Abstract
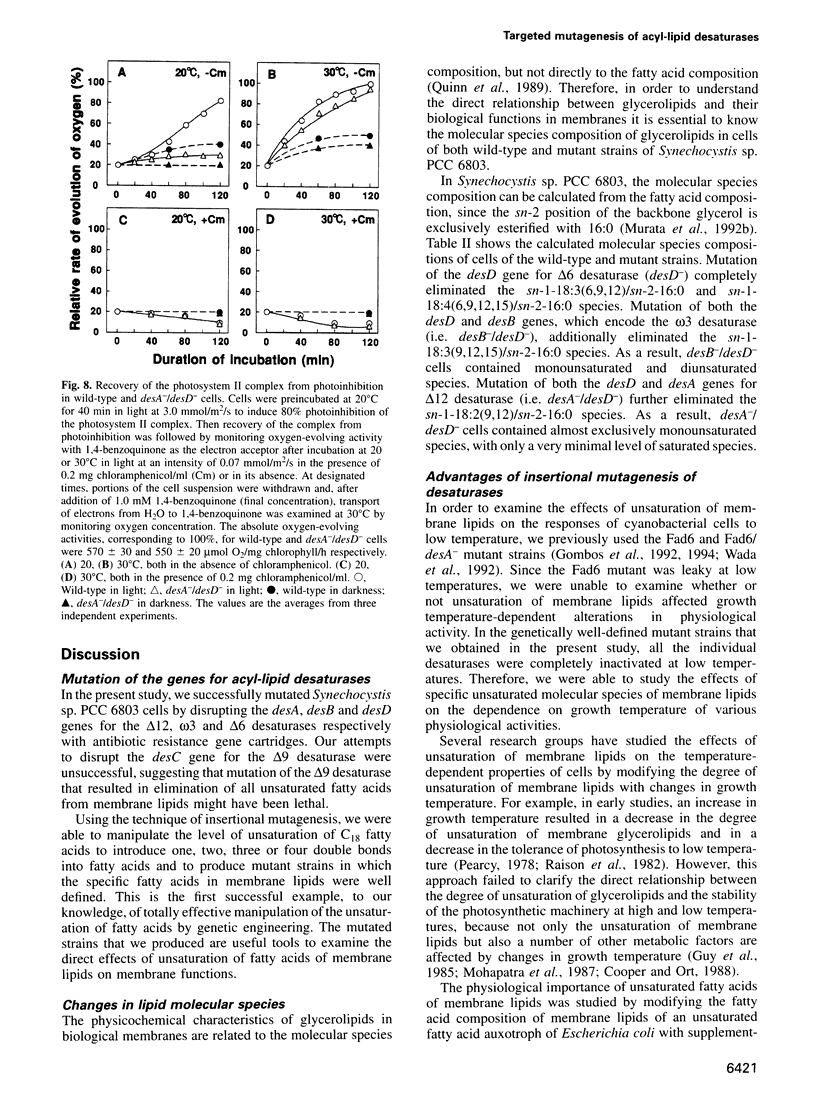
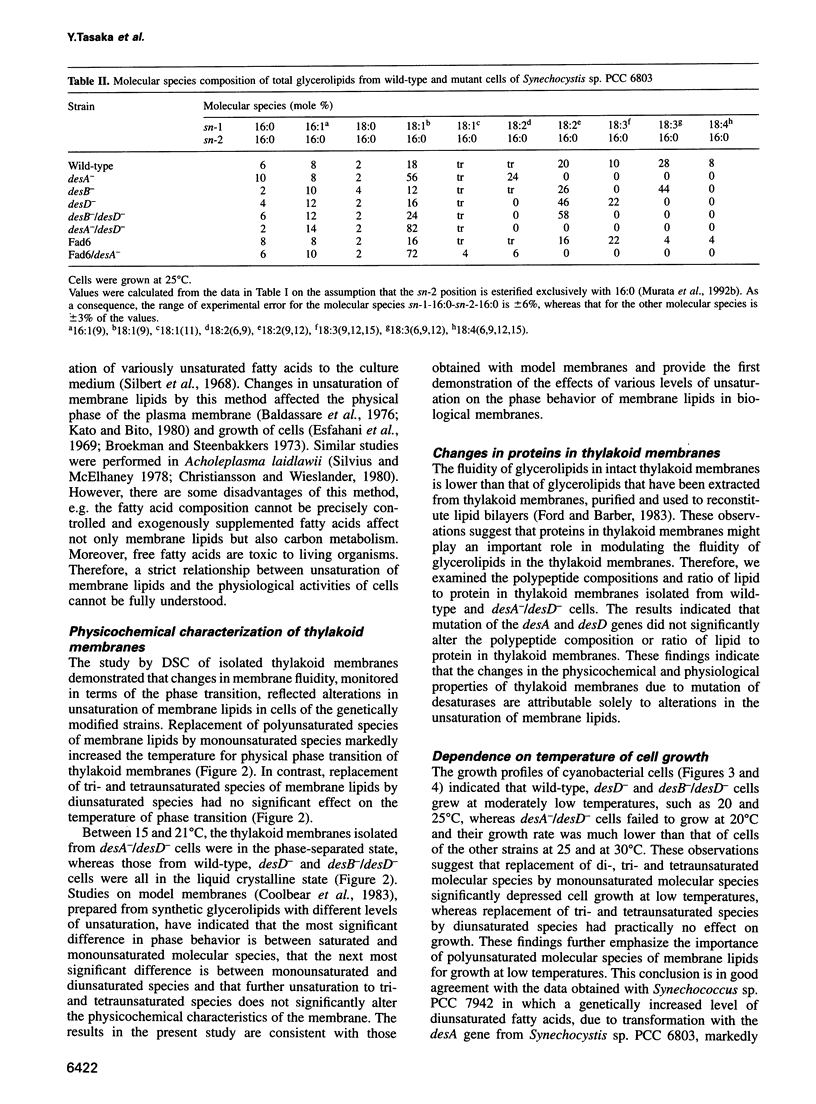
Acyl-lipid desaturases introduce double bonds (unsaturated bonds) at specifically defined positions in fatty acids that are esterified to the glycerol backbone of membrane glycerolipids. The desA, desB and desD genes of Synechocystis sp. PCC 6803 encode acyl-lipid desaturases that introduce double bonds at the delta12, omega3 and delta6 positions of C18 fatty acids respectively. The mutation of each of these genes by insertion of an antibiotic resistance gene cartridge completely eliminated the corresponding desaturation reaction. This system allowed us to manipulate the number of unsaturated bonds in membrane glycerolipids in this organism in a step-wise manner. Comparisons of the variously mutated cells revealed that the replacement of all polyunsaturated fatty acids by a monounsaturated fatty acid suppressed growth of the cells at low temperature and, moreover, it decreased the tolerance of the cells to photoinhibition of photosynthesis at low temperature by suppressing recovery of the photosystem II protein complex from photoinhibitory damage. However, the replacement of tri- and tetraunsaturated fatty acids by a diunsaturated fatty acid did not have such effects. These findings indicate that polyunsaturated fatty acids are important in protecting the photosynthetic machinery from photoinhibition at low temperatures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Aro E. M., Virgin I., Andersson B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993 Jul 5;1143(2):113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Rhinehart K. B., Silbert D. F. Modification of membrane lipid: physical properties in relation to fatty acid structure. Biochemistry. 1976 Jul 13;15(14):2986–2994. doi: 10.1021/bi00659a008. [DOI] [PubMed] [Google Scholar]
- Broekman J. H., Steenbakkers J. F. Growth in high osmotic medium of an unsaturated fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):285–289. doi: 10.1128/jb.116.1.285-289.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansson A., Wieslander A. Control of membrane polar lipid composition in Acholeplasma laidlawii a by the extent of saturated fatty acid synthesis. Biochim Biophys Acta. 1980 Jan 25;595(2):189–199. doi: 10.1016/0005-2736(80)90083-8. [DOI] [PubMed] [Google Scholar]
- Coolbear K. P., Berde C. B., Keough K. M. Gel to liquid-crystalline phase transitions of aqueous dispersions of polyunsaturated mixed-acid phosphatidylcholines. Biochemistry. 1983 Mar 15;22(6):1466–1473. doi: 10.1021/bi00275a022. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ort D. R. Changes in protein synthesis induced in tomato by chilling. Plant Physiol. 1988 Oct;88(2):454–461. doi: 10.1104/pp.88.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. F., Yu C. A. Preparation and reconstitution of a phospholipid deficient cytochrome b6-f complex from spinach chloroplasts. Biochem Biophys Res Commun. 1985 Sep 16;131(2):700–706. doi: 10.1016/0006-291x(85)91294-x. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Barnes E. M., Jr, Wakil S. J. Control of fatty acid composition in phospholipids of Escherichia coli: response to fatty acid supplements in a fatty acid auxotroph. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1057–1064. doi: 10.1073/pnas.64.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Gombos Z., Wada H., Murata N. The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: a mechanism of chilling tolerance. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8787–8791. doi: 10.1073/pnas.91.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos Z., Wada H., Murata N. Unsaturation of fatty acids in membrane lipids enhances tolerance of the cyanobacterium Synechocystis PCC6803 to low-temperature photoinhibition. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9959–9963. doi: 10.1073/pnas.89.20.9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C. L., Niemi K. J., Brambl R. Altered gene expression during cold acclimation of spinach. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3673–3677. doi: 10.1073/pnas.82.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Bito Y. Gel to liquid-crystalline phase transitions of lipids and membranes isolated from Escherichia coli cells. Microbiol Immunol. 1980;24(8):703–716. doi: 10.1111/j.1348-0421.1980.tb02871.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Los D., Horvath I., Vigh L., Murata N. The temperature-dependent expression of the desaturase gene desA in Synechocystis PCC6803. FEBS Lett. 1993 Feb 22;318(1):57–60. doi: 10.1016/0014-5793(93)81327-v. [DOI] [PubMed] [Google Scholar]
- Miller R. W., de la Roche I., Pomeroy M. K. Structural and functional responses of wheat mitochondrial membranes to growth at low temperatures. Plant Physiol. 1974 Mar;53(3):426–433. doi: 10.1104/pp.53.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Changes in Protein Patterns and Translatable Messenger RNA Populations during Cold Acclimation of Alfalfa. Plant Physiol. 1987 Aug;84(4):1172–1176. doi: 10.1104/pp.84.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Low-temperature effects on cyanobacterial membranes. J Bioenerg Biomembr. 1989 Feb;21(1):61–75. doi: 10.1007/BF00762212. [DOI] [PubMed] [Google Scholar]
- Murata N., Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J. 1995 May 15;308(Pt 1):1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy R. W. Effect of Growth Temperature on the Fatty Acid Composition of the Leaf Lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1978 Apr;61(4):484–486. doi: 10.1104/pp.61.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Joo F., Vigh L. The role of unsaturated lipids in membrane structure and stability. Prog Biophys Mol Biol. 1989;53(2):71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Reddy A. S., Nuccio M. L., Gross L. M., Thomas T. L. Isolation of a delta 6-desaturase gene from the cyanobacterium Synechocystis sp. strain PCC 6803 by gain-of-function expression in Anabaena sp. strain PCC 7120. Plant Mol Biol. 1993 May;22(2):293–300. doi: 10.1007/BF00014936. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Los D. A., Higashi S., Wada H., Nishida I., Ohmori M., Murata N. Cloning of omega 3 desaturase from cyanobacteria and its use in altering the degree of membrane-lipid unsaturation. Plant Mol Biol. 1994 Oct;26(1):249–263. doi: 10.1007/BF00039536. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Wada H., Nishida I., Ohmori M., Murata N. delta 9 Acyl-lipid desaturases of cyanobacteria. Molecular cloning and substrate specificities in terms of fatty acids, sn-positions, and polar head groups. J Biol Chem. 1994 Oct 14;269(41):25576–25580. [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Growth and membrane lipid properties of Acholeplasma laidlawii B lacking fatty acid heterogeneity. Nature. 1978 Apr 13;272(5654):645–647. doi: 10.1038/272645a0. [DOI] [PubMed] [Google Scholar]
- Vigh L., Los D. A., Horváth I., Murata N. The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H., Gombos Z., Murata N. Contribution of membrane lipids to the ability of the photosynthetic machinery to tolerate temperature stress. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4273–4277. doi: 10.1073/pnas.91.10.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H., Gombos Z., Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature. 1990 Sep 13;347(6289):200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]
- Wada H., Murata N. Temperature-Induced Changes in the Fatty Acid Composition of the Cyanobacterium, Synechocystis PCC6803. Plant Physiol. 1990 Apr;92(4):1062–1069. doi: 10.1104/pp.92.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Hof R., van Klompenburg W., Pilon M., Kozubek A., de Korte-Kool G., Demel R. A., Weisbeek P. J., de Kruijff B. The transit sequence mediates the specific interaction of the precursor of ferredoxin with chloroplast envelope membrane lipids. J Biol Chem. 1993 Feb 25;268(6):4037–4042. [PubMed] [Google Scholar]



