Abstract
Basal ganglia structures comprise a portion of the neural circuitry that is hypothesized to coordinate the selection and suppression of competing responses. Parkinson’s disease (PD) may produce a dysfunction in these structures that alters this capacity, making it difficult for patients with PD to suppress interference arising from the automatic activation of salient or overlearned responses. Empirical observations thus far have confirmed this assumption in some studies, but not in others, due presumably to considerable inter-individual variability among PD patients. In an attempt to help resolve this controversy, we measured the performance of 50 PD patients and 25 healthy controls on an arrow version of the Eriksen flanker task in which participants were required to select a response based on the direction of a target arrow that was flanked by arrows pointing in the same (congruent) or opposite (incongruent) direction. Consistent with previous findings, reaction time (RT) increased with incongruent flankers compared to congruent or neutral flankers, and this cost of incongruence was greater among PD patients. Two novel findings are reported. First, distributional analyses, guided by dual-process models of conflict effects and the activation-suppression hypothesis, revealed that PD patients are less efficient at suppressing the activation of conflicting responses, even when matched to healthy controls on RT in a neutral condition. Second, this reduced efficiency was apparent in half of the PD patients, whereas the remaining patients were as efficient as healthy controls. These findings suggest that although poor suppression of conflicting responses is an important feature of PD, it is not evident in all medicated patients.
Keywords: Parkinson’s disease, Basal ganglia, Interference control, Response inhibition, Flanker task, Action selection
Responding optimally in a visual environment often requires attentional navigation of relevant and irrelevant stimuli as well as the capacity to control responses that might be signaled by these stimuli. In some instances, a response to a visual stimulus is over-learned and activation related to this response may be triggered automatically by the presence of the stimulus, even if the stimulus is irrelevant to the task at hand. Depending on the circumstances, this automatic stimulus–response activation can be advantageous or disadvantageous to performance. For instance, when automatic response activation anticipates a preferred course of action, the speed and accuracy of selecting the preferred response is facilitated (Burle, van den Wildenberg, & Ridderinkhof, 2005). In contrast, conflict between an automatically activated response and a preferred response interferes with the speed and accuracy of selecting the preferred response (Botvinick, Braver, Barch, Carter, & Cohen, 2001). In the case of conflict, cognitive control is necessary to suppress the automatic response activation in order to minimize interference with the selection of the preferred action (Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004a; Ridderinkhof, van den Wildenberg, Wijnen, & Burle, 2004b). Anyone who has attempted to type a sentence quickly using a keyboard with an unfamiliar key arrangement can appreciate the amount of cognitive control required to suppress the automatic, overlearned keystrokes (e.g., typing with a Dvorak key arrangement after learning a Qwerty layout).
Frontal-basal ganglia circuits are hypothesized to play an important role in the executive control of action, including the capacity to suppress unwanted response tendencies (Aron & Poldrack, 2006; Aron, Robbins, & Poldrack, 2004; Band & van Boxtel, 1999; Frank, 2005; Mink & Thach, 1993; Mink, 1996; Ridderinkhof, van den Wildenberg, Segalowitz, et al., 2004; van den Wildenberg et al., 2006). Indeed, many influential models of basal ganglia function have proposed that direct, indirect, and, recently, hyper-direct pathways within the basal ganglia architecture implement the selection and suppression of competing response alternatives (Aron & Poldrack, 2006; Chevaler & Deniau, 1990; Groves, 1983; Hikosaka, 1998; Jackson & Houghton, 1995; Krauthamer, 1979; Kropotov & Etlinger, 1999; Middleton & Strick, 2000a; Middleton & Strick, 2000b; Oberg & Divac, 1979; Redgrave, Prescott, & Gurney, 1999; Robbins & Brown, 1990; Taylor & Saint-Cyr, 1995). This conceptualization implies that the complementary basal ganglia pathways may be uniquely adapted to implement interference control during action selection. That is, when two response alternatives are concurrently competing for the control of action, and the non-preferred response option happens to be the more overlearned or strongly signaled of the two responses, the basal ganglia may play a key role in suppressing this alternative and amplifying the selection of the preferred response (Bogacz, 2007). One test of this idea is to determine if individuals with known basal ganglia dysfunction have greater difficulty resolving response interference and, if so, whether the difficulty arises from stronger activation of competing responses, poorer suppression of competing responses, or some combination of these two processes.
Parkinson’s disease (PD) is a neurodegenerative condition that leads to progressive loss of dopamine-producing neurons in the substantia nigra compacta of the basal ganglia. The substantial dopamine loss due to PD dramatically alters information flow through the basal ganglia, producing well-known changes in motor function (e.g., bradykinesia, tremor, rigidity) as well as various deficits in so-called executive cognitive capacities (Cools, 2006). According to the interference control model of the basal ganglia described above, one possible source of difficulty for PD patients may be related to greater interference during action selection. For instance, PD patients may experience stronger activation of undesired response tendencies that are signaled by external stimuli or have greater difficulty suppressing unwanted response activations (Gauggel, Rieger, & Feghoff, 2004; Praamstra & Plat, 2001; Praamstra, Stegeman, Cools, & Horstink, 1998; Seiss & Praamstra, 2004). In other words, basal ganglia dysfunction produced by PD may create a response selection traffic jam that requires extra time and greater effort to resolve. In the current study, we investigate this possibility by measuring the effects of PD during response selection when an automatically activated response conflicts with a preferred course of action.
1. Interference control and the Eriksen flanker task
A well-established procedure for measuring interference control is the Eriksen flanker task (Eriksen & Eriksen, 1974). In the arrow version of the task, participants are asked to make speeded responses to the direction of a target arrow (e.g., left pointing arrow = left hand button press). Additional arrows, or flankers, are positioned along the horizontal and/or vertical plane that point either in the same or opposite direction as the target, thus signaling a manual response that is congruent or incongruent, respectively, with the response signaled by the target. Reaction time (RT) slows and error rates increase when target and flankers signal incongruent as opposed to congruent responses (i.e., there is a cost of incongruence or interference effect). The presence of incongruent flankers is associated with changes in the properties of the lateralized readiness potential (LRP) derived from the electroencephalogram. The LRP provides an “on line” comparison (i.e., subtraction) of activation recorded from scalp sites over both motor cortices. A shift in the balance of activation in favor of the response signaled by the incongruent flankers occurs prior to the appearance of a shift in LRP activation that favors the motor cortex controlling the response signaled by the target (e.g., Kopp, Rist, & Mattler, 1996; Mattler, 2003; Wascher, Reinhard, Wauschkuhn, & Verleger, 1999; Willemssen, Hoormann, Hohnsbein, & Falkenstein, 2004). This pattern is consistent with the view that the response signaled by the flankers is rapidly and automatically activated before the controlled response to the target is activated and selected. Suppression of the activation induced by incongruent flankers is a time-consuming process that slows overall RT, but ensures selection of the response that is signaled by the target. Based on these dynamics, the flanker interference task provides a powerful context for examining interference control during response selection, and individual differences in interference effects can be used to draw inferences about the efficiency of cognitive control processes engaged to resolve the interference, including the suppression of automatic response activation.
There are now six studies that have investigated the effects of PD on performance in the flanker task. In each study, it was predicted that dysfunction of the basal ganglia would make individuals with PD more vulnerable than healthy matched controls to the interference produced by incongruent flankers. Praamstra et al. (Praamstra et al., 1998; Praamstra, Plat, Meyer, & Horstink, 1999) were the first to demonstrate and replicate the finding that medication-withdrawn PD patients (n = 8,1998; n = 10, 1999) show larger interference effects than do healthy controls. Using the LRP as an index of differential motor system activation for the competing response hands, they also found that changes in the properties of the LRP supported the conclusion that the enhanced interference effects in PD are driven by a stronger, automatic activation of cortical motor areas that control the conflicting response. A few years later, we (Wylie, Stout, & Bashore, 2005) replicated the behavioral effect reported by Praamstra et al. in a sample of 16 medicated PD patients. In addition, we found that the greater interference induced by incongruent flankers for PD patients could be harnessed to benefit RT if the response activated by the incongruent flankers became the preferred response (i.e., when instructions required a response in the direction opposite to that indicated by the target arrow). In contrast to these studies, greater interference among PD patients was not supported in an early study of 10 medicated PD patients by Lee, Wild, Hollnagel, and Grafman (1999) or in recent studies of 15 medicated PD patients by Falkenstein, Willemssen, Hohnsbein, and Hielscher (2006) and of 20 medicated PD patients by Cagigas, Filoteo, Stricker, Rilling, and Friedrich (2007).
Based on these mixed reports, Falkenstein et al. (2006) considered the potential impact of several experimental variables, including clinical characteristics of sampled PD patients and differences in task design and procedures, but no clear and consistent factor could account for the discrepant findings. For example, across all studies, the severity of the disorder in PD patients was mild to moderate as measured by standard clinical rating systems, the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Hoehn and Yahr Scale (1967), and all of the patients studied were free of dementia. Even in studies that found exaggerated interference effects among PD participants, no relationship between disease severity and interference effects was found. Dopaminergic medication status at the time of testing, i.e., during a patient’s normal medication “on” state or in a practically defined “off” state following overnight medication withdrawal, appeared non-contributory as both medicated and medication-withdrawn patients showed larger interference effects in some studies, but normal effects in others.
Here we examine whether individual differences in the efficiency of crucial cognitive processes involved in performing the flanker task (i.e., individual differences in the sensitivity to incongruent response activation and/or in the proficiency of inhibiting this response activation) can account for interference effects in PD. We measured interference effects in a much larger sample of PD patients (n = 50) than has been previously investigated. Based on previous findings from Praamstra et al. and our own work, we predicted that individuals with PD would show larger interference effects compared to healthy controls. Next, we utilized a specific model and related analytic techniques to zoom in on cognitive processes associated with response activation and response inhibition (Ridderinkhof, 2002; Ridderinkhof, van den Wildenberg, Wijnen, et al., 2004). As a secondary goal, we aimed to determine if individual differences in key demographic variables, such as clinical symptom severity and age at disease onset, are related to these cognitive processes and may help explain why some individuals with PD show enhanced interference effects while others do not.
2. A theoretical account of flanker interference effects: dual processing and the activation-suppression hypothesis
To explain conflict effects in the flanker task, several investigators have appealed to a dual-processing model which posits that the target and flankers are processed in parallel along two processing routes, a deliberate and a direct route (e.g., Eimer, Hommel, & Prinz, 1995; Kornblum, Hasbroucq, & Osman, 1990; Ridderinkhof, van der Molen, & Bashore, 1995). The target, upon which a correct response is based, dominates processing along a slower, more controlled stimulus–response translation route (i.e., the deliberate route) so as to ensure correct response selection. The direct route involves a faster, automatic activation of responses associated with the target and flankers, with the degree of activation closely related to perceptual salience and the strength of the stimulus–response (S–R) association (Miller, 1991). Because the arrows in the flanks outnumber the target arrow (i.e., have greater perceptual salience), direct response activation is greater for the response signaled by the flankers. As noted above, this phenomenon has been supported by psychophysiological investigations that demonstrate differential activation over motor cortex contralateral to the response hand signaled by the incongruent flankers (i.e., LRP) that precedes a shift in motor cortex activation contralateral to the response hand signaled by the target (Coles, Gratton, Bashore, Eriksen, & Donchin, 1985; Eimer, 1998; Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988; Leuthold, 2003; Smid, Mulder, & Mulder, 1990; Spencer & Coles, 1999).
Thus, in an incongruent situation, the build-up of initial response activation along the direct route is afforded a head start over the response activated along the deliberate route. The convergence of the two routes at the level of response activation is assumed by the dual-process model to produce interference or conflict when the flankers activate an incongruent response. If left unchecked, according to this model, response activation along the direct route approaches the threshold for response execution. If the threshold is exceeded, the result is a response error. If activation along the direct route approaches but does not exceed the threshold, the build-up of activation produces greater conflict with the response signaled by the target and slows the latency of the correct response (i.e., produces a larger interference effect). According to the activation–suppression hypothesis, inhibitory processes are engaged to counter activation along the direct route, and the time course and strength of the inhibition process are critical for determining interference effects (Ridderinkhof, 2002). If the net effect of inhibition is weak, the interference produced along the direct route will be larger, either resulting in fast errors or slow correct responses. With strong net inhibition, activation along the direct route will be less interfering to response latency, particularly for the slowest RTs in the distribution.
The activation–suppression hypothesis was developed to capture these dynamics, i.e., incorrect response activation followed by suppression of this activation, by analyzing the pattern of interference effects as a function of the entire individual RT distribution (de Jong, Liang, & Lauber, 1994; Ridderinkhof, 2002; Ridderinkhof, van den Wildenberg, Wijnen, et al., 2004; Wiegand & Wascher, 2007). For fast reactions, rapid response activation along the direct route is less likely to be countered by the suppression process. This is because suppression takes time to build-up within a trial. Thus, the activation–suppression hypothesis predicts that most errors in the flanker task will occur before the development of effective suppression. In other words, errors are most likely to be fast errors. Moreover, the frequency of fast errors is expected to vary with the strength of the initial activation of the incongruent response. Group or individual differences in the strength of response activation can therefore be inferred by the pattern of error rates at the fastest segment of the RT distribution. Groups experiencing stronger initial activation of the incongruent response will produce more fast errors.
Because inhibition takes time to build-up after presentation of the stimulus array, its impact on interference effects manifests itself in the slower segments of the RT distribution. In the case of efficient inhibition, the increase of interference effects across earlier segments of the distribution is reduced, and in some instances, reversed at the slower segments of the distribution. In some studies of conflict effects, the suppression is effective enough to reverse interference effects completely such that RT for response incongruent trials is faster than RT for response congruent trials (Burle, Possamaï, Vidal, Bonnet, & Hasbroucq, 2002). Moreover, individual differences in the modulation of interference effects at the slowest segments of the RT distribution have recently been associated with the amount of activation in the right ventrolateral prefrontal cortex, an area that is linked to inhibitory processes during action selection in the Simon task (Forstmann et al., in press). That is, individuals who show greater reduction in interference effects at the slow end of the RT distribution demonstrate greater right ventrolateral prefrontal activation during conflict trials. In the flanker task, the reduction in interference effects is less dramatic than in other conflict tasks (e.g., Simon task), but nonetheless useful for distinguishing group differences in the efficiency of inhibitory control (Bub, Masson, & Lalonde, 2006; Ridderinkhof, Scheres, Oosterlaan, & Sergeant, 2005; Wylie, Ridderinkhof, Eckerle, & Manning, 2007). Specifically, inefficient suppression leads to steeper increases of interference effects at the slowest segments of the RT distribution.
In the present study, we examined whether distributional analyses, guided by the activation-suppression hypothesis, could provide new insights into the mechanisms responsible for exaggerated flanker interference effects in PD. Data were acquired from a much larger sample than in previous studies to capture the considerable inter-individual variability among PD patients. We made very specific predictions about the effects of PD on flanker interference effects and the distributional dynamics. First, in line with previous findings, we predicted that PD patients would show larger interference effects compared to healthy controls. If the source of greater interference results from stronger initial response activation, this would be observed behaviorally as a pattern of increased error rates at the fastest segments of the RT distribution. While some LRP studies suggest that PD patients may experience earlier and stronger activation of the incongruent response, no studies have found PD patients to make more errors under incongruent flanker trials compared to healthy controls. Thus, we tested whether PD patients show a pattern of increased fast errors compared to healthy controls. Greater flanker interference among PD patients could also be accounted for by less efficient response inhibition. Based on previous findings and the hypothesized role of basal ganglia in inhibitory control, our stronger prediction was that PD patients would show poorer suppression of interference. Specifically, we predicted that PD patients would show steeper increases or less reduction in interference effects at the slow end of the RT distribution compared to healthy controls.
3. Methods
3.1. Participants
Fifty individuals diagnosed with PD and 25 healthy controls (HC) similar in age and education (p values > 0.05) participated in this study. Table 1 shows group demographics. Notably, the groups did not differ on a measure of global cognitive status (mini-mental status exam (MMSE); p>0.05) or on ratings of depression (Center for Epidemiological Studies Depression Scale, CES-D; p >0.05). Participants with PD were recruited from the Movement Disorders Clinic at the University of Virginia and diagnosed with PD by a neurologist specializing in movement disorders. They voluntarily completed the study on the same day or within 2 weeks of their regularly scheduled Movement Disorders Clinic visit. Patients completing study participation on the same day as their clinic visit were screened for adverse clinical events or issues (e.g., drastic medication changes, fatigue, distress) that might have affected their task performance. All PD patients were rated a Hoehn and Yahr Stage III or less, with stage II the most common rating. All PD patients were taking medications to improve dopaminergic function and tested during the “on” state of their medication cycle. Healthy elderly controls were spouses or family members of PD patients as well as individuals recruited from the local community via advertisement. Exclusion criteria included the following: history of other neurological condition; untreated or unstable mood disorder; history of bipolar affective disorder, schizophrenia, or other psychiatric condition known to compromise executive cognitive functioning; untreated or unstable medical condition known to interfere with cognitive functioning (e.g., diabetes, pulmonary disease). All participants had corrected-to-normal vision. They all provided informed consent prior to participating in the study, which was fully compliant with standards of ethical conduct in human research as regulated by the University of Virginia human investigation committee.
Table 1.
Demographic and performance data for PD and HC groups
| HC | PD | |
|---|---|---|
| Sample size | 25 | 50 |
| Age (years) | 67.7 (9.5) | 64.1 (8.3) |
| Education (years) | 16.1 (2.3) | 15.2 (2.8) |
| Gender (M:F) | 9:16 | 34:16 |
| MMSE (raw score) | 29.4 (0.8) | 28.9(1.5) |
| Years since diagnosis | - | 6.6 (4.9) |
| Hoehn and Yahr rating | - | 2.1 (0.4) |
| Flanker task | ||
| Neutral (NT) | ||
| RT (ms) | 466 (64) | 526(92) |
| Accuracy (%) | 99.8 (0.5) | 99.3(1.4) |
| Congruent (CG) | ||
| RT (ms) | 475(70) | 524(91) |
| Accuracy (%) | 99.7 (0.4) | 99.4(1.0) |
| Incongruent (IG) | ||
| RT (ms) | 547(76) | 635(135) |
| Accuracy (%) | 98.1 (2.8) | 95.7(5.6) |
| Flanker effect (IG-CG) | ||
| RT (ms) | 72(23) | 111 (66) |
| Accuracy (%) | −1.6(2.7) | −3.7(5.3) |
Standard deviations shown in parentheses.
3.2. Tasks and procedures
The flanker task was designed and implemented using E-prime software (www.pstnet.com; Psychology Software Tools Inc.) and an IBM-compatible computer with a 17-in. digital display monitor. The computer screen, placed at a distance of 91 cm, was positioned so that stimuli appeared at eye level. Stimuli consisted of white arrows (pointing in the left or right direction) or white diamonds against a black background. Responses to stimuli were right or left thumb presses made on buttons located in a rectangular box placed comfortably on a table in front of the participant.
Each trial began with the presentation of a fixation point in the center of the computer screen. After 500 ms, the fixation point was extinguished and replaced by a stimulus array that remained on the screen until the participant made a response. Each array consisted of 5 stimuli spanning 22.5 cm (visual angle = 14°), including a target arrow located in the same center location as the fixation cross, and two distractor stimuli (i.e., flankers) located on each side of the target arrow (each arrow subtended a visual angle of 2.5°; width = 4.0 cm, height = 3.5 cm). The edge-to-edge separation between the target and nearest flankers was less than 1° visual angle. Participants were instructed to make a button press in the direction indicated by the target arrow (e.g., right pointing arrow = right button press; left pointing arrow = left button press). After a response, the stimulus array disappeared and the screen remained blank for 750 ms (i.e., the intertrial interval) until a fixation cross appeared and signaled the next trial.
Each trial was defined by one of three levels of flanker congruence (Fig. 1). In the Neutral condition, flankers consisted of diamond shapes that did not correspond to a particular response. For the Congruent condition, flankers consisted of arrows pointing in the same direction as the target arrow, thus signaling the same response as the target. For the Incongruent condition, flanker arrows pointed in the opposite direction of the target arrow and signaled the conflicting manual response. Each arrow array (neutral, congruent, incongruent) appeared randomly and with equal probability within a block of trials. Following a block of 30 practice trials, each participant completed three experimental blocks of 103 trials for a total of 309 experimental trials.
Fig. 1.
Examples of flanker arrays used in the present experiment.
Reaction times (RT) and accuracy rates (arcsine transformed) were the primary dependent variables of interest. Extreme RT values, due to anticipatory or excessively delayed responses, were removed from the analysis based on a conservative trim procedure (e.g., RT values >3 standard deviations above or below the mean) and after visual inspection of each trial within each level of flanker congruence to verify each value as a clear outlier. This resulted in the elimination of fewer than 2% of trials per subject per flanker condition. RT and accuracy data were submitted to separate overall mean analyses (repeated measures ANOVA with Huyhn-Feldt corrections for violations of sphericity) to determine the effects of Flanker Congruence (neutral, congruent, incongruent) and Group (PD, HC). Uncorrected degrees of freedom are reported for easy of interpretation. Planned comparisons of interference effects adjust for the one-sided hypothesis test that PD patients experience greater interference than healthy controls. The distributional analyses are described in detail below.
4. Results
4.1. Overall interference effects in PD: comparison to healthy controls
Consistent with the prevalence of PD, the ratios of males to females comprising the PD and HC samples were different, with a larger proportion of males included in the PD sample and the opposite pattern in the HC sample. We included gender as a between-subjects variable in the initial repeated measures ANOVA. There was no main effect of gender on RT, F(1, 71), = 0.39, p = 0.53), and neither the gender by flanker congruence interaction, F(2, 70), = 0.70, p = 0.43, the gender by group interaction, F(1, 71) = 0.81, p = 0.37, nor the gender by flanker congruence by group interaction, F(2, 70), = 0.18, p = 0.72, approached statistical significance. Therefore, in the following analyses, the data were collapsed across gender.
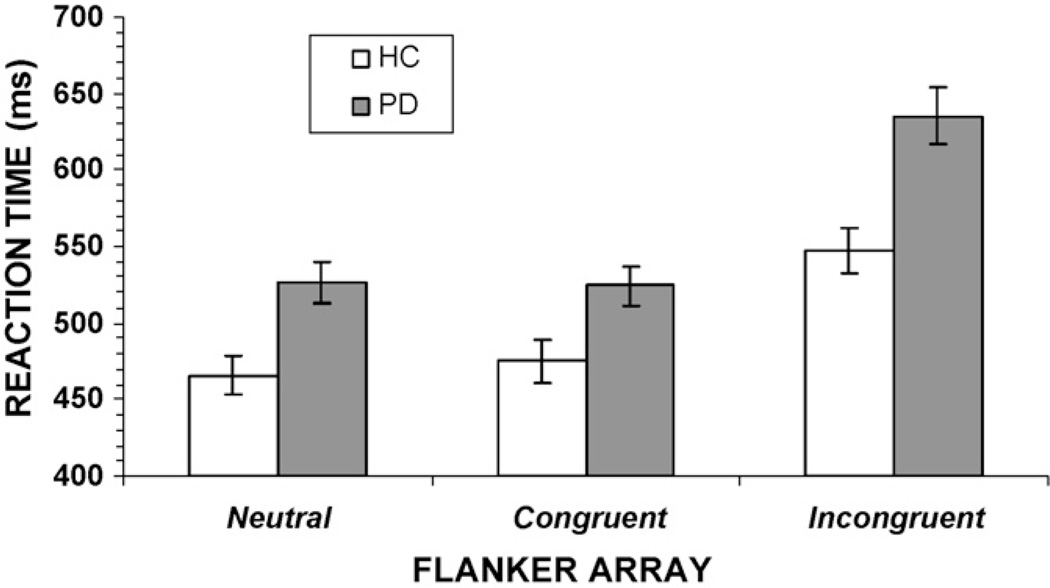
Overall analysis of mean RT showed a significant flanker congruence effect, [F(2, 146) = 182.31, p< 0.001]. For both groups, incongruent flankers produced a significant slowing of mean RT compared to neutral and congruent flankers (Fig. 2). There was also a significant effect of group [F(1, 73) = 8.29, p<0.01]; overall RT was slower among PD patients (562 ms) than among HC subjects (496 ms). The omnibus test revealed a significant group by flanker congruence interaction [F(2, 146) = 6.20, p = 0.01], with planned contrasts showing that the slowing induced by the incongruent flankers was greater among PD patients than among HC subjects [F(1, 73) = 7.95, p < 0.01]. Overall, the difference in RT between congruent and incongruent conditions (i.e., the interference effect) was 72 ms for HC subjects and 111ms for PD patients.
Fig. 2.
Overall mean reaction times (RT) for individuals with Parkinson’s disease (PD) and healthy controls (HC). Compared to the HC group, the PD group shows a significantly larger increase in RT when flankers are incongruent as opposed to congruent.
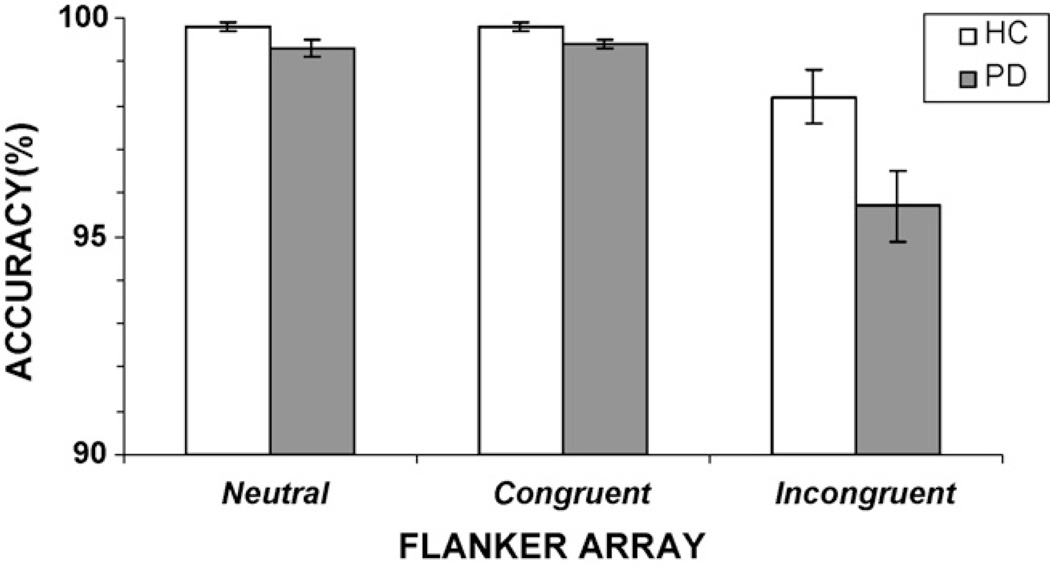
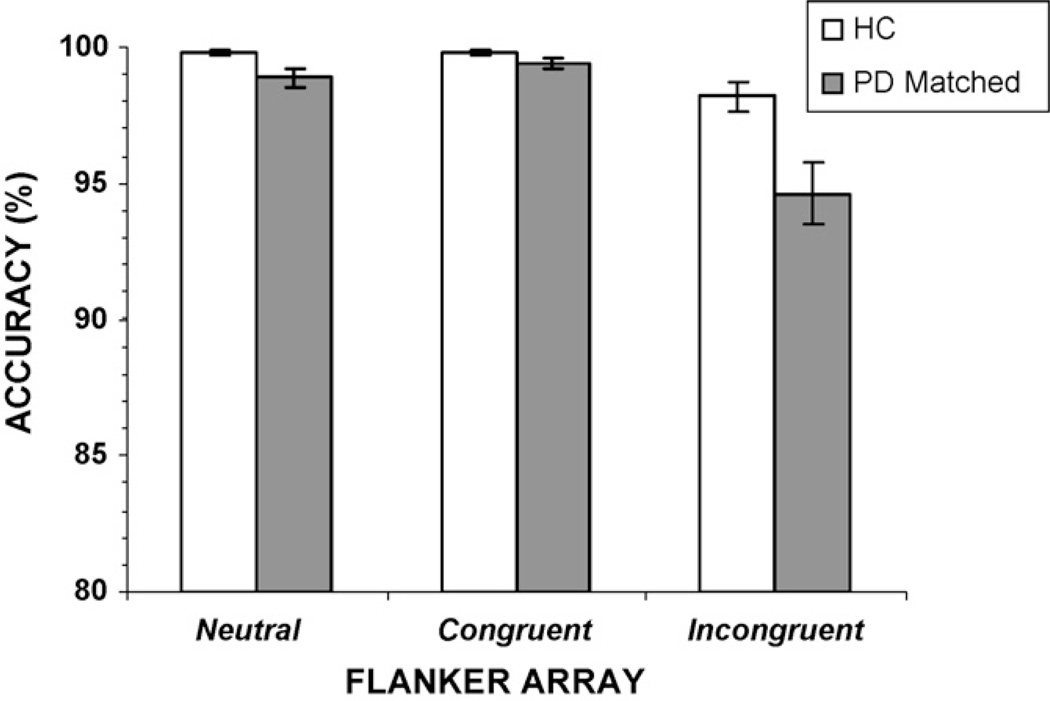
Overall, participants from both groups performed the flanker task with a high degree of accuracy (Fig. 3). A significant interference effect was evident in accuracy rates, [F(2, 146) = 47.49, p < 0.001]; the presence of incongruent flankers reduced accuracy in both groups compared to the presence of neutral and congruent flankers (Fig. 3). The groups differed in overall accuracy rates, F(1, 73) = 5.81, p <0.05, with PD patients making more errors than HC subjects. There was a trend for the effects of flanker congruence on accuracy rates to vary as a function of group, group x flanker congruence interaction, F(2, 146) = 2.72, p = 0.08 (Table 1). A planned contrast showed that PD patients made more errors than healthy controls to the presence of incongruent flankers, F(1, 73) = 5.67, p = 0.01. Notably, for PD patients, but not for HC, the magnitude of the interference effect on RT (calculated as the difference in RT between incongruent and congruent conditions) was negatively correlated with the interference effect on accuracy rates (r= −0.39, p <0.01), indicating that as interference effects increased among patients accuracy levels decreased when flankers were incongruent. This finding supports the idea that performance differences in the PD group were related importantly to the degree of interference induced by incongruent flankers.
Fig. 3.
Overall accuracy rates (%) for individuals with Parkinson’s disease (PD) and healthy controls (HC). Accuracy rates decline for the incongruent flanker array, and there is a trend for PD patients to make more errors in this condition compared to HC.
4.2. Distinguishing the effects of PD on response activation and response inhibition
Based on the activation–suppression hypothesis, a group that experiences stronger initial activation of the incorrect response would make more fast errors at the fastest segment of the RT distribution when flankers are incongruent. The slope between the first two bins of the conditional accuracy function is postulated to reflect the strength of response capture, with a steeper increase in the slope associated with stronger initial response activation. Because inhibition is engaged after the initial activation of a conflicting response and takes time to build-up, group differences in the efficiency of inhibition are argued to be most discernible at the slowest segments of the RT distribution. Thus, groups with less efficient inhibition are expected to show a larger slope increase in the interference effect across the RT distribution compared to groups with more efficient inhibition, who are expected to show greater reduction of these effects. To examine these patterns, we applied distributional analyses (de Jong et al., 1994; Ridderinkhof, 2002; Ridderinkhof, van den Wildenberg, Wijnen, et al., 2004). Because we had specific predictions that PD would produce larger slopes compared to healthy controls, contrast analyses use a p-value adjustment for a one-tailed distribution.
4.2.1. Response activation
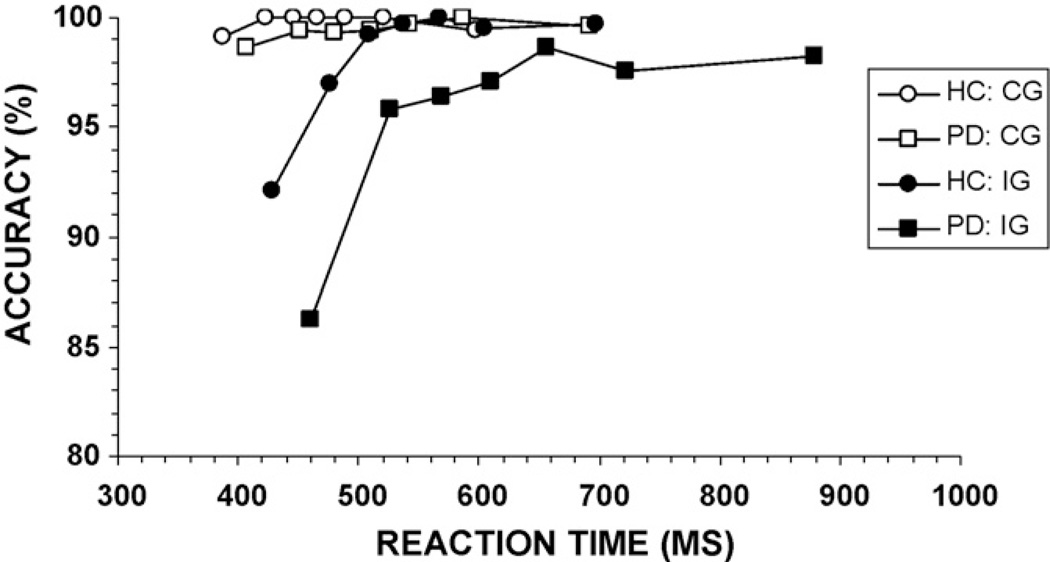
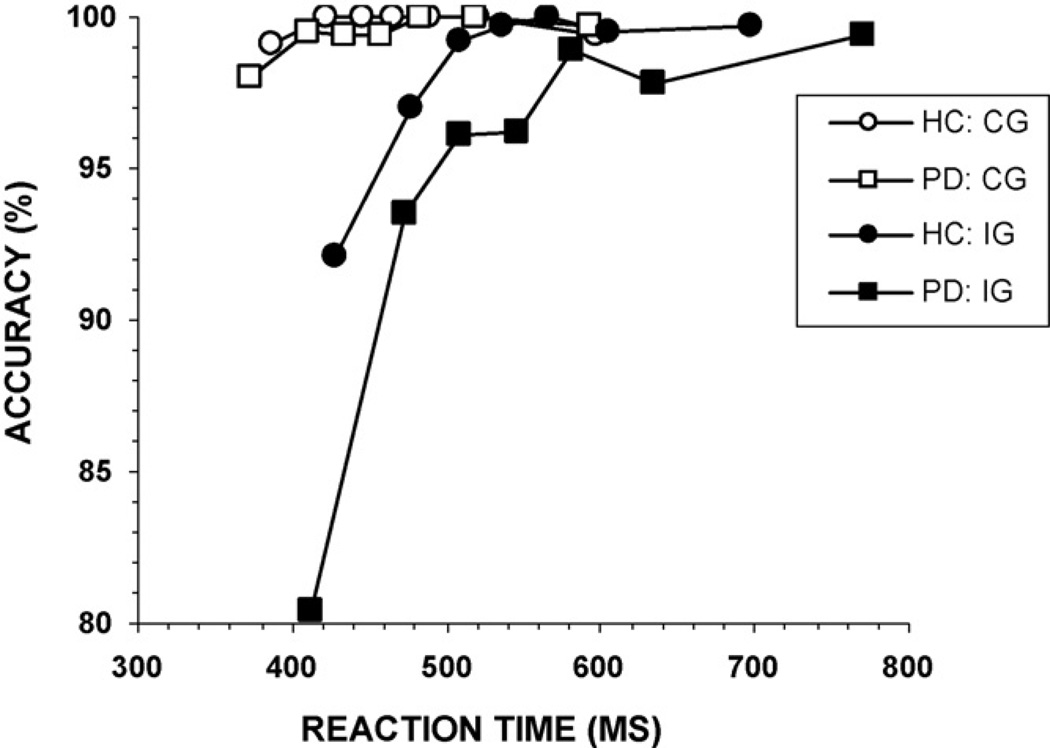
Conditional accuracy functions (CAF) were calculated and plotted to analyze the pattern of fast errors. Specifically, RTs for all responses at each level of flanker congruence were rank-ordered and partitioned into seven equal size bins (septiles; bin1-bin7). Next, accuracy rates were calculated separately for each bin, thus generating seven accuracy values for both congruent and incongruent flanker trials. These accuracy rates were plotted against the average RT for each bin (see Fig. 4). As seen in the figure, errors for both groups were most pronounced for fast reactions under incongruent flanker trials. That is, most errors were fast responses. Slow RTs were associated with near perfect accuracy. Graphically, the PD group appears to show a slightly steeper accuracy slope between the fastest two bins of the incongruent flanker CAF, with similar slopes across the rest of the distribution. However, the group comparison of slopes between the accuracy rates for the first two bins for the incongruent flanker CAF showed a trend toward a significant difference, but was not statistically different after correcting for multiple comparisons, F(1, 73) = 2.61, p = 0.05 (bonferroni corrected alpha = 0.008 for all six possible slope comparisons). A direct comparison of accuracy rates in the first bin also showed a trend toward more fast errors among PD patients (p = 0.03; bonferroni corrected alpha = 0.007 for all seven possible bin comparisons). According to the activation–suppression hypothesis, these results suggest that PD patients may have experienced stronger initial response activation associated with the incorrect response.
Fig. 4.
Conditional accuracy functions for individuals with Parkinson’s disease (PD) and healthy controls (HC). For both groups, errors are associated with the fastest reaction times, and the pattern of error rates is suggestive that PD patients make more fast errors than HC. CG: congruent, IG: incongruent.
4.2.2. Response inhibition
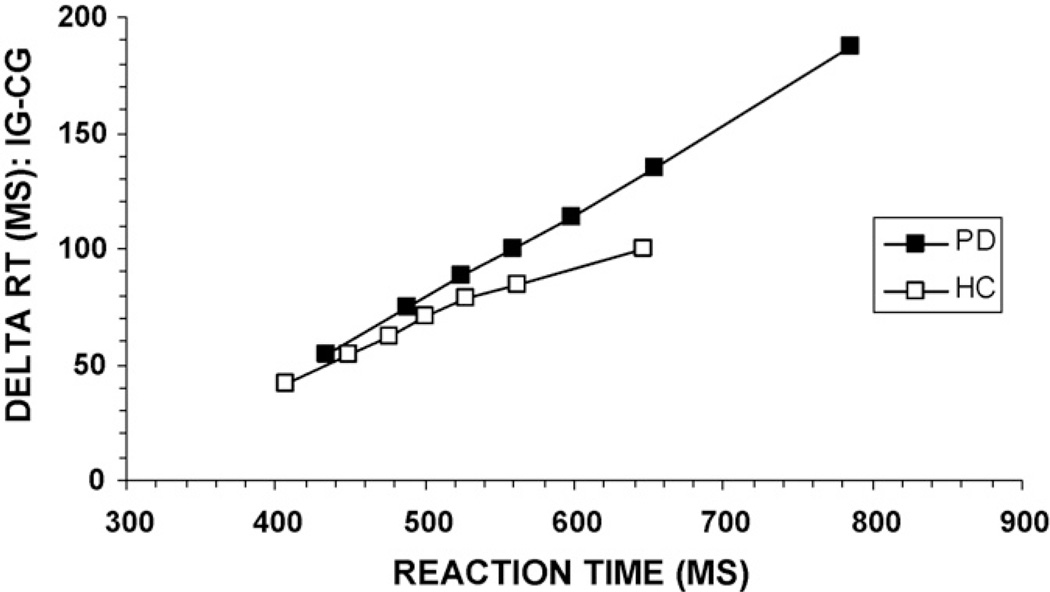
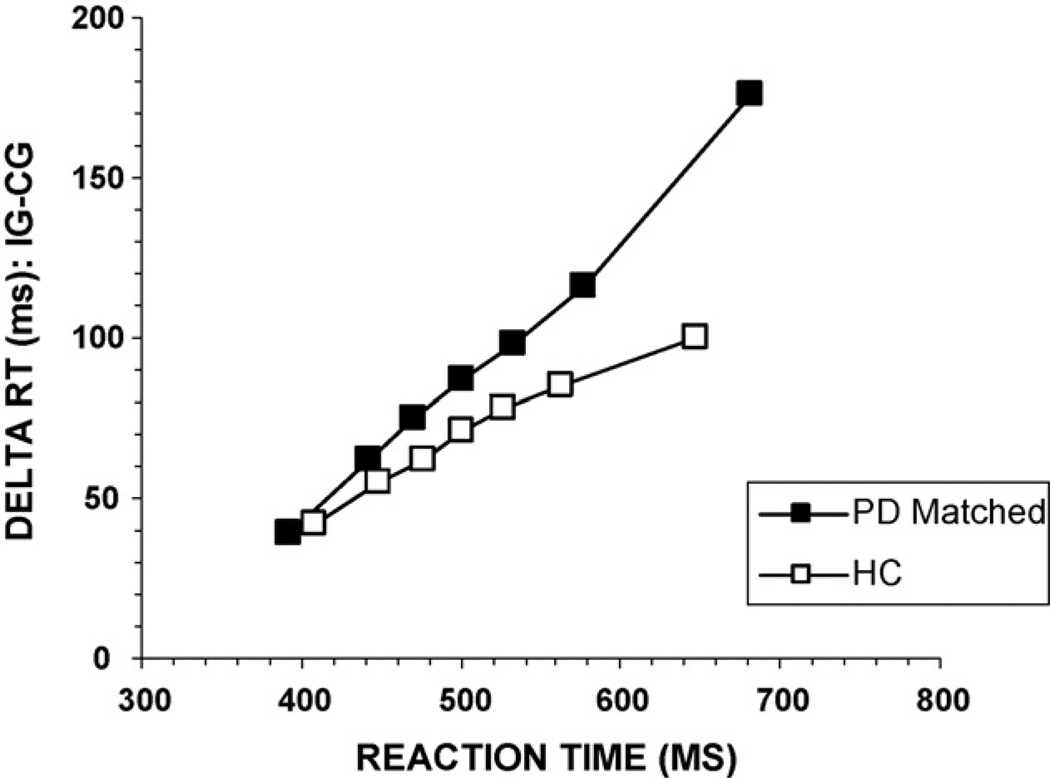
To analyze the effectiveness of inhibition engaged to counter the interference produced by the incongruent information, we analyzed the pattern of interference effects across the RT distribution. We first constructed delta plots, which plot interference effects as a function of mean RT. For each participant, RTs for all responses at each level of flanker congruence were rank-ordered and partitioned into seven equal size bins (septiles; bin1–bin7). Next, mean RT was calculated for each septile. Seven interference effect sizes (delta values) were generated by subtracting mean RT for the congruent condition from mean RT for the incongruent condition for each septile. Delta plots for RT were then constructed by plotting interference effect sizes (i.e., delta values) as a function of average RT for the corresponding septile. For the RT delta plot analysis, we focused on contrasts between slopes, anticipating that the slopes at the slowest segments of the RT distribution (where the build-up of inhibition is hypothesized to be maximal) would differentiate PD and HC groups. Specifically, we predicted that PD would be associated with a more positive-going slope compared to HC.
The analysis of RT delta plot slopes shows a distinguishable pattern of interference effects between the two groups (Fig. 5). For both groups, the interference effect increases at a similar rate across the fastest and intermediate segments of the RT distribution (i.e., the interference effect increases with slower RT). However, the group delta slopes appear to diverge between the final three septiles, with HC participants showing a reduction of the interference effect and PD patients showing a greater increase in flanker interference. Group contrasts between slopes with Bonferroni correction (alpha = 0.008) showed a divergence in the slope between the 5th and 6th bins that approached significance, [bin5–bin6, F(1, 73) = 4.22, p = 0.02], and a clear divergence between the final septiles, [bin6–bin7, F(1, 73) = 6.15, p = 0.007]. According to the activation–suppression hypothesis, the larger delta slope in the PD group toward the slow end of the RT distribution argues for group differences in the efficiency of inhibition engaged to control or counter the response interference. Specifically, the PD group shows less effective inhibitory control of the incorrect response activation compared to the HC group.
Fig. 5.
Reaction time delta plots for PD and HC groups. Group delta slopes diverge at the slow end of the distribution, suggesting poorer inhibitory control of incorrect response activation among PD patients. CG: congruent, IG: incongruent.
4.3. Interference effects in PD: relationship to clinical features
Within the PD group, we examined the relationship between clinical features (e.g., disease ratings, age at PD onset, disease duration) and each of the three critical cognitive variables: (1) overall interference effect, (2) response activation revealed by the slope between the first two bins of the conditional accuracy function, and (3) response inhibition revealed by the slope between the final two bins of the RT delta plot. Consistent with previous findings, flanker interference effects were unrelated to Hoehn and Yahr ratings, age at disease onset, disease duration, or global cognitive status (all p > 0.10). Forty of the PD patients had also been rated on the motor subtest of the UPDRS, which includes separate ratings of bradykinesia and tremor. Notably, the interference effect size for this subset of patients did not differ from that evident among the entire PD group. Consistent with the overall analysis, there were no significant correlations between interference effects and the total motor subtest, bradykinesia, or tremor ratings among this subset of patients (p >0.10). Finally, the slopes representing response activation and response inhibition did not correlate with any of the clinical features (all p > 0.10).
All PD patients were taking dopaminergic medications (e.g., l-dopa) at the time of testing; however, a subset of patients was also taking a dopamine agonist (e.g., pramipexol). There were no differences in the size of the interference effect between PD patients taking a dopamine precursor and those patients taking a precursor plus a dopamine agonist (p >0.10).
4.4. Interference effects and PD subgroups
Inspection of the distribution of interference effect sizes within the PD group revealed an intriguing pattern. For exactly 50% of the PD patients, the size of the interference effect exceeded the entire distribution of interference effect values for the HC group, whereas the remaining 50% of patients appeared to show interference effects that overlapped the distribution of interference effects shown by the HC group. Given the mixed findings reported thus far concerning interference effects in PD, we decided to look at these subgroups more closely. Based on this apparent median split, we partitioned the PD group into two subgroups, including individuals demonstrating low flanker interference (PD Low) and those showing high flanker interference (PD High). Important from a clinical perspective, these subgroups did not differ in terms of the severity of motor symptoms, disease duration, mental status, age of disease onset, or present age (all p > 0.10).
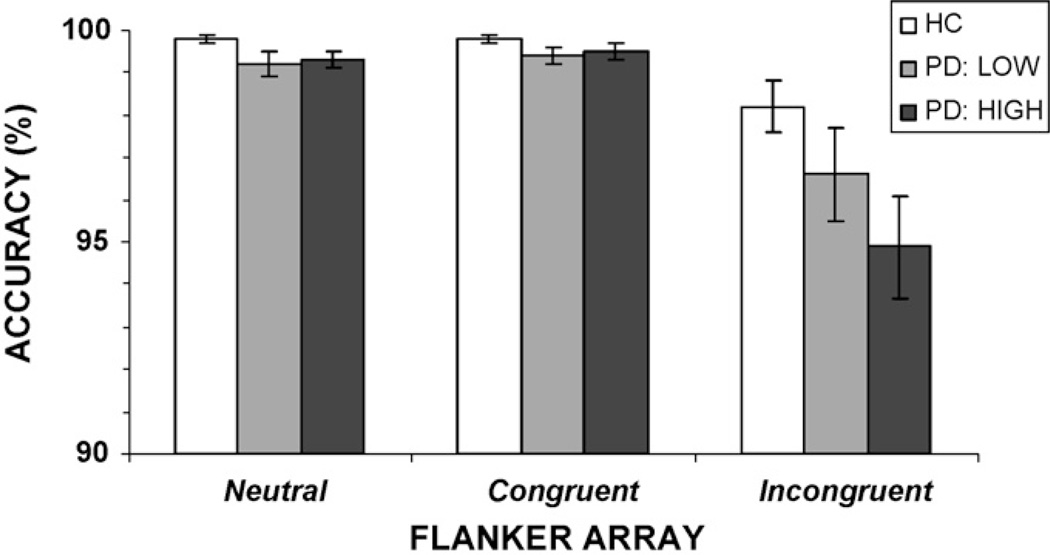
We compared the accuracy rates, conditional accuracy functions, and RT delta slopes between the PD subgroups and the HC group. For accuracy rates, the effect of flanker congruence was significant, F(2, 144) = 63.48, p < 0.001, with planned contrast analyses confirming that accuracy rates were reduced when incongruent as compared to congruent or neutral flanker arrays were presented (p <0.001). There was a significant main effect of subgroup on accuracy rates, F(2, 72) = 3.21, p <0.05, with the PD High group making more errors compared to the HC group (p <0.05). The PD Low group showed accuracy rates intermediate to the PD High and HC groups, although no differences were statistically significant. The interaction between flanker congruence and subgroup was also significant, F(4, 144) = 2.59, p < 0.05, with post hoc comparisons showing that the effect was confined to poorer accuracy rates in the PD High group compared to the HC group (p = 0.02) when flankers were incongruent. Again, however, accuracy rates for the PD Low group were intermediate to the PD High and HC groups, but did not statistically differ from either group (Fig. 6). These patterns indicate that the PD patients who experienced the greatest RT interference from the presence of incongruent flankers were also more prone to select the incorrect response signaled by these flankers.
Fig. 6.
Overall accuracy rates (%) for PD high interference (PD High), PD low interference (PD Low), and healthy control (HC) groups. All groups show reduced accuracy when flankers were incongruent, and the PD High group makes more errors in this condition than the HC group. CG: congruent, IG: incongruent.
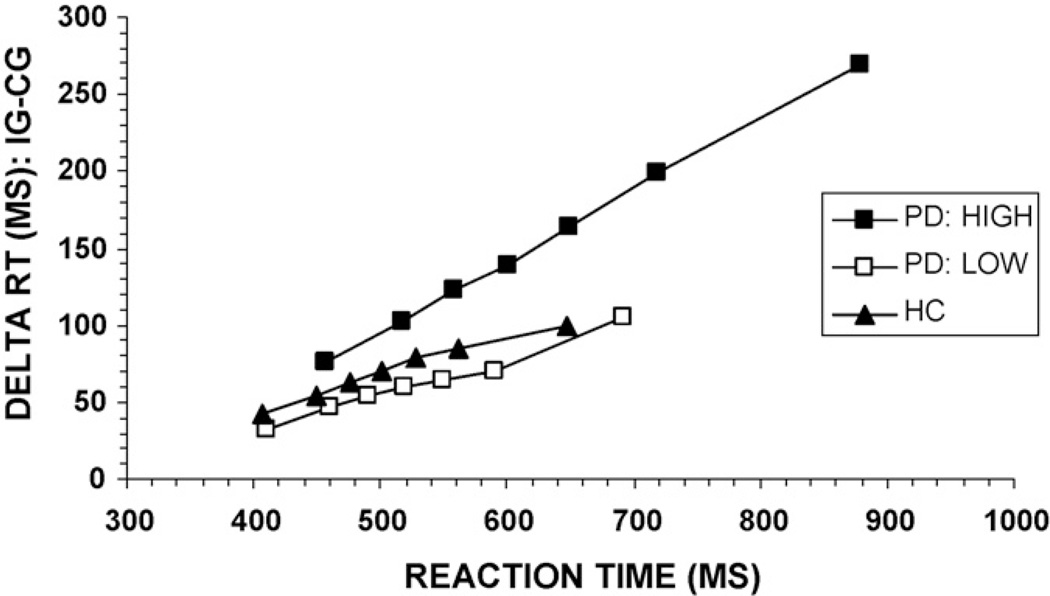
Of particular interest was whether the apparent PD subgroups, especially the PD High interference group, could be distinguished from the healthy control group on the basis of specific cognitive patterns (e.g., response activation, response inhibition). To examine performance differences between these subgroups further, we used distributional analyses to determine if the groups differed on the basis of response activation (revealed in the slope of the conditional accuracy function between the first two septiles, bin1–bin2) or suppression of this activation (revealed in the RT delta slopes between the slowest septiles, bin6–bin7; note: we chose the last RT delta slope because this measure differentiated the PD and HC control groups). The accuracy slope between the first two septiles (bin1–bin2) did not differ among the three groups, F(2, 72) = 1.29, p > 0.10, which according to the activation-suppression model, suggests similar levels of initial response activation. However, there was a significant group effect on the RT delta slope between the slowest septiles (bin6–bin7), F(2, 72) = 3.85, p < 0.05 (Fig. 7). Bonferroni corrected planned contrasts confirmed a steeper positive slope in the PD High group compared to the HC group. While the delta slope of the PD Low group was intermediate to the PD High and HC groups, the difference was not statistically different from either group. This pattern suggests that only the PD High subgroup could be distinguished from the HC group on the basis of suppression effects toward the slow end of the RT distribution.
Fig. 7.
Reaction time delta plots for healthy controls (HC), PD High, and PD Low interference groups. The PD High group shows a much more positive-going slope between the final two bins, a pattern consistent with reduced inhibitory control of incorrect response activation.
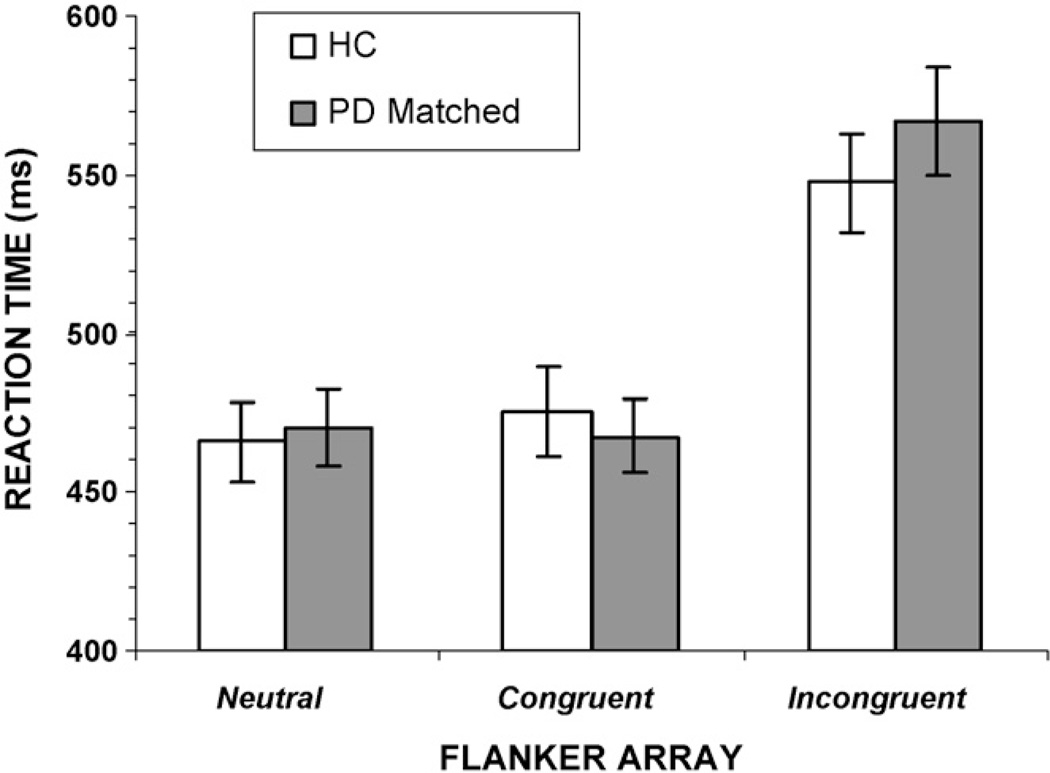
The subgroup of PD patients showing exaggerated flanker interference effects was significantly slower in overall RT compared to the HC group and the PD low interference group. To rule out the possibility that this difference in the interference effect was due to generalized slowing in PD, we selected a new group of 25 PD patients by matching baseline RT in the neutral condition with each of the 25 participants from the HC group. The two groups did not differ in age (PD = 65.2; HC = 67.7) or education (all p values > 0.10). For neutral flanker trials, the two groups differed by 5ms in RT (PD-matched = 471, HC = 466) and by 5ms in variability of RT (i.e., standard deviation; PD-matched = 59.6, HC = 64.4).
Repeated-measures ANOVA applied to the RTs obtained for Congruent and Incongruent flanker trials showed a significant flanker interference effect, F(1, 48) = 258.2, p < 0.001). As Fig. 8 depicts, RT to incongruent flanker arrays was slowed compared to congruent flanker arrays. There was no main effect of group on RT, F(1, 48) = 0.09, p >0.10, further confirming that the two groups showed similar overall RTs. Consistent with the previous analysis, the flanker congruence by group interaction was significant, F(1, 48) = 6.31, p < 0.05; the RT slowing induced by the presence of incongruent flankers was greater for the PD-matched group than for the HC group. For accuracy rates, both groups showed reduced accuracy to incongruent flanker arrays, F(1, 48) = 68.24, p < 0.001. Not only did the PD-matched group make more overall errors than the HC group, F(1, 48) = 7.66, p <0.01, they also made more errors to incongruent flankers than HC participants, [group by flanker congruence, F(1, 48) = 8.09, p < 0.01] (Fig. 9).
Fig. 8.
Overall mean reaction times (RT) for a subset of 25 individuals with Parkinson’s disease (PD-matched) who were matched to the healthy control (HC) group on the basis of baseline RT in the neutral flanker condition. Compared to the HC group, the PD-matched group shows a significantly larger increase in RT for the incongruent flanker compared to the congruent flanker condition.
Fig. 9.
Overall accuracy rates (%) for the PD-matched group (PD-matched) and healthy controls (HC). Accuracy rates decline for incongruent arrays, and the PD-matched group made more errors in this condition than did the HC group.
To determine group differences on the basis of the dynamics of specific cognitive patterns (e.g., response activation, response inhibition), we compared the conditional accuracy functions and RT delta slopes. Comparing the accuracy slopes between the first two septiles (bin1–bin2) showed a strong trend toward a group difference, F(1, 48) = 3.01, p = 0.04, suggesting that the strength of initial response activation may have been stronger among the PD-matched group (Fig. 10). Directly comparing the accuracy rates for the fastest bin of RTs in the incongruent condition showed that the PD-matched group made more fast errors than the HC group, F(1, 48) = 6.90, p = 0.006 (one-sided hypothesis test). Thus, differences in initial response activation emerge between PD and HC groups matched on the basis of baseline RT. There was also a significant group effect on the RT delta slope for the slowest septiles (bin6–bin7), F(1, 48) = 10.67, p = 0.001 (Fig. 11).This shows that even among PD patients with similar baseline RT to HC participants, a pattern consistent with less effective suppression is revealed by delta plots.
Fig. 10.
Conditional accuracy functions for the PD-matched and healthy control (HC) groups. For both groups, errors are associated with the fastest reaction times in the incongruent flanker condition, but the PD-matched group shows significantly more fast errors. CG: congruent, IG: incongruent.
Fig. 11.
Reaction time delta plots for PD-matched and HC groups. Consistent with the overall PD group analysis, the group delta slopes diverge at the slow end of the distribution, suggesting poorer inhibitory control of incorrect response activation among PD-matched patients compared to HC. CG: congruent, IG: incongruent.
5. Discussion
Basal ganglia structures are hypothesized to play an important role in the focused selection and inhibition of responses (Aron & Poldrack, 2006; Hikosaka, 1998; Mink, 1996). Based on this idea, we and others have predicted that basal ganglia dysfunction produced by PD alters the coordination of these processes, thus making it more difficult for patients with PD to select a response efficiently when an alternative response is activated concurrently (Praamstra & Plat, 2001; Praamstra et al., 1998, 1999; Wylie et al., 2005). More specifically, it has been hypothesized that this inefficiency stems from a reduced capacity to suppress the automatic activation of conflicting responses, which in turn increases interference during response selection (Praamstra & Plat, 2001; Praamstra et al., 1998). Previous studies of medicated and medication-withdrawn PD patients have found mixed evidence for increased response interference, and a role for diminished suppression has been inferred on the basis of increased interference in some studies.
The current study extends this literature in two important ways. First, we addressed the mixed findings by investigating response interference in a sample of PD patients nearly three times larger than the samples used in previous studies. Second, we used a theory-based analysis of RT distributions to investigate more directly the hypothesis that PD compromises the inhibition of conflicting responses. The present results demonstrated larger flanker interference effects for the PD group compared to healthy controls that was independent of baseline RT. We also showed that the size of interference effects within the PD group is quite variable, and there appears to be a more vulnerable subset of PD patients. The distributional analyses pointed strongly to a specific deficit among PD patients in the ability to suppress the unintended, prepotent response activation induced by incongruent flankers.
5.1. Effects of PD on flanker interference: comparison to previous findings
The present findings replicated the results from three previous studies that showed greater flanker interference effects for PD patients relative to healthy controls (Praamstra et al., 1998, 1999; Wylie et al., 2005). That is, PD patients were disproportionally slower to respond to a target stimulus when flankers signaled and presumably activated a conflicting response (i.e., were incongruent). The patterns of error rates, including the proportion of fast errors, supported a trend that PD patients were more susceptible to selecting the conflicting response resulting in a fast error than the healthy control group. Together, these findings suggest that PD patients tend to be more susceptible to the rapid selection of unintended, but prepotent, responses but also take longer to resolve the interference from prepotent responses even when the intended response is selected correctly.
These findings are in agreement with the notion that basal ganglia dysfunction due to PD alters the efficiency of interference control during action selection. That the deficit involves pronounced response interference by visual distractors is supported by additional findings from previous studies. For example, we hypothesized previously that greater response interference due to incongruent flankers in PD could actually facilitate performance if the task was modified so that the incongruent flankers corresponded to the correct response rather than a competing, incorrect response (Wylie et al., 2005). This was studied by manipulating stimulus-response compatibility in the context of the flanker task. Participants made compatible (i.e., choose the response in the same direction as the target arrow; right pointing arrow = right hand response) and incompatible (i.e., choose the response in the opposite direction of the target arrow; right pointing arrow = left hand response) responses. Consistent with a larger literature, participants produced slower RTs when making incompatible responses (i.e., there was a cost of incompatibility). However, the cost of incompatibility was modulated by the configuration of flankers (Ridderinkhof et al., 1995). As expected, all participants showed the normal slowing of RT when making compatible responses in the presence of incongruent flankers. However, the cost of incompatibility was smaller in the presence of incongruent flankers. That is, when participants were instructed to make the response opposite the direction of the target arrow (i.e., the incompatible response), the RT slowing typical of this demand was reduced when the flankers pointed in the opposite direction of the target, thus signaling and presumably activating the incompatible response. Moreover, the reduction in the cost of incompatibility was greater among PD patients, further highlighting the exaggerated interference from incongruent flankers on action selection among these patients.
The studies by Praamstra et al. (1998, 1999) offered greater insight into a possible source of increased response interference in their demonstration of a shorter latency and an enhancement in the amplitude of the initial deflection of the LRP (which corresponds to the differential activation of motor cortex that controls the response signaled by the incongruent flankers relative to motor cortex controlling the response signaled by the target) among PD patients relative to healthy controls. Moreover, this enhancement was associated with the increase in the interference effect measured in the behavioral data, suggesting a stronger overall influence from the direct activation of the response signaled by the incongruent flankers among PD patients.
While these findings in PD converge on a pattern of exaggerated response interference from incongruent flankers, not all studies have found these effects among PD patients with mild to moderate disease severity (Falkenstein et al., 2006; Cagigas et al., 2007; Lee et al., 1999). In fact, Falkenstein et al. (2006) observed smaller flanker interference effects coupled with a delayed and smaller amplitude LRP component among PD patients compared to healthy controls, findings in striking contrast to those reported by Praamstra et al. (1998, 1999). Consistent with their findings, Falkenstein et al. suggested that PD patients experience a reduction in the interference induced by incongruent flankers. As Falkenstein et al. (2006) pointed out in their detailed discussion, there are no clear methodological or patient characteristics that seem to explain the discrepant flanker interference effects in PD patients across studies. Here, we expand on their thoughtful discussion by considering: (1) specific design differences between the current study and past studies, and (2) potentially relevant patient factors across studies that might prove to be important in explaining discrepant findings between studies.
5.2.1. Interference effects in PD: task design considerations
One of the more obvious differences across studies is the use of different visual displays. For example, some tasks have used arrows or arrowheads as stimuli (Falkenstein et al., 2006; Praamstra et al., 1998, 1999; Wylie et al., 2005; current study), which benefit from overlearned associations with response direction (e.g., left pointing arrow signals a left directional response). In contrast, other studies (Cagigas et al., 2007; Lee et al., 1999) used arbitrary associations between responses and features of a stimulus (e.g., color of a circle, letter identification). Notably, studies showing larger interference effects among PD patients used arrow stimuli, whereas studies using arbitrary stimulus–response associations found no differences between PD and healthy control groups. Thus, the automaticity of the stimulus–response association may be a critical factor that could be tested directly in a study that mixes over-learned and arbitrary stimulus–response associations. Then again, Falkenstein et al. (2006) used arrowheads for stimuli and found reduced flanker interference effects in PD patients. However, flanking arrowheads were organized in the vertical plane as opposed to a horizontal plane used in studies by Wylie et al. and a display in which flanker arrows completely surrounded the target in studies by Praamstra et al. (1998, 1999). The other difference between the latter studies was that Falkenstein et al. used two flanker arrowheads in their display, whereas the Wylie et al. and Praamstra et al. studies used four and eight flanker arrows in their displays, respectively. This suggests that the salience of the flankers relative to the target may also be a critical factor in accounting for the discrepant results. The sensitivity of PD motor performance to the presence of strong, salient visual cues is well documented (Cunnington, Iansek, & Bradshaw, 1999; Oliveira, Gurd, Nixon, Marshall, & Passingham, 1997). An argument against this possibility is that the elicitation of stronger incorrect response activation by more salient incongruent flankers should produce larger interference effects and errors rates, and across studies there does not appear to be differences in flanker effect sizes or error rate patterns that conform to this prediction. One way to test this possibility in future studies would be to vary systematically the salience of flankers relative to the target, for example, by manipulating relative size (large versus small), number of flankers, stimulus intensity (bright versus dim), or stimulus quality (degraded versus non-degraded).
A related difference concerns the visual angle of the flanker array, which varied across studies. In the current study, the visual angle of the flanker array was much larger than previous studies. While visual angle may influence the magnitude of interference effects, it seems unlikely that it played a crucial role in determining the mixed PD effects across studies (Miller, 1991). First, the size of the flanker effect in the current study is similar to other studies. Second, the edge-to-edge separation between the target arrow and the nearest flanker arrows was less than 1° visual angle, a distance that is similar to other studies and associated with enhanced interference effects compared to larger edge-to-edge visual angles (Parquet, 2001; Parquet & Craig, 1997; Miller, 1991). Another design feature that differed in the current study and past studies was the duration of the stimulus array. Past studies have used a fixed duration for displaying the flanker array, whereas in the current study, the duration of the flanker array was response-terminated. In previous work, stimulus duration (i.e., fixed versus response-terminated) did not affect the elicitation or magnitude of flanker interference effects in healthy adults (Sevilla et al., 2003). Thus, it seems unlikely that the duration of the flanker display can account for the mixed findings in PD.
Another factor concerns the effect of speed-accuracy strategy on flanker interference effects. In all but one study, task instructions appear to have emphasized a balance of speed and accuracy. In contrast, Falkenstein et al. (2006) placed higher stress on speed of responding by requiring participants to respond within 600ms. Even with this speed emphasis, PD patients did not show larger interference effects. In a recent study of 28 PD patients and 17 healthy controls, we found that speed-accuracy instructions played an important role in modulating flanker interference effects among PD patients (Wylie et al., submitted). Instructions that emphasized “accuracy” of performance led to equivalent RT, accuracy rates, and interference effects between PD and HC groups. In contrast, an emphasis on “speed” of performance produced distinct patterns of interference effects between the groups. Although both groups decreased their response latencies, the decrease in the PD group was about half that of the HC group. Despite this difference, the groups showed a similar increase in error rates when flankers signaled an incongruent response. Most importantly, the PD group also showed a significant increase in the interference effect compared to the HC group. An implication of this study is that manipulations that increase response interference and place greater demands on the suppression of incorrect response activation may exacerbate interference control deficits among PD patients.
5.2.2. Interference effects in PD: clinical features and emergence of subgroups
Consistent with previous studies, there was no relationship between the magnitude of flanker interference and clinical features of PD, including disease duration, age at symptom onset, current age, global mental status, and motor symptom rating. Whereas our previous investigation pointed to a possible link between symptoms of bradykinesia and flanker interference effects, this association was not supported by the current results. In part, we suspect that a limitation of such associations stems from the fact that conventional ratings of disease severity based on clinical examination, while sensitive to gross differences in motor dysfunction, may not be sufficiently sensitive and/or comprehensive for comparisons with certain complex cognitive processes. Thus, more detailed methods for quantifying PD symptoms or pathology may be needed to fully understand the relationship between difficulties with interference control and disease characteristics.
One might suspect that interference effects among PD patients are sensitive to medication effects or effectiveness. It is possible that the subgroup of patients who show flanker interference effects similar to healthy controls benefit to a greater degree from their medications. A limitation of the current study is that our results cannot address the impact of dopaminergic medications on interference effects. Notably, studies failing to find differences in interference effects studied PD patients on their usual medications, whereas studies from Praamstra et al. (1998, 1999) found exaggerated effects among PD patients who had withdrawn from dopaminergic medications overnight. Our two studies of medicated PD patients show exaggerated flanker interference effects. A study design that compares the performance of PD patients while taking their prescribed dopaminergic medication and after overnight withdrawal might offer clues as to why some PD patients show larger interference effects and others do not. A recent study by Willemssen, Muller, Schwarz, Hohnsbein, and Falkenstein (2008) provides some insight into medication effects. A flanker task was administered to PD patients while taking their usual dopaminergic medications and after overnight withdrawal, and flanker interference effects were unaffected by medication status and did not differ from healthy controls. The design differed from previous studies in that the probability of incongruent flanker trials (20%) within a block of trials was significantly less than the probability of congruent flanker trials (60%), with no-go trials comprising the remaining 20% of trials within a block. However, it could be argued that the higher proportion of congruent flanker trials would create stronger prepotent response activation for the response signaled by flankers, thus increasing the amount of conflict when patients encountered infrequent incongruent flanker trials. A replication of medication effects on the flanker task with incongruent and congruent flanker trials occurring with equiprobability and under various stimulus array frequencies would further strengthen these initial findings.
A second possible explanation for the mixed findings across PD studies is that pathological processes producing the clinical expression of motor dysfunction are distinct from processes involved in the expression of executive cognitive control deficits. This would certainly be consistent with our understanding of distinguishable frontal–basal ganglia circuits devoted to motor and cognitive processes (Alexander, Crutcher, & DeLong, 1990; Alexander, DeLong, & Strick, 1986; Cummings, 1993). The idea for distinct subgroups of PD patients with similar motor symptoms and clinical features but dissociable deficits on measures of executive cognitive functioning has been supported in several recent investigations (Graham & Sagar, 1999; Lewis, Cools, et al., 2003; Lewis, Dove, Robbins, Barker, & Owen, 2003). For example, Lewis et al. identified subgroups of PD patients with similar motor and disease characteristics who could be distinguished on the basis of performance on a measure of executive planning and problem-solving. In a complementary fMRI study, a subgroup of PD patients who showed abnormally poor performance on measures of planning and working memory displayed hypoactivation in prefrontal and neostriatal regions compared to a subgroup of PD patients with similar motor and disease characteristics but normal performance on these executive cognitive measures (Lewis, Cools, et al., 2003; Lewis, Dove, et al., 2003). Furthermore, the subgroup of PD patients without executive cognitive deficits also showed similar brain activation patterns as the healthy controls during task performance.
We observed a similar pattern in the current study in that half of the PD patients displayed seemingly normal interference effects, while the remaining half of patients showed interference effects that exceeded the entire distribution of these effects found in the healthy control group. Comparisons of these PD subgroups revealed no differences in motor symptom severity or clinical features, including disease duration and age at disease onset. Moreover, the low interference PD subgroup performed similarly to the healthy control group (i.e., showed a similar interference effect), thus supporting the conclusion of Falkenstein et al. (2006) that response interference deficits may not be an inevitable fate of PD. This pattern also helps to explain why studies with smaller samples of PD patients have found mixed findings. Dopamine depletion in PD is known to affect putaminal regions of the striatum in the earliest stages of the disease, with progression eventually affecting more anterior circuits of the caudate nucleus (Kish, Shannak, & Hornykiewicz, 1988; Nurmi et al., 2001). Although speculative, we wonder if dopamine depletion in patients who show larger interference effects is related to greater disruption in the latter circuits, which would include projections from dorsolateral and ventrolateral prefrontal cortices that are linked to inhibitory control. The extent to which PD patients with poor interference control also show greater deficits in other aspects of executive cognition that would be supported by prefrontal–caudate circuits, such as the planning and working memory deficits found in the subgroup studied by Lewis et al., is an important issue for future studies to address.
5.2. Distributional analyses and the activation–suppression hypothesis
The strength of the response activated by incongruent flankers represents a potential source of variability in accounting for interference effects. The conditional accuracy functions strongly hinted that PD patients may experience stronger incorrect response activation as revealed by more fast errors when flankers signaled an incongruent response. This pattern was most clear when comparing a subset of PD patients with healthy controls after matching on the basis of baseline RT. Based on the activation–suppression model, this subgroup of PD patients showed stronger initial, incorrect response activation (i.e., more fast errors) coupled with a reduced ability to suppress this activation (i.e., steeper delta slopes) compared to healthy controls. The suggestion that PD patients experience stronger initial activation of the incongruent response is consistent with the LRP findings by Praamstra et al. (1998,1999) that showed motor activation corresponding to the response signaled by incongruent flankers had an earlier onset and higher amplitude among PD patients.
When a situation signals conflicting responses, executive control is necessary to suppress the incorrect response activation. According to the activation–suppression hypothesis, the activation and subsequent suppression of an incorrect response can be tracked in the pattern of interference effects across the RT distribution (i.e., delta plots). Because slow RTs are thought to benefit more from the build-up of suppression, the slopes between delta points at slower RT segments are used to infer the effectiveness of the suppression mechanism, with steeper slopes corresponding to less effective suppression. Delta slope reductions and even reversals (e.g., negative-going slopes) have been demonstrated in several studies of response conflict and tied to inhibitory ability (Burle et al., 2002; de Jong et al., 1994; Forstmann et al., in press; Ridderinkhof, 2002; Ridderinkhof, van den Wildenberg, Wijnen, et al., 2004; Ridderinkhof et al., 2005; Wiegand & Wascher, 2007).
In the current study, delta slopes showed a similar increase for both groups across early segments of the RT distribution. However, the slopes for PD and HC groups began to diverge between the 5th and 6th septiles and were clearly divergent between the final two septiles. Specifically, the PD group showed a much steeper delta plot slope. According to the activation–suppression hypothesis, the divergence of delta slopes toward the slow end of the distribution indicates that PD patients are less efficient at suppressing conflicting response-relevant information than are HC participants. Importantly, these patterns remained when comparing a subset of PD patients who were matched to the HC group on the basis of baseline RT. This confirms that the differences in flanker interference and reduced inhibitory control were unrelated to global RT slowing.
It is important to note that the reduction of the delta slopes among HC is significant, but not very dramatic (e.g., not reversing or negative-going). In other response conflict tasks, such as the Simon task, more dramatic reversals of the delta slopes are found (Burle et al., 2002; Ridderinkhof, 2002; Stürmer, Leuthold, Soetens, Schröter, & Sommer, 2002), clearly indicating strong suppression effects. Moreover, the most dramatic reversals of the delta slopes are found for the subset of trials on which partial EMG activation in the incorrect response hand is found (Burle et al., 2002). Therefore, despite the lack of dramatic reduction of the delta slopes in the flanker task, the presence of reduction is still consistent with tenets of the activation–suppression hypothesis and findings from other studies using similar methods (Bub et al., 2006; Ridderinkhof et al., 2005; Wylie et al., 2007).
The distributional analyses also provided some insight into the possible differences between subgroups of PD patients showing normal interference effects and those showing exaggerated effects. The slopes across the earliest septiles in the delta plot did not differ between the control group and either the high and low interference PD groups, but the delta slopes between the HC and PD Low groups diverged at the slowest segments of the distribution. Again, this is consistent with differences in the efficiency of response suppression engaged to counter the build-up of incorrect response activation. Notably, the delta slopes between the final two septiles were clearly largest for the PD High group and lowest for the HC group, with the PD Low group showing a slope intermediate to these groups, but not statistically different from either. Thus, there is an emerging pattern of poor inhibitory control in a subset of PD patients, but near normal inhibitory control in other PD patients with similar disease presentations.
Overall, the activation–suppression model appears to effectively accommodate the observed differences between PD patients and controls in terms of response activation and selective inhibition. However, it cannot be excluded that alternative models can be extended such that they could also accommodate these findings. For instance, random-walk or accumulator models of reaction times might be modified such that they predict negative-going delta plots if congruent and incongruent stimuli were associated with different drift rates at which they approach a response boundary. Future formal modeling and simulation efforts should establish whether such models can describe the details of RT distribution with levels of precision similar to the activation–suppression model and whether predictions can be derived from such a model that distinguish it from the activation–suppression model.
The finding of reduced inhibitory control during action selection adds to other studies that have postulated similar deficits in PD (Brown & Marsden, 1998; Filoteo, Rilling, & Strayer, 2002; Gauntlett-Gilbert, Roberts, & Brown, 1999; Hayes, Davidson, Keele, & Rafal, 1998; Henik, Singh, Beckley, & Rafal, 1993; Jackson & Houghton, 1995; Joti, Kulashekhar, Behari, & Murthy, 2007; Praamstra & Plat, 2001; Praamstra et al., 1998; Robbins & Brown, 1990; Stam et al., 1993; van den Wildenberg et al., 2006).The neural mechanisms by which dopamine depletions caused by PD produce poor inhibitory control are not clearly understood. One possibility is that the reduced neuromodulation from dopamine at the corti-costriatal interface disrupts the coordination of direct and indirect pathways that implement the focused selection and inhibition of competing motor programs (Mink, 1996). Consistent with this idea is the demonstration that the selectivity of inhibited (i.e., leading to action) and disinhibited (i.e., leading to suppression) movement-related basal ganglia output neurons is diminished by dopamine depletion (Leblois, Boraud, Meissner, Bergman, & Hansel, 2006).
Another possibility is that dopamine depletions alter the interface of the so-called hyperdirect pathway that links ventrolateral prefrontal cortex (VLPFC) to the subthalamic nucleus (STN) (Aron, 2007; Frank, 2006; Nambu, Tokuno, & Takada, 2002). It is well known that STN activity is abnormal in PD and a primary target for deep brain stimulation electrode placement (Blandini, Nappi, Tassorelli, & Martignoni, 2000). The STN is uniquely positioned to excite inhibitory output neurons of the basal ganglia, thus keeping action commands in check or stopping ongoing action selection (Aron, 2007; Frank, 2006). In healthy adults, activation of VLPFC and STN increases during trials of a stop-signal task that require the controlled inhibition of an activated, but not yet emitted, response (Aron & Poldrack, 2006). In PD, deep brain stimulation of the STN has been shown to improve inhibitory control during stop-signal performance (van den Wildenberg et al., 2006). Thus, the poor inhibitory control found in the present study among PD patients may reflect fundamental alterations to the hyperdirect pathway that is engaged to suppress unwanted response commands. As further evidence of this possibility, a recent fMRI study of the Simon response conflict task showed that steeper negative-going delta slopes at the slow end of the RT distribution, which correspond to greater inhibitory control, were associated with stronger activation in the right ventrolateral prefrontal cortex (Forstmann et al., in press). Thus, absence of a reduction in the delta slope at the slow end of the RT distribution among PD patients in the current study may correspond to poor inhibitory control along the hyperdirect pathway engaged to suppress the response command triggered by the incongruent flankers.
6. Conclusions
The action selection and inhibition model of basal ganglia function offers an influential and intriguing framework for understanding some of the cognitive deficits produced by PD. Here we demonstrate that PD can produce poor interference control during action selection relative to healthy controls, and the basis of this problem stems from less effective suppression of what appears to be a more strongly activated incorrect response. Not all patients show a strong pattern of poor inhibitory control; however, when controlling for baseline RT, there is a hypothesized pattern of stronger activation of an incorrect response followed by poorer suppression of this response among PD patients. Future work is needed to determine what neuropathological processes are responsible for cognitive control deficits among PD patients, what factors contribute to the variability of cognitive control deficits among PD patients, and whether all PD patients eventually show similar deficits.
Acknowledgement
The work of KRR and WPMvdW is supported respectively by a VICI grant and a VENI grant from the Netherlands Organization for Scientific Research (NWO).
References
- Alexander GE, Crutcher MF, DeLong MR. Basal ganglia–thalamocortical circuits: Parallel substrates for motor, oculomotor, ‘prefrontal’ and ‘limbic’ functions. Progress in Brain Research. 1990;85:119–147. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Band GPH, van Boxtel GJM. Inhibitory motor control in stop paradigms: Review and reinterpretation of neural mechanisms. Acta Psychologica. 1999;101:179–211. doi: 10.1016/s0001-6918(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Progress in Neurobiology. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Bogacz R. Optimal decision making theories: Linking neurobiology with behaviour. Trends in Cognitive Sciences. 2007;11:118–125. doi: 10.1016/j.tics.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. What do the basal ganglia do? Lancet. 1998;351:1801–1804. doi: 10.1016/s0140-6736(97)11225-9. [DOI] [PubMed] [Google Scholar]
- Bub DN, Masson ME, Lalonde CE. Cognitive control in children: Stroop interference and suppression of word reading. Psychological Science. 2006;17:351–357. doi: 10.1111/j.1467-9280.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- Burle B, Possamaï CA, Vidal F, Bonnet M, Hasbroucq T. Executive control in the Simon effect: An electromyographic and distributional analysis. Psychological Research. 2002;66:324–336. doi: 10.1007/s00426-002-0105-6. [DOI] [PubMed] [Google Scholar]
- Burle B, van den Wildenberg WPM, Ridderinkhof KR. Dynamics of facilitation and interference in cue-priming and Simon tasks. European Journal of Cognitive Psychology. 2005;17:619–641. [Google Scholar]
- Cagigas XE, Filoteo JV, Stricker JL, Rilling LM, Friedrich FJ. Flanker compatibility effects in patients with Parkinson’s disease: Impact of target onset delay and trial-by-trial stimulus variation. Brain and Cognition. 2007;63:247–259. doi: 10.1016/j.bandc.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaler G, Deniau J. Disinhibition as a basic process in the expression of striatal functions. Trends in Neurosciences. 1990;13:277. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:529–553. doi: 10.1037//0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson’s disease. Neuroscience and Biobehavioral Reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal–subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Bradshaw J. Movement-related potentials in Parkinson’s disease: External cues and attentional strategies. Movement Disorders. 1999;14:63. doi: 10.1002/1531-8257(199901)14:1<63::aid-mds1012>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- de Jong R, Liang CC, Lauber E. Conditional and unconditional automaticity: A dual-process model of effects of spatial stimulus–response correspondence. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:731–750. doi: 10.1037//0096-1523.20.4.731. [DOI] [PubMed] [Google Scholar]
- Eimer M. The lateralized readiness potential as an on-line measure of central response activation processes. Behavior Research Methods, Instruments and Computers. 1998;30:146–156. [Google Scholar]
- Eimer M, Hommel B, Prinz W. S-R compatibility and response selection. Acta Psychologica. 1995;90:301–313. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of target letters in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Willemssen R, Hohnsbein J, Hielscher H. Effects of stimulus–response compatibility in Parkinson’s disease. A psychophysiological analysis. Journal of Neural Transmission. 2006;113:1449–1462. doi: 10.1007/s00702-005-0430-1. [DOI] [PubMed] [Google Scholar]
- Filoteo J, Rilling L, Strayer D. Negative priming in patients with Parkinson’s disease: Evidence for a role of the striatum in inhibitory attentional processes. Neuropsychology. 2002;16:230–241. doi: 10.1037//0894-4105.16.2.230. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, van den Wildenberg WPM, Ridderinkhof KR. Neural mechanisms, temporal dynamics, and individual differences in interference control. Journal of Cognitive Neuroscience. in press doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and non-medicated Parkinsonism. Journal of Cognitive Neuroscience. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Networks. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, Feghoff TA. Inhibition of ongoing responses in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntlett-Gilbert J, Roberts R, Brown V. Mechanisms underlying attentional set-shifting in Parkinson’s disease. Neuropsychologia. 1999;37:605. doi: 10.1016/s0028-3932(98)00049-9. [DOI] [PubMed] [Google Scholar]
- Graham JM, Sagar HJ. A data-driven approach to the study of heterogeneity in idiopathic Parkinson’s disease: Identification of three distinct subtypes. Movement Disorders. 1999;14:10–20. doi: 10.1002/1531-8257(199901)14:1<10::aid-mds1005>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Sirevaag EJ, Eriksen CW, Donchin E. Pre-and poststimulus activation of response channels: A psychophysiological analysis. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Groves P. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Research Reviews. 1983;5:109. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Hayes AE, Davidson MC, Keele SW, Rafal RD. Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience. 1998;10:178–198. doi: 10.1162/089892998562645. [DOI] [PubMed] [Google Scholar]
- Henik A, Singh J, Beckley DJ, Rafal RD. Disinhibition of automatic word reading in Parkinson’s disease. Cortex. 1993;29:589–599. doi: 10.1016/s0010-9452(13)80283-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Neural systems for control of voluntary action—A hypothesis. Advances in Biophysics. 1998;35:81–102. [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Jackson S, Houghton G. Sensorimotor selection and the basal ganglia: A neural network model. In: Houk J, Davis J, Beiser D, editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 337–368. [Google Scholar]
- Joti P, Kulashekhar S, Behari M, Murthy A. Impaired inhibitory oculomotor control in patients with Parkinson’s disease. Experimental Brain Research. 2007;177:447–457. doi: 10.1007/s00221-006-0687-0. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak It, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease: Pathophysiologic and clinical implications. New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: Cognitive basis for stimulus–response compatibility—A model and taxonomy. Psychological Review. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33:282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Krauthamer G. Sensory functions of the neostriatum. In: Divac I, Oberg R, editors. The neostriatum. Oxford: Pergamon Press; 1979. p. 263. [Google Scholar]
- Kropotov JD, Etlinger S. Selection of actions in the basal ganglia–thalamocortical circuits: Review and model. International Journal of Psychophysiology. 1999;31:197–217. doi: 10.1016/s0167-8760(98)00051-8. [DOI] [PubMed] [Google Scholar]
- Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. Journal of Neuroscience. 2006;26:3567–3583. doi: 10.1523/JNEUROSCI.5050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Wild It, Hollnagel C, Grafman J. Selective visual attention in patients with frontal lobe lesions or Parkinson’s disease. Neuropsychologia. 1999;37:595–604. doi: 10.1016/s0028-3932(98)00081-5. [DOI] [PubMed] [Google Scholar]
- Leuthold H. Programming of expected and unexpected movements: Effects on the onset of the lateralized readiness potential. Acta Psychologica. 2003;114:83–100. doi: 10.1016/s0001-6918(03)00051-9. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41:645–654. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. Journal of Cognitive Neuroscience. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyper-direct” pathway. Neuroscience Research. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Mattler U. Delayed flanker effects in lateralized readiness potentials. Experimental Brain Research. 2003;151:272–288. doi: 10.1007/s00221-003-1486-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research Reviews. 2000a;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain and Cognition. 2000b;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Miller J. The flanker compatibility effect as a function of visual angle, attentional focus, visual transients, and perceptual load: A search for boundary conditions. Perception and Psychophysics. 1991;49:270–288. doi: 10.3758/bf03214311. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: Focused selection an inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia intrinsic circuits and their role in behavior. Current Opinion in Neurobiology. 1993;3:950–957. doi: 10.1016/0959-4388(93)90167-w. [DOI] [PubMed] [Google Scholar]
- Nurmi E, Ruottinen HM, Bergman J, Haaparanta M, Solin O, Sonninen P, et al. Rate of progression in Parkinson’s disease: A 6-[18F]fluoro-l-dopa PET study. Movement Disorders. 2001;16:608–615. doi: 10.1002/mds.1139. [DOI] [PubMed] [Google Scholar]
- Oberg R, Divac I. Cognitive functions of the neostriatum. In: Divac I, Oberg R, editors. The neostriatum. Oxford: Pergamon Press; 1979. p. 291. [Google Scholar]
- Oliveira R, Gurd J, Nixon P, Marshall J, Passingham R. Micrographia in Parkinson’s disease: The effect of providing external cues. Journal of Neurology, Neurosurgery & Psychiatry. 1997;63(4):429–433. doi: 10.1136/jnnp.63.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parquet L. Eliminating flanker effects and negative priming in the flankers task: Evidence for early selection. Psychonomic Bulletin and Review. 2001;8:301–306. doi: 10.3758/bf03196165. [DOI] [PubMed] [Google Scholar]
- Parquet L, Craig GL. Evidence for selective target processing with a low perceptual load flankers task. Memory & Cognition. 1997;25:182–189. doi: 10.3758/bf03201111. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat EM. Failed suppression of direct visuomotor activation in Parkinson’s disease. Journal of Cognitive Neuroscience. 2001;13:31–43. doi: 10.1162/089892901564153. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat EM, Meyer AS, Horstink MW. Motor cortex activation in Parkinson’s disease: Dissociation of electrocortical and peripheral measures of response generation. Movement Disorders. 1999;14:790–799. doi: 10.1002/1531-8257(199909)14:5<790::aid-mds1011>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman D, Cools A, Horstink MW. Reliance on external cues for movement inititation in Parkinson’s disease: Evidence from movement-related potentials. Brain. 1998;121:167. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: A vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: Activation and suppression in conflict tasks. Psychological Research. 2002;66:312–323. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Scheres A, Oosterlaan J, Sergeant JA. Delta plots in the study of individual differences: New tools reveal response inhibition deficits in AD/HD that are eliminated by methylphenidate treatment. Journal of Abnormal Psychology. 2005;114:197–215. doi: 10.1037/0021-843X.114.2.197. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Wijnen J, Burle B. Response inhibition in conflict tasks is revealed in delta plots. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford Press; 2004. pp. 369–377. [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Bashore TR. Limits on the application of additive factors logic: Violation of stage robustness suggests a dual-process architecture to explain flanker effects on target processing. Acta Psychologica. 1995;90:29–48. [Google Scholar]
- Robbins TW, Brown VJ. The role of the striatum in the mental chronometry of action: A theoretical review. Reviews in the Neurosciences. 1990;2:181–213. doi: 10.1515/REVNEURO.1990.2.4.181. [DOI] [PubMed] [Google Scholar]
- Seiss E, Praamstra P. The basal ganglia and inhibitory mechanisms in response selection: Evidence from subliminal priming of motor responses in Parkinson’s disease. Brain. 2004;127:330–339. doi: 10.1093/brain/awh043. [DOI] [PubMed] [Google Scholar]
- Sevilla JG, Linares MJ, Menéndez GC, Caparrόs DA, Oviedo PM, Martínez PR. Effects of exposure time in a flanker task for different conditions of target-flanker distance. Anales de Psicologia. 2003;19:27–36. [Google Scholar]
- Smid HG, Mulder G, Mulder LJ. Selective response activation can begin before stimulus recognition is complete: A psychophysiological and error analysis of continuous flow. Acta Psychologica. 1990;74:169–201. doi: 10.1016/0001-6918(90)90005-z. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Coles MGH. The lateralized readiness potential: Relationship between human data and response activation in a connectionist model. Psychophysiology. 1999;36:364–370. doi: 10.1017/s0048577299970749. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Visser SL, Op de Coul AA, de Sonneville LM, Schellens RL, Brunia CHM, et al. Disturbed frontal regulation of attention in Parkinson’s disease. Brain. 1993;116:1139–1158. doi: 10.1093/brain/116.5.1139. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Leuthold H, Soetens E, Schröter H, Sommer W. Control over location-based response activation in the Simon task: Behavioral and electrophysiological evidence. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:1345–1363. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- Taylor A, Saint-Cyr J. The neuropsychology of Parkinson’s disease. Brain & Cognition. 1995;28:281. doi: 10.1006/brcg.1995.1258. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WPM, van Boxtel GJM, van der Molen MW, Bosch DA, Speelman JD, Brunia CHM. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. Journal of Cognitive Neuroscience. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- Wascher E, Reinhard M, Wauschkuhn B, Verleger R. Spatial S–R compatibility with centrally presented stimuli: An eventrelated asymmetry study on dimensional overlap. Journal of Cognitive Neuroscience. 1999;11:214–229. doi: 10.1162/089892999563346. [DOI] [PubMed] [Google Scholar]
- Wiegand It, Wascher E. Response coding in the Simon task. Psychological Research. 2007;71:219–233. doi: 10.1007/s00426-005-0027-1. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Hoormann J, Hohnsbein J, Falkenstein M. Central and parietal event-related lateralizations in a flanker task. Psychophysiology. 2004;41:762–771. doi: 10.1111/j.1469-8986.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Muller T, Schwarz M, Hohnsbein J, Falkenstein M. Error processing in patients with Parkinson’s disease: The influence of medication state. Journal of Neural Transmission. 2008;115:461–468. doi: 10.1007/s00702-007-0842-1. [DOI] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WPM, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, Wooten GF. doi: 10.1016/j.neuropsychologia.2009.02.025. The effect of speed-accuracy strategy on response interference control in Parkinson’s disease (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Eckerle MIt, Manning CA. Inefficient response inhibition in individuals with mild cognitive impairment. Neuropsychologia. 2007;45:1408–1419. doi: 10.1016/j.neuropsychologia.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Stout JC, Bashore TR. Activation of conflicting responses in Parkinson’s disease: Evidence for degrading and facilitating effects on response time. Neuropsychologia. 2005;43:1033–1043. doi: 10.1016/j.neuropsychologia.2004.10.008. [DOI] [PubMed] [Google Scholar]