. The presence of serum may be a factor, as we used a low concentration of 0.1% during induction, whereas in the studies by Webber, et al., there was no serum present [5, 6]. There could also be variability among human donors and their fibroblasts. For example, fibroblast clones from patients with fibrosis produced highly variable amounts of hyaluronan and were not significantly different from non-fibrotic cells [33]. In other studies, diminished hyaluronan was implicated in the anti-proliferative response of the oral (non-scarring, low hyaluronan producing) fibroblasts, whereas dermal fibroblasts, which made more hyaluronan, proliferated in response to TGF-β1 [34]. Thus, distinct populations of fibroblasts can have different responses to TGF-β1 and this can be associated with differences in hyaluronan generation, turnover, or matrix integrity. We found that the increase in fibronectin deposition after hyaluronan disruption was consistent among the two isolates of HLF that were tested. Regardless, it suggests that the role of hyaluronan in myofibroblasts is complex and requires more study.
CD44 and β1 integrin receptors generally co-distributed in the cell membrane and along some stress fibers. However, CD44 and hyaluronan were distinctly segregated from focal adhesions, whereas β1 integrins were concentrated along stress fibers and focal adhesions. This distribution in myofibroblasts is consistent with previous observations in embryonic avian fibroblasts [18]. That study suggested that the thick hyaluronan matrix, with bound chondroitin sulfate proteoglycans and other hyaladherins, sterically prevents the membrane from contacting the culture surface.
Our data suggests that CD44 and pericellular hyaluronan are associated primarily with the cortical actin network, although some co-alignment with β1 integrins along stress fibers is seen. This localization, combined with observations that cells do not adhere when plated on purified hyaluronan, suggests that hyaluronan and bound proteoglycans may promote de-adhesion, or by their swelling properties, sterically block receptors in the cell membrane from attaching to other matrix components. However, time-lapse experiments showing cells retract partially after hyaluronidase treatment clearly indicated that the intact hyaluronan matrix also mediates partial adhesion of the membrane and the associated cortical actin cytoskeleton between stress fibers and focal contacts. The material properties of fibronectin and hyaluronan are probably very different. We speculate that the relative degree of adhesion through fibronectin, integrins, and stress fibers versus adhesion through hyaluronan, CD44, and cortical actin affects the overall tensile state of the cells, the ability to further mechanically activate TGF-β1, and ultimately, the overall integration of mechanical signals. The observation that fibronectin was deposited as a dense mat under the cells after treatment with 4-MU, and (albeit to a lesser extent) the other reagents, suggests these treatments result in either closer proximity of the membrane to the culture surface, or more membrane surface area, or fibronectin surface area available for the β1 integrins to bind and organize the fibronectin, or a combination of the above.
A recent study showed that brief digestion with hyaluronidase caused a dramatic temporary reversal in the spread morphology of myofibroblastic synoviocytes induced by the viral mimic, poly I:C, suggesting that hyaluronan plays a direct role in myofibroblast adhesion [35]. Microvillous protrusions appear to function as hyaluronan synthesizing organelles, and help to scaffold the pericellular matrix [21, 36]. These protrusions, if unattached, will also retract upon digestion of the pericellular matrix with hyaluronidase [36]. The rapid retraction of cellular membrane and cytoplasmic structures induced by brief hyaluronidase digestion also strongly suggests that there is some level of tensional pre-stress in the hyaluronan-dependent matrix that involves CD44 anchorage to cytoskeletal elements [37, 38] that partially regulates cytoskeletal organization and organelle distribution. Interestingly, one earlier study showed that local application of high molecular weight hyaluronan to an inactive cell edge promoted lamellipodia outgrowth from the cortical actin [39]. This may be related to why increased hyaluronan synthesis promotes the formation and maintenance of microvillous protrusions [36, 40].
With less swelling pressure from the hyaluronan matrix after the disruptive treatments, there is less steric hindrance, enabling β1 integrins on the cells to engage fibronectin to pull it into a mat of fibers and develop extensive focal adhesions. This may depend on the degree to which hyaluronan coats the fibronectin. If the cells have a stiffer mat of fibrillar matrix under them, they can conceivably develop more tension, which, by itself, is known to upregulate αSMA expression and promote further TGF-β1 activation [1]. The increased fibronectin availability or increased tension may then signal to upregulate αSMA expression. Along with redistribution of CD44, local fragmentation of the hyaluronan in the pericellular matrix can cause the cortical actin or unanchored filopodia to retract, and thus directly alter cytoskeletal dynamics. This potentially affects the ability of a cell to form new lamellipodia or filopodia, or perhaps, facilitates redistribution of cortical actin to the stress fibers and focal adhesions.
In conclusion, hyaluronan plays a complex role in myofibroblast differentiation, adhesion, and matrix assembly. Hyaluronan matrix antagonizes the deposition of fibrillar ECM components, and, in our hands, its loss further promoted the myofibroblastic phenotype. Hyaluronan is ideally situated to modulate adhesion to and remodeling of fibronectin, but is not positioned to participate in major contractile force generation. Further critical examination of the interplay between hyaluronan and fibronectin and their receptors may provide useful strategies for preventing or reducing fibrosis.
Materials and Methods
Antibodies and reagents
Monoclonal antibody to human αSMA (clone 1A4, cat # MO851) was used at a 1:200 dilution and was from Dako North America, Inc. (Carpinteria, CA). Monoclonal antibody to cellular fibronectin (EDA-fibronectin) was used at 1:2000 and was from Sigma-Aldrich (St. Louis, MO) (clone FN-3E2, cat # F6140). This antibody does not recognize plasma fibronectin. Antibody to β1 integrin subunit was from Millipore and was used at 1:500 (Billerica, MA) (cat # MAB1951Z). Monoclonal antibody to paxillin was from BD Transduction Laboratories, used at 1:500 (San Jose, CA) (cat # 610620). The rat monoclonal antibody to CD44 (Hermes I, used at 1:500) was kindly provided by Charles Underhill, Department of Biochemistry and Molecular and Cellular Biology, Georgetown University. Antibody to type I collagen was from Abcam, used at 1:500 (Cambridge, MA) (cat # 34710). Fluorescein-avidin, AF-488 phalloidin, and AF-488 or AF-546-labeled secondary antibodies were from Molecular Probes, Life Technologies, (Grand Island, NY). Streptomyces hyaluronidase, (4-MU), other chemicals and reagents were from Sigma. A biotinylated hyaluronan binding protein preparation (b-HABP) from cartilage was prepared as described (Underhill et al. 1993).
Fluorescein-hyaluronan was prepared using grade I high molecular weight hyaluronan (Sigma-Aldrich) as described previously [41]. Briefly, hyaluronan (10 mg, Grade I; Sigma-Aldrich) was dissolved in 8 ml dH2O and added to 4 ml DMSO. A mixture of acetaldehyde (5 μl)/cyclohexyl isocyanide (5 μl)/fluoresceinamine (5 mg; all from Sigma-Aldrich) in 0.3 ml DMSO was added to the hyaluronan and the mixture was incubated at room temperature with stirring for 5 h. This was added to 160 ml cold 100% ethanol and precipitation was performed with ~2 ml saturated NaCl. Precipitates were centrifuged at 3000 rpm for 2 min, pooled in 10 ml dH2O, and added to 100 ml cold 100% ethanol. Centrifugation was repeated, and precipitates were resuspended in 5 ml dH2O and dialyzed exhaustively against PBS, 0.2% sodium azide. The final preparation had an average molecular weight of approximately 500 kD and contained approximately 0.8 mg fluorescein/mg of uronate. The labeled hyaluronan was susceptible to Streptomyces hyaluronidase digestion, indicating that it was biologically capable of interacting with a known binding partner (data not shown).
Cell culture
HLFs were derived from explants of the lung, following removal of both the pleura and parenchyma, and were a generous gift from Professor Ganesh Raghu, Division of Pulmonary and Critical Care Medicine, University of Washington, Seattle. The cells were isolated as described previously in accordance with approval from the institution’s human subjects review committee (Raghu et al. 1988). HLFs were maintained in DMEM high-glucose medium supplemented with 10% fetal bovine serum (FBS; HyClone; Logan, UT)), 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 0.43 mg/ml GlutMAX-1, and penicillin-streptomycin(penicillin G sodium, 100 U/ml, and streptomycin sulfate, 0.10 mg/ml; Invitrogen Life Technologies, Carlsbad, CA) at 37 °C in 5% CO2. Cells were passaged with trypsin-EDTA (0.05% trypsin and 0.53 mM tetrasodium EDTA) and were used for experiments between passages 9 and 17. A second isolate of adult HLFs was also used and was purchased from Lonza (Allendale, NJ) and used between passages 2 and 3. HLFs were incubated in medium containing 0.1% FBS for 48 h prior to use unless otherwise indicated.
Myofibroblast induction and treatments
Cells were seeded on 22 mm glass coverslips (Corning) at 3.5 × 105/well in 6-well plates or in 60 mm tissue culture dishes at 6 × 105 cells per dish in 10% FBS in Dulbecco’s Modified Eagle Medium (DMEM). After 24 h, cells were growth arrested in 0.1% FBS/DMEM for 48 h, at which point the cells were about 80–90% confluent. Cells were stimulated with TGF-β1 (10 ng/ml). DMSO (dimethylsulfoxide) vehicle (0.1%), 4-MU (400 μM in 0.1% DMSO vehicle), hyaluronan oligosaccharides (50 μg/ml, no DMSO), Streptomyces hyaluronidase (4 U/ml, no DMSO), were added immediately after the TGF-β1, and cells were incubated with TGF-β1 and the reagents together for 24 h or 4 days. As controls, non-induced fibroblasts were treated similarly.
Preparation of hyaluronan oligosaccharides
Two hundred milligrams of high molecular weight hyaluronan (pharmaceutical grade, Genzyme, Cambridge, MA) was dissolved in 20 mL of 0.1 mol/L ammonium acetate, pH 6.0, and digested for 72 h with 50 U of Streptomyces hyaluronidase. The enzyme was inactivated by boiling for 20 min and the hyaluronan fragments were fractionated by ultrafiltration through Centricon microconcentrators (Millipore, Billerica, MA) with molecular weight cutoffs of 50 kDa, followed by 3 kDa. Hyaluronan fragments passing through the Centricon 3 are designated HA6. Analysis by chromatography on Biogel P4 (Bio-Rad, Hercules, CA) showed that the oligosaccharide fraction consisted primarily of hexasaccharides (~ 45%) and tetrasaccharides (~ 30%) with small amounts of octa-, deca-, and dodecasaccharides comprising the remainder (data not shown). Samples were lyophilized, resuspended in phosphate buffered saline (PBS), and filter-sterilized. Uronic acid concentration was measured by the orcinol method [42].
Quantitative rtPCR
All reagents were supplied by Life Technologies (Grand Island, NY) unless otherwise noted. Relative quantitation of HAS2 gene expression was performed using Taqman Gene Expression Assay Hs00193435_m1. Briefly, 25 ng cDNA was amplified in 1X Taqman Gene Expression Master Mix with 250 nM Taqman probe in a 20 μl reaction. ColI, Fn, and αSMA amplifications were performed using 25 ng cDNA in 1X SYBR Select Master Mix and 1 μM primers. Expression was normalized to eukaryotic 18S rRNA Endogenous Control part no.4333760. All reactions were run using the Standard program for 40 cycles on an ABI 7500 Fast Real-Time thermocycler. All samples were done in duplicate unless otherwise indicated. Copy number estimates were generated from a standard curve created by using a selected reference cDNA template and Taqman probe [43] Primers for ColI, Fn, and αSMA, designed with NCBI Primer-BLAST and synthesized by Sigma, are as follows: ColI F 5′acatgttcagctttgtggacc3′, ColI R 5′tgtacgcaggtgattggtgg3′; Fn F 5′aggcaacggccgaggagagt3′, Fn R 5′ggaggaggctgggggtgagg3′; αSMA F 5′ctacaatgagcttcgtgttgcc3′, αSMA R 5′acatacatggctgggacattga3′.
Immunocytochemistry
Cells on coverslips were fixed for 10 min at 22 °C by addition of 0.5 ml of 10% formalin to 2 ml of medium. Where appropriate (f-actin, αSMA staining), cells were also permeabilized in 0.5 % Triton X-100 in PBS for 10 min. Following rinsing in PBS, cells were stained for hyaluronan using b-HABP (4 μg/ml) followed by fluorescein-avidin (Molecular Probes, Life Technologies) in PBS containing 1% bovine serum albumin as previously described (Evanko and Wight 1999). Nuclei were stained during mounting with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml in Fluorogel mounting medium (Electron Microscopy Sciences, Hatfield, PA). As controls, cells were incubated with normal IgG or predigested with hyaluronan-specific Streptomyces hyaluronidase, which abolished staining with the b-HABP.
Cells were examined using a Leica DMIRB microscope under epifluroescence optics using a 63 × 0.70 numerical aperture objective, and images were acquired using a Spot™-cooled CCD camera and imaging program. Better resolution of structural details within the pericellular coats and the matrix deposited on the substrate was afforded by linear adjustment of brightness and contrast levels of the highlights and mid-tonal values of the entire original image using Adobe Photoshop Elements 2.0. Identical adjustments were made for each image when separate treatments were compared. Images were sharpened slightly in Photoshop using unsharp mask at 75%, with a radius of 5 pixels, and threshold equal to zero. Images presented are representative of at least three independent experiments.
Hyaluronan binding and uptake
For hyaluronan binding studies, cells were incubated with fluorescein-labeled hyaluronan, at 2.5 μg/ml for 30 min at 37 °C, prior to fixation and co-staining for fibronectin, CD44, or β1 integrin. As a control, cells were incubated with excess unlabeled hyaluronan, which abolished the binding (data not shown). For measuring uptake of hyaluronan, cells were incubated with fluorescein-hyaluronan during the first 24 h after TGF-β1 induction in the presence of 100 μg/ml RGD peptide, known to inhibit fibronectin fiber formation. RGE peptide was used as a control. Cells were trypsinized and intracellular hyaluronan measured by flow cytometry. As an alternate method, trypsinized cells were re-plated for 2 h, photographed and the amount of intracellular hyaluronan quantitated from thresholded images as described below for fibronectin and collagen.
Fibronectin and collagen deposition
The amount of deposited fibronectin and collagen I fibers was quantitated from micrographs after immunostaining of fixed cells. Images were acquired from 20 randomly selected fields per condition and the staining intensity was quantitated from thresholded images and expressed as positive pixels per field. In some cases where the cell density among treatments was variable, the number of DAPI-stained nuclei was counted for each field and data were expressed as pixels per cell. All images were acquired and analyzed identically.
Time-lapse microscopy
Cells induced for 4 days with TGF-β1 were examined immediately after treatment with Streptomyces hyaluronidase using a Leica DMIRB microscope under phase contrast optics on a stage maintained at 37 °C in DMEM buffered with 50 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). Sequential images were acquired every 10 seconds using a Spot™ cooled CCD camera and imaging program. The myofibroblasts were filmed for 2–5 min prior to addition of hyaluronidase and followed for an additional 25–60 minutes. Representative images from the sequences are presented in Results. Corresponding videos are also provided in Supplemental Material.
Statistical analysis
Data were analyzed by one way ANOVA with a Tukey’s multiple comparison test using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
Supplementary Material
Highlights.
We examined the role of hyaluronan during TGF-β1-induction of lung myofibroblasts.
Hyaluronan interacts with fibronectin in the ECM of induced myofibroblasts.
Inhibiting HA synthesis/disrupting matrix promoted fibronectin, collagen deposition.
HA disruption also enhanced the myofibroblast phenotype and α-SMA expression.
HA-CD44-actin adhesion balances fibronectin-β1 integrin-mediated focal adhesion.
Acknowledgments
We would like to thank Dr. Ganesh Raghu, Division of Pulmonary and Critical Care Medicine, University of Washington, Seattle, for providing human lung fibroblasts, and Dr. Charles Underhill, Department of Biochemistry and Molecular and Cellular Biology, Georgetown University for providing the CD44 antibody. The authors would also like to thank Dr. Virginia M. Green for careful reading and editing of the manuscript. This work is supported by National Institutes of Health grants HL098067, AI101984, EB012558 to T.N.W.
Abbreviations used
- TGF-β1
transforming growth factor-beta 1
- αSMA
alpha smooth muscle actin
- ECM
extracellular matrix
- EDA
extra domain A
- HLFs
human lung fibroblasts
- 4-MU
4-methyl umbelliferone
- HAS2
hyaluronan synthase 2
- b-HABP
biotinylated hyaluronan binding protein preparation
- FBS
fetal bovine serum
- DMEM
Dulbecco’s Modified Eagle Medium
- DMSO
dimethylsulfoxide
- PBS
phosphate buffered saline
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–6. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 2.Follonier L, Schaub S, Meister JJ, Hinz B. Myofibroblast communication is controlled by intercellular mechanical coupling. J Cell Sci. 2008;121:3305–16. doi: 10.1242/jcs.024521. [DOI] [PubMed] [Google Scholar]
- 3.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins RH, Thomas GJ, Williams JD, Steadman R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J Biol Chem. 2004;279:41453–60. doi: 10.1074/jbc.M401678200. [DOI] [PubMed] [Google Scholar]
- 5.Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175:148–60. doi: 10.2353/ajpath.2009.080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284:9083–92. doi: 10.1074/jbc.M806989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman E, Geiger B, Addadi L. Initial stages of cell-matrix adhesion can be mediated and modulated by cell-surface hyaluronan. Biophys J. 2002;82:1848–57. doi: 10.1016/S0006-3495(02)75535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis JA, Hughes MG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: Role of CD44 and implications for constrictive remodeling. Circ Res. 2001;88:77–83. doi: 10.1161/01.res.88.1.77. [DOI] [PubMed] [Google Scholar]
- 9.Huang-Lee LL, Nimni ME. Fibroblast contraction of collagen matrices with and without covalently bound hyaluronan. J Biomater Sci Polym Ed. 1993;5:99–109. doi: 10.1163/156856294x00680. [DOI] [PubMed] [Google Scholar]
- 10.Barnhart BJ, Cox SH, Kraemer PM. Detachment Variants of chinese hamster cells. Hyaluronic acid as a modulator of cell detachment. Exp Cell Res. 1979;119:327–32. doi: 10.1016/0014-4827(79)90360-4. [DOI] [PubMed] [Google Scholar]
- 11.Brecht M, Mayer U, Schlosser E, Prehm P. Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem J. 1986;239:445–50. doi: 10.1042/bj2390445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman PJ. Evidence for a role of hyaluronan in the spacing of fibrils within collagen bundles in rabbit synovium. Biochim Biophys Acta. 2002;1571:173–82. doi: 10.1016/s0304-4165(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 13.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–99. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Laterra J, Culp LA. Differences in hyaluronate binding to plasma and cell surface fibronectins. Requirement for aggregation. J Biol Chem. 1982;257:719–26. [PubMed] [Google Scholar]
- 15.Yamada KM, Kennedy DW, Kimata K, Pratt RM. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem. 1980;255:6055–63. [PubMed] [Google Scholar]
- 16.Bates RC, Elith CA, Thorne RF, Burns GF. Engagement of variant CD44 confers resistance to anti-integrin antibody-mediated apoptosis in a colon carcinoma cell line. Cell Adhes Commun. 1998;6:21–38. doi: 10.3109/15419069809069758. [DOI] [PubMed] [Google Scholar]
- 17.Verfaillie CM, Benis A, Iida J, McGlave PB, McCarthy JB. Adhesion of committed human hematopoietic progenitors to synthetic peptides from the C-terminal heparin-binding domain of fibronectin: cooperation between the integrin alpha 4 beta 1 and the CD44 adhesion receptor. Blood. 1994;84:1802–11. [PubMed] [Google Scholar]
- 18.Yamagata M, Saga S, Kato M, Bernfield M, Kimata K. Selective distributions of proteoglycans and their ligands in pericellular matrix of cultured fibroblasts. Implications for their roles in cell-substratum adhesion. J Cell Sci. 1993;106:55–65. doi: 10.1242/jcs.106.1.55. [DOI] [PubMed] [Google Scholar]
- 19.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan and versican rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–13. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 20.Kultti A, Rilla K, Tiihonen R, Spicer AP, Tammi RH, Tammi MI. Hyaluronan synthesis induces microvillus-like cell surface protrusions. J Biol Chem. 2006;281:15821–8. doi: 10.1074/jbc.M512840200. [DOI] [PubMed] [Google Scholar]
- 21.Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J Histochem Cytochem. 2009;57:1041–60. doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. 2009;48:137–61. doi: 10.1016/s0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- 23.Sekiguchi K, Hakomori S, Funahashi M, Matsumoto I, Seno N. Binding of fibronectin and its proteolytic fragments to glycosaminoglycans. Exposure of cryptic glycosaminoglycan-binding domains upon limited proteolysis. J Biol Chem. 1983;258:14359–65. [PubMed] [Google Scholar]
- 24.Funahashi M, Nakamura T, Kakizaki I, Mizunuma H, Endo M. Stimulation of small proteoglycan synthesis by the hyaluronan synthesis inhibitor 4-methylumbelliferone in human skin fibroblasts. Connect Tissue Res. 2009;50:194–202. doi: 10.1080/03008200802684615. [DOI] [PubMed] [Google Scholar]
- 25.Kultti A, Pasonen-Seppanen S, Jauhiainen M, Rilla KJ, Karna R, Pyoria E, et al. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–23. doi: 10.1016/j.yexcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Mattey DL, Garrod DR. Role of glycosaminoglycans and collagen in the development of a fibronectin-rich extracellular matrix in cultured embryonic corneal epithelial cells. J Cell Sci. 1984;67:189–202. doi: 10.1242/jcs.67.1.189. [DOI] [PubMed] [Google Scholar]
- 27.Lutz R, Sakai T, Chiquet M. Pericellular fibronectin is required for RhoA-dependent responses to cyclic strain in fibroblasts. J Cell Sci. 2010;123:1511–21. doi: 10.1242/jcs.060905. [DOI] [PubMed] [Google Scholar]
- 28.Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, et al. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A. 2009;106:18267–72. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kisiel M, Martino MM, Ventura M, Hubbell JA, Hilborn J, Ossipov DA. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials. 2013;34:704–12. doi: 10.1016/j.biomaterials.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Chopra A, Lin V, McCollough A, Atzet S, Prestwich GD, Wechsler AS, et al. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J Biomech. 2012;45:824–31. doi: 10.1016/j.jbiomech.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, et al. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003;285:H722–6. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 32.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2013;35:71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westergren-Thorsson G, Sime P, Jordana M, Gauldie J, Sarnstrand B, Malmstrom A. Lung fibroblast clones from normal and fibrotic subjects differ in hyaluronan and decorin production and rate of proliferation. Int J Biochem Cell Biol. 2004;36:1573–84. doi: 10.1016/j.biocel.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Meran S, Thomas DW, Stephens P, Enoch S, Martin J, Steadman R, et al. Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J Biol Chem. 2008;283:6530–45. doi: 10.1074/jbc.M704819200. [DOI] [PubMed] [Google Scholar]
- 35.Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rilla K, Tiihonen R, Kultti A, Tammi M, Tammi R. Pericellular hyaluronan coat visualized in live cells with a fluorescent probe is scaffolded by plasma membrane protrusions. J Histochem Cytochem. 2008;56:901–10. doi: 10.1369/jhc.2008.951665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underhill C, Toole B. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979;82:475–84. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacy BE, Underhill CB. The hyaluronate receptor is associated with actin filaments. J Cell Biol. 1987;105:1395–404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliferenko S, Kaverina I, Small JV, Huber LA. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J Cell Biol. 2000;148:1159–64. doi: 10.1083/jcb.148.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rilla K, Lammi MJ, Sironen R, Torronen K, Luukkonen M, Hascall VC, et al. Changed lamellipodial extension, adhesion plaques and migration in epidermal keratinocytes containing constitutively expressed sense and antisense hyaluronan synthase 2 (Has2) genes. J Cell Sci. 2002;115:3633–43. doi: 10.1242/jcs.00042. [DOI] [PubMed] [Google Scholar]
- 41.de Belder AN, Wik KO. Preparation and properties of fluorescein-labelled hyaluronate. Carb Res. 1975;44:251–7. doi: 10.1016/s0008-6215(00)84168-3. [DOI] [PubMed] [Google Scholar]
- 42.Brown AH. Determination of pentose in the presence of large quantities of glucose. Arch Bioch Biophys. 1946;11:269–78. [PubMed] [Google Scholar]
- 43.Shih SC, Smith LE. Quantitative multi-gene transcriptional profiling using real-time PCR with a master template. Exp Mol Pathol. 2005;79:14–22. doi: 10.1016/j.yexmp.2005.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.