Abstract
Background
Due to the recent increased use of the McMaster (MM) fecal egg counting method for assessing benzimidazole drug efficacy for treating soil-transmitted helminth (STH) infections, the aim of the current study was to determine the operational value of including the MM method alongside the Kato-Katz (KK) fecal thick smear to increase the diagnostic sensitivity when STHs are co-endemic with trematode helminths (e.g., Schistosoma mansoni).
Methods
A cross-sectional study was conducted in school-aged children aged 4-18 years in the northeastern region of the State of Minas Gerais (Brazil), where Necator americanus, Ascaris lumbricoides, Trichuris trichiura, and S. mansoni are co-endemic. One fecal sample from each participant was collected and transported to the field laboratory for analysis. Coprological diagnosis was performed on each fecal sample by three different methods: Formalin-Ether Sedimentation (FES), KK and the MM technique. The diagnostic sensitivity and negative predictive value (NPV) of each technique was calculated using the combination of all three techniques as the composite standard. In order to determine the agreement between the three techniques Fleiss´ kappa was used. Both the Cure Rate (CR) and the Fecal Egg Count Reduction (FECR) were calculated using the two quantification techniques (i.e., the MM and KK).
Results
Fecal samples from 1260 children were analyzed. The KK had higher diagnostic sensitivity than the MM for the detection of both A. lumbricoides (KK 97.3%, MM 69.5%) and hookworm (KK 95.1%, MM 80.8%). The CR of a single dose of mebendazole varied significantly between the KK and MM for both A. lumbricoides (p = 0.016) and hookworm (p = 0.000), with lower rates obtained with the KK. On the other hand, the FECR was very similar between both techniques for both A. lumbricoides and hookworm.
Conclusion
The MM did not add any diagnostic value over the KK in areas where both STHs and trematodes were co-endemic. The lower sensitivity of the MM would have an important impact on the administration of selective school-based treatment in this area since if only the MM were used, 36 (13.9%) children diagnosed with A. lumbricoides would have gone untreated.
Author Summary
Diagnosis of intestinal helminths and Schistosoma mansoni infections is based on the detection of eggs in feces. There are many techniques available for both detection and quantification of infection. For the quantification of helminth infections, the methods traditionally used are the Kato-Katz (KK) fecal think smear in humans, and the McMaster (MM) counting method in animals. Recently, the MM has been used for assessing the efficacy of benzimidazole drugs for treating soil-transmitted helminth (STH) infections in humans. In most parts of the world, however, STHs occur simultaneously with other helminth species, and the MM does not detect other helminth eggs. Therefore, in this study we sought to determine if the use of the MM in an area of Brazil were both STHs and S. mansoni are co-endemic, added any value to the current standard of diagnosis using the KK.
Introduction
Despite numerous technological advances in the diagnosis of soil-transmitted helminth (STH) infections, such as the development of multiplex and multi-parallel fecal DNA assays [1,2], coprological microscopy techniques remain the standard for diagnosing these infections in humans [3], including the McMaster (MM) counting method [4] and the Kato-Katz (KK) fecal thick smear [5]. This is due to their simplicity, ease-of-use in the field, and low cost. Moreover, neither method requires expensive or highly calibrated instrumentation such as a real-time PCR instrument (multiplex and multi-parallel fecal DNA assay), so both can be performed on site in resource-limited endemic areas.
The KK method has been the standard technique for the detection and quantification of STH and intestinal trematode infections globally for nearly forty years as reviewed in [6–12] and is recommended by the World Health Organization (WHO) for the detection of these infections [13]. However, as pointed out by Levecke et al. (2011) [14], the KK method has limitations with respect to its qualitative and quantitative diagnostic performance when used to assess STHs in areas endemic for multiple helminth species. These limitations are most apparent when hookworm (e.g., Necator americanus) is one of the co-endemic STHs. With the use of the malachite green-glycerol solution in the KK method, helminth eggs are visualized at different time intervals or “clearing times” after the preparation of a slide. The clearing time for hookworm on a KK slide is limited to 30 minutes after preparation, whereas it is one hour for A. lumbricoides or S. mansoni. However, after 60 minutes, the glycerol solution begins to desiccates the hyaline shell of the hookworm eggs, distorting and eventually collapsing the eggs, so that they are mistaken as something else (e.g., a vegetable spore) or are reported as entirely absent, hence affecting the specificity and sensitivity of the KK for the detection hookworm infection.
In contrast, the eggs from A. lumbricoides or S. mansoni can be visualized after hours or even days after the KK slide has been prepared [15]. Moreover, the quantification of egg excretion (or the intensity of infection) by the KK method as determined by fecal egg counts (FEC) is based on a small fixed volume of feces (41.7 mg) and not on the mass of the feces submitted [5]. Hence, the quantitative performance of the KK method is controversial as the density of the feces can vary while the intensity of the eggs excreted is expressed the same, as the number of eggs per gram (EPG) of stool [16]. This potential error in FECs obtained by the KK is considered critical in programs monitoring drug efficacy, where it is thought to “introduce variation in the results and broaden the confidence levels of the resulting statistical parameters” [14]. These properties have also impeded standardization of the KK method in large-scale studies conducted at different study sites. For these reasons, the MM was used as the diagnostic method in several recent large-scale multi-site trials [17,18] of efficacy of the anthelminthics albendazole and mebendazole in treating humans for STH infections.
While the MM is the standard coprological method to assess STHs in veterinary parasitology, its estimates of FEC, ease of use, and suitability for poorly equipped laboratories, also make it a good choice for public health monitoring of human STHs. The aim of the current study was to determine the operational value of including the MM method in addition to the KK for routine diagnosis in areas co-endemic for several species of helminths (Fig 1). To this end, the MM and the KK methods were performed on the same fecal sample in a cross-sectional study of over 1,200 children aged 4–18 years of age in the northeastern region of the state of Minas Gerais, Brazil, where N. americanus, A. lumbricoides, T. trichiura, and S. mansoni are co-endemic, as shown in our previous studies [19,20].
Fig 1. Global distribution of STHs and other trematodes species of medical importance including Schistosoma mansoni, S. japonicum, Fasciola hepatica, F. gigantica, Fasciolopsis buski, Paragonimus westermani, Heterophyes heterophyes, Clonorchis sinensis and Opisthorchis viverrini.
More specifically, our objectives were to assess: (i) the qualitative diagnostic performance of the KK and MM (e.g., diagnostic sensitivity and negative predictive value); (ii) the quantitative diagnostic performance of the KK and MM (fecal egg counts); and (iii) the accuracy of both methods for estimating drug efficacy. While recent multinational studies have focused on the relative performances of the MM and KK for monitoring drug efficacy for treatment of STH infections [17,18], to our knowledge, this is the first study to describe the operational value of combining these two techniques for routine public health monitoring of helminth endemicity.
Methods
Ethics Statement
The study was approved by the National Ethics Committee of Brazil (Protocol 454/2009) and the Institutional Review Board of the Centro de Pesquisas René Rachou (Belo Horizonte, Brazil). Written informed consent was obtained from a parent or guardian of every child.
Study Area and Study Population
This study was conducted in northeastern, Minas Gerais state, Brazil. Details of the study region have been extensively detailed elsewhere [21,22]. Prior studies have confirmed that N. americanus is the sole hookworm species endemic in this region [23,24].
Study Design
The study was conducted as a school-based survey including children between the ages of 4 and 18 years old, inclusive. First, meetings were held with community members to explain the purpose of the study. For the parasitological survey, school personnel and students were informed 24 hours in advance of sample collection and labeled plastic containers were provided. Students were instructed to deposit one fecal sample of each child into the container and return it to school on the following day. Fresh fecal samples were transported at 4°C and those returned later than 48h after date of distribution were not accepted and new containers were issued. Refrigerated samples were transported to the nearby field laboratory located in Americaninhas, Minas Gerais state, Brazil were they were processed within 24 hours of receipt. Children positive for a STH by any of the three detection techniques described below were treated with a single oral dose of 500 mg mebendazole. Children were asked to provide a second fecal sample 7 to 14 days after treatment to monitor treatment efficacy; these samples were also analyzed by all three coprological techniques.
Stool Examination
The presence of A. lumbricoides, T. trichiura, hookworm and S. mansoni eggs in feces was determined by trained lab personnel in compliance with standard operating procedures for the 3 different techniques routinely used in the field laboratories of the Centro de Pesquisas René Rachou: a) formalin-ether sedimentation (FES) [25]; b) KK fecal thick smear [5]; and MM counting technique [4].
Formalin-ether sedimentation
Fecal samples were homogenized and 3 grams were weighed and diluted in 10 ml dH2O. After mixing the feces with a wooden spatula, the contents were then filtered through a funnel containing gauze into a conical 15 ml glass tube. After the addition of 3 ml ethyl ether, the tube was capped and vigorously agitated by hand. After careful removal of the cap (done slowly to relieve any pressure build-up), the sample was centrifuged at room temperature and 1000 rpm for 1 minute. Four layers were formed with the sediment in the very bottom of the tube. The first 3 layers were decanted and then 50 μl 5% formalin was added to the sediment. Three slides were prepared from each sample by placing 50 μl of sediment between the slide and cover slip and then viewed under the microscope.
Kato-Katz
A solution of 3% malachite green-glycerol solution was prepared in advance and cellophane strips the size of a slide were immersed in the solution for 24 hours prior to use. After thorough homogenization of the samples, approximately 1 gram of feces was placed on a tissue paper and covered with a wire mesh. With the aid of a spatula, pressure was applied and the feces that passed through the mesh were deposited onto a standard template holding 41.7 mg of feces located on a glass slide. The template was removed and a strip of cellophane paper embedded in the 3% malachite green-glycerol solution was placed on top of the feces. Pressure was gently applied over the cellophane strip with the aid of another slide in order to spread out the sample. Two KK slides were prepared from each sample ≤24 h after receipt and examined by microscope using 100x magnification after 30–60 minutes of clearing time. Each slide was observed in its entirety and for each sample; the EPG of feces was obtained by multiplying the mean number of eggs per slide by 24. The clearing time is defined as the period after the application of the malachite green-glycerol solution until eggs can be visualized. Due to the osmotic force on the thin hyaline layer of hookworm eggs, the slide must be read within 30–60 minutes after preparation or these eggs will desiccate rapidly and become deformed beyond recognition as the KK slide dries. Nonetheless, during that time interval, it is possible to detect and quantify eggs from all three STHs and S. mansoni.
McMaster counting technique
Two grams of homogenized fresh fecal sample were suspended in 30 ml saturated sodium chloride (NaCl) solution (specific gravity [SG] = 1.22) prepared in advance by dissolving 333 g of NaCl in 1L of distilled water and observing precipitation. The suspension was poured three times through a wire mesh with the aid of a wooden spatula to remove large debris. The filtered portion of the sample was then homogenized for one minute using a magnetic bar and stirrer. Immediately after stirring, 0.5 ml aliquots were removed from the very top surface of the supernatant using a Pasteur pipette and then added into 2 chambers of a MM slide. The filled chambers were allowed to stand for two minutes and were examined under a light microscope using 100x magnification and the EPG of feces for each helminth species was obtained by multiplying the total number of eggs counted on both chambers by 50. Of note, the MM technique does not detect eggs of S. mansoni.
Statistical Analysis
All data were double-entered in Microsoft Excel (2010 Microsoft Corporation). Statistical analyses were performed using SPSS Statistics for Windows, Version 19.0 (Copyright IBM Corporation 1994, 2013) and GraphPad Prism, Version 6.0 (2015 GraphPad Software, Inc.)
Diagnostic sensitivity and egg counts
Intensities of infection for the STHs and S. mansoni were stratified according to WHO guidelines into light, moderate or heavy categories [26]; for A. lumbricoides these were defined as between 1–4,999, 5,000–49,999, and ≥50,000 EPG, respectively; for T. trichiura, 1–999, 1,000–9,999, and ≥10,000 EPG; for N. americanus, 1–1,999, 2,000–3,999, and ≥4,000 EPG; and, for S. mansoni, 1–99, 100–399, and ≥400 EPG. A related-samples Wilcoxon Ranked Signed test was used to evaluate differences in mean EPG counts obtained by the KK and MM methods. Scatter plots were used to assess the linearity of the relationship between average eggs counts and their correlation was analyzed using Spearman’s correlation coefficient.
Following the methods used by [14,27–38], the sensitivity and negative predictive value (NPV) were assessed for each diagnostic technique in comparison to a composite reference standard, which was defined as being positive if any of the 3 tests were positive. The specificity of the composite standard was assumed to be 100% given the small number of false positives found when experienced technicians examine the slides microscopically [39]. The sensitivity of both the KK and MM were also calculated by intensity class and compared against the composite reference standard.
The degree of agreement between the different diagnostic techniques (compared two by two) was assessed by calculating Cohen’s kappa (κ) statistic [40] and the agreement between all three techniques was assessed by calculating Fleiss’ kappa statistic [41], which allows comparison between more than two raters. The following cut-offs were used to calculate the degree of agreement: κ < 0, poor agreement; κ between 0 and 0.20, slight agreement; κ between 0.21 and 0.40, fair agreement; κ between 0.41 and 0.60, moderate agreement; κ between 0.61 and 0.80, substantial agreement; and, κ between 0.81 and 1.0, almost perfect agreement. For the KK and MM, Cohen’s kappa statistic was also used to assess the agreement in the assignment of the cases to the different classes of infection intensity (e.g., light, moderate or heavy) according to WHO guidelines [26].
Drug efficacy
Both the Cure Rate (CR) and the Fecal Egg Count Reduction (FECR) were calculated using the two quantification techniques (i.e., the MM and KK). The CR measures the percent of those treated who become egg negative after drug administration and is calculated by dividing the number of individuals who are egg positive post-treatment by the number who are positive pre-treatment and then multiplying by 100 [42]. The calculated CRs were compared using a two-sample test of proportion, setting the significance level at p < 0.05.
The FECR measures the percent reduction in egg intensity after drug treatment using the arithmetic mean (AR) of pre- and post-treatment egg counts. It is calculated by subtracting the AR of post-treatment FECs from the pre-treatment FECs, dividing it by the AR of pre-treatment FEC and then multiplying by 100 [17].
Results
Study Population
A total of 1,260 children from 23 schools were included in the study, of which 635 (50.4%) were female and 625 (49.6%) were male. The ages ranged from 4 to 18 years of age, with a mean age of 10.1 years. All the children included in the study provided a sufficient volume of feces to perform all 3 diagnostic procedures. A total of 311 children were found to be positive for at least 1 of the 3 STHs and were therefore treated with a single dose of 500 mg mebendazole. Of these, 302 provided fecal samples at follow-up 7–14 days following treatment.
Prevalence of Helminth Species
A total of 312 children were infected with S. mansoni. Since the MM is a flotation technique and therefore does not detect eggs of S. mansoni, the overall prevalence for this helminth (24.8%) was calculated using only the FES and KK techniques. Moreover, 46.2% of the children diagnosed with S. mansoni were co-infected with at least 1 STH. By combining the results from the 3 different techniques into a composite standard outcome measure, the prevalence for each STH was: 22.8% for hookworm, 20.6% for A. lumbricoides and 1.2% for T. trichiura. The overall prevalence and the individual prevalence obtained using each technique is detailed in Table 1.
Table 1. Prevalence, sensitivity and negative predictive value (NPV) derived by the different diagnostic methods for STHs and S. mansoni.
| Parasite | Technique | Prevalence (%) | Sensitivity (%) | NPV (%) |
|---|---|---|---|---|
| Hookworm | Composite* | 22.8 | 100 | 100 |
| Formalin-Ether Sedimentation | 21.8 | 95.8 | 98.8 | |
| Kato-Katz | 21.7 | 95.1 | 98.6 | |
| McMaster | 18.4 | 80.8 | 94.6 | |
| A. lumbricoides | Composite* | 20.6 | 100 | 100 |
| Formalin-Ether Sedimentation | 19.4 | 94.2 | 98.5 | |
| Kato-Katz | 20.0 | 97.3 | 99.3 | |
| McMaster | 14.3 | 69.5 | 92.7 | |
| T. trichiura | Composite* | 1.2 | 100 | 100 |
| Formalin-Ether Sedimentation | 1.0 | 86.7 | 99.8 | |
| Kato-Katz | 1.0 | 86.7 | 99.8 | |
| McMaster | 0.6 | 46.7 | 99.4 | |
| S. mansoni | Composite* | 24.8 | 100 | 100 |
| Formalin-Ether Sedimentation | 20.4 | 82.4 | 94.5 | |
| Kato-Katz | 23.7 | 95.5 | 98.5 | |
| McMaster | - | - | - |
*Composite: positive result from any of the three coprological tests.
Method Comparison for the Diagnosis of STHs and S. mansoni
Table 1 summarizes the relative performance characteristics of the 3 different fecal techniques used for the diagnosis of STH infections. Although the technique with the best performance for the diagnosis of hookworm proved to be FES, the kappa statistic (Table 2) showed an almost perfect agreement between all 3 techniques, with a Fleiss’ κ of 0.887. On the other hand, for A. lumbricoides, the technique with the best performance was the KK. Again, Fleiss’ kappa statistics comparing the techniques showed almost perfect agreement between them with a κ of 0.843, although Cohen’s kappa statistics showed only substantial agreement between the KK and MM (κ = 0.794) and between the FES and MM (κ = 0.791). Regarding the diagnosis of T. trichiura, both FES and KK were equally useful, with almost perfect agreement between them (Cohen’s kappa of 0.845). On the other hand, the agreement between the KK and MM techniques and between the MM and FES was only substantial (κ = 0.629 and 0.698, respectively). The overall agreement between all 3 techniques for T. trichiura as measured by Fleiss’ kappa was of substantial agreement (κ = 0.694). The comparison of the 2 techniques used for the diagnosis of S. mansoni demonstrated that the KK performed better than the FES, although Cohen’s kappa statistic showed almost perfect agreement between them with a κ of 0.841 (Table 2).
Table 2. Agreement between the different diagnostic techniques for the detection of STHs and S. mansoni.
| Parasite | Techniques compared | Cohen’s Kappa (κ) | Fleiss’ Kappa (κ)* |
|---|---|---|---|
| Hookworm | KK vs FES | 0.944 | |
| KK vs MM | 0.854 | 0.887 | |
| MM vs FES | 0.860 | ||
| A. lumbricoides | KK vs FES | 0.935 | |
| KK vs MM | 0.794 | 0.843 | |
| MM vs FES | 0.791 | ||
| T. trichiura | KK vs FES | 0.845 | |
| KK vs MM | 0.629 | 0.694 | |
| MM vs FES | 0.698 | ||
| S. mansoni | KK vs FES | 0.841 |
KK = Kato-Katz direct fecal smear; MM = McMaster counting technique; FES = Formalin-Ether Sedimentation.
*Used for the comparison of all three techniques.
Quantitative Fecal Egg Counts
The maximum EPG count, geometric and arithmetic mean of the EPG counts, standard deviation from the mean, and lower and upper 95% confidence intervals for STHs and S. mansoni obtained with the KK and MM counting techniques are summarized in Table 3. This table also summarizes the infection intensities of the positive samples, stratified into light, moderate or heavy infections. The mean EPG was significantly higher with the KK than the MM, as evaluated using the related-samples Wilcoxon Ranked Signed Test (p<0.001), for hookworm (207.39 vs. 86.53 EPG) and A. lumbricoides (3940.01 vs. 1329.33 EPG) but not for T. trichiura (p = 0.396). The maximum EPG value was lower with the KK for T. trichiura (252 vs. 400 EPG), although it was higher for both A. lumbricoides (129,120 vs. 51,250 EPG) and for hookworm (20,616 vs. 3850 EPG). The relationship between the EPG values obtained for each sample by each technique followed a linear trend as shown by scatter plots presented in Fig 2. The correlation between the KK and MM based on Spearman’s correlation coefficient was significant for all three STHs (0.86 for A. lumbricoides, 0.88 for hookworm and 0.63 for T. trichiura).
Table 3. Characteristics of STH and S. mansoni infections among children in Americaninhas, Minas Gerais, Brazil, as determined by the Kato-Katz (KK) and McMaster counting method (MM).
| Parasite | Arithmetic Mean EPG | Max EPG count | SD | Lower, Upper 95% CI | GeoMean EPG | Wilcoxon Ranked Signed test | Method |
|---|---|---|---|---|---|---|---|
| Hookworm | 207.4 | 20616 | 1149 | 143, 270. | 285.0 | <0.001 | KK |
| 86.5 | 3850 | 304 | 69, 103 | 282.9 | MM | ||
| A. lumbricoides | 3940.0 | 129120 | 13254 | 3940, 3207 | 6564.5 | <0.001 | KK |
| 1329.3 | 51250 | 5065 | 1049, 1609 | 4016.5 | MM | ||
| T. trichiura | 0.83 | 252 | 11 | 0.22–1.44 | 55.6 | 0.396 | KK |
| 0.75 | 400 | 13 | 0.02–1.49 | 101.7 | MM | ||
| S. mansoni | 76.5 | 10272 | 481 | 50–103 | 84.2 | NA | KK |
EPG refers to eggs per gram of feces. SD refers to the standard deviation of the arithmetic mean. CI refers to Confidence interval. Geomean refers to geometric mean.
Fig 2. Scatter plots of arithmetic egg counts (eggs per gram of feces—EPG) comparing the Kato-Katz direct fecal smear (KK) and the McMaster counting method (MM).
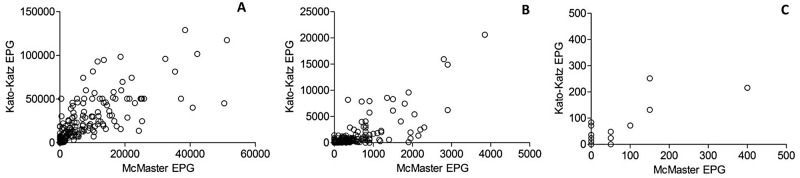
The graphs are presented separately for each soil-transmitted helminths: A. Ascaris lumbricoides, B. Hookworm, C. Trichuris trichiura. Correlation was measures using Spearman’s correlation coefficient: 0.86 for A. lumbricoides, 0.88 for hookworm and 0.63 for T. trichiura.
Moreover, when the sensitivity of the KK and MM was calculated according to the classification by infection intensity, the KK proved to be more sensitive than the MM for light, medium and heavy infections. This was true for all three STHs detected in the study (Table 4).
Table 4. Diagnostic sensitivity of the Kato-Katz (KK) and McMaster (MM) techniques according to intensity of infection.
| Intensity of infection | Parasite | |||||
|---|---|---|---|---|---|---|
| A. lumbricoides | Hookworm | T. trichiura | ||||
| Method | MM | KK | MM | KK | MM | KK |
| Light | 51.2 | 88.9 | 79.1 | 93.3 | 42.9 | 78.6 |
| Moderate | 52.9 | 92.3 | 12.1 | 43.3 | - | - |
| Heavy | 2.4 | 79.6 | - | 48.5 | - | - |
In general, the KK technique detected more cases of moderate and heavy intensity infections when compared to the MM counting technique (Table 5). Using Cohen’s kappa statistics (κ), the agreement in the classification between the MM and KK for the STHs showed differences depending on the species being studied (Table 5). For hookworm, the agreement was substantial (κ = 0.70) for light-intensity infections, slight (κ = 0.11) for moderate-intensity infections and poor (κ = 0.00) for heavy-intensity infections. For Ascaris, the agreement for both light- and heavy-intensity infections was slight (κ = 0.20 and 0.02, respectively) while for moderate-intensity infections it was fair (κ = 0.33). The difference observed in A. lumbricoides infection intensities is probably due to the presence of infertile A. lumbricoides eggs since the MM does not detect them because they are too heavy to float. During this study, the presence of infertile A. lumbricoides eggs was annotated and it was observed that the MM does not detect them. In 41 of the 259 cases of A. lumbricoides that were detected (15.8% of all cases detected); the presence of infertile eggs was observed, but only by the KK method. In 5 of these samples, the MM detected only fertile eggs while the KK detected both fertile and unfertile eggs; the remaining 36 cases that contained only infertile eggs were missed by the MM. For Trichuris, only light-intensity infections were diagnosed by both methods and the agreement between them was slight (κ = 0.19).
Table 5. Cohen’s kappa statistics (κ) to evaluate the agreement in the classification of STHs according to classes of intensity as determined by the Kato-Katz direct fecal smear (KK) and McMaster counting method (MM).
| No. of children stratified by infection intensity | |||||
|---|---|---|---|---|---|
| Parasite | Negative | Light | Moderate | Heavy | Method |
| Hookworm | 987 | 244 | 13 | 16 | KK |
| 1028 | 224 | 8 | 0 | MM | |
| Proportions of Agreement (κ) | 0.94 | 0.70 | 0.11 | 0.00 | |
| A. lumbricoides | 1008 | 94 | 121 | 37 | KK |
| 1080 | 88 | 90 | 2 | MM | |
| Proportions of Agreement (κ) | 0.93 | 0.20 | 0.33 | 0.02 | |
| T. trichiura | 1247 | 13 | 0 | 0 | KK |
| 1253 | 7 | 0 | 0 | MM | |
| Proportions of Agreement (κ) | 0.99 | 0.43 | NA | NA | |
NA: Not applicable
Efficacy of Treatment
As shown in Table 6, the CR was significantly lower when assessed by the KK technique in comparison to the MM for both hookworm (p < 0.001) and A. lumbricoides (p = 0.016). Therefore, 22 of the 189 children (11.6%) who were treated were determined to be cured from A. lumbricoides by the MM technique but were in fact still infected as determined by the KK. Also, after treatment, the MM failed to detect 38 light hookworm infections out of the 212 children treated (17.9%) that were diagnosed using the KK. On the other hand, 4 children (1.9%) with light hookworm infections after treatment went undetected by the KK method but were detected by the MM. Nonetheless, this difference in egg detection did not have an impact on the FECR since it was very similar between both techniques. The CR and FECR for T. trichiura were not calculated due to the small number of positive samples found.
Table 6. Cure Rates (CR) and Fecal Egg Count Reductions (FECR) after treatment with Mebendazole.
| McMaster Counting Method | Kato-Katz Direct Fecal Smear | |||
|---|---|---|---|---|
| STH | CR (%) | FECR (%) | CR (%) | FECR (%) |
| Hookworm | 61.0 a | 84.0 | 32.0 a | 86.0 |
| A. lumbricoides | 74.0 b | 97.0 | 59.0 b | 96.0 |
a Two sample test of proportions: p < 0.001
b Two sample test of proportions: p = 0.016
Discussion
The McMaster counting method (MM) is an inexpensive and easily implemented flotation technique, extensively used in veterinary parasitology and, more recently, in human studies to estimate cure rates for anthelminthics of the benzimidazole class, including albendazole [18] and mebendazole [17]. While the diagnostic sensitivity and specificity of the MM method for detecting nematode infections has long been reported to be excellent in humans and animals, its recent application in multi-nation studies for monitoring drug efficacy for STHs prompted us to consider incorporating it as a routine component in our epidemiological and public health surveys for STH infections in endemic areas of northeastern Minas Gerais state, Brazil [21–24,43]. As many regions are co-endemic for STHs and the trematode S. mansoni, which cannot be detected by the conventional MM, the principal rationale for adding the MM into our survey workflow for STH infections would be improved diagnostic sensitivity. Hence, the objective of the current study was to assess the added value of a combination detection strategy, which includes both the MM and KK methods.
Overall, the observed diagnostic performances of the MM and KK in this study were comparable for STHs, which is consistent with prior studies including a recent meta-analysis [44], and alone argues against the extra cost, time, and labor of including the MM in our diagnostic workflow. However, of particular concern was our observation that the MM method fell far short of its intended use in our combined diagnostic strategy in some critical respects, most importantly leading to several false negative results. In fact, the KK outperformed the MM for each of the three STHs studied, especially for the detection of A. lumbricoides eggs, for which there was a marked difference in egg detection between the 2 methods (Table 1). The KK method was far more sensitive (97.3%) for diagnosing A. lumbricoides infections than the MM method (69.5%), with the KK method detecting 36 more A. lumbricoides cases than the MM method. Although this difference in sensitivity has already been observed in other comparative studies [14,31,32,45], this is the first time that the extent that infertile A. lumbricoides eggs are undetected by the MM has been documented. A marked difference between the two methods was also observed when classifying A. lumbricoides infections by intensity according to WHO guidelines [13] since the KK method classified more cases into the moderate (κ = 0.33, fair agreement) and heavy (κ = 0.02, slight agreement) categories.
The reason for the difference in the performance of the two methods in regards to A. lumbricoides lay mainly with the presence of infertile Ascaris eggs in human feces. This is due to the fundamental mechanism of the conventional saline used in the MM, which relies on suspending a homogenized fecal sample in a NaCl solution that is pipetted into a chambered slide, where the specific gravity of the solution “floats” STH eggs to the chamber’s upper surface, where they can be counted under low magnification (usually 100X). While the MM method readily detects fertilized A. lumbricoides eggs given their specific gravity of 1.13 [46] (which is well within range of flotation for the MM method), it does not detect infertile A. lumbricoides eggs. The latter observation is due to a combination of both the high specific gravity (SG = 1.18) of infertile A. lumbricoides eggs [47], which is outside the range of flotation for the MM method, and the absence of a lipoidal membrane that prevents the equilibration of the interior of the egg with the external flotation media [47], so that infertile eggs sink instead of float. This deficiency in the MM method had a significant impact on the diagnosis of this STH in the current study: if the MM method had been used without the KK, the presence of A. lumbricoides eggs would have gone undetected in 36 of 259 children with this infection. Moreover, as shown in Table 6, the CR for A. lumbricoides was significantly lower when assessed using the KK method given that the MM method failed to detect the presence of eggs in the fecal samples of 22 children that had been treated with mebendazole.
The use of the MM method for the detection of hookworm eggs also added little extra diagnostic value to our study since the KK was found to be more sensitive (95.1%) than the MM (80.8%), although the best performance characteristics were obtained with FES (95.8%) (Table 1). For the quantification of hookworm infection, the KK method was able to detect not only more light intensity hookworm infections than the MM method, but also more moderate and heavy infections. This difference was statistically significant as shown by the kappa statistic (Table 5) since the agreement between the techniques for moderate and heavy infections was “slight” and “poor”, respectively. Moreover, similar to our observations for A. lumbricoides, the CR for hookworm was significantly lower when the KK method was used since the MM method failed to detect the presence of eggs in the fecal samples of 38 children (17.9%) that had been treated with mebendazole. It must be pointed out, however, that the KK failed to detect hookworm eggs that were observed by MM in 4 children who had been treated.
Finally, the strongest agreement between the 3 helminth detection techniques studied was found between the FES and KK techniques. The advantage of using both the FES and KK techniques is that it allows the detection of both protozoa and helminths (using the non-quantitative FES) and the quantification of several helminth species (using the KK), including S. mansoni, an important helminth in our area of study (S1 Fig). Based on our experience and the results reported herein, we recommend the use of both of these techniques in areas where STHs and S. mansoni are endemic, with the FES used as a screening test and KK used to quantifying infections. Furthermore, we do not recommend relying solely on the single solution MM for the study of STHs due to the important implications on treatment. In our opinion, the use of the KK would be a better choice for STHs and a more useful one from a public health perspective, minimizing the number of false negatives, ensuring treatment of infected individuals and, consequently, minimizing transmission.
Our analysis is subject to several limitations. First, our evaluation of helminths diagnostic test performance was done in the absence of a “gold” standard to estimate the ‘true’ unmeasured infection status and allowing for conditional dependency between the test outcomes. Numerous evaluations of STH diagnostics have relied on a composite reference standard as done in the current study, which is generated by combining the results of the MM, KK and the FEC. However, this approach has been widely criticized [48,49], with the absence of an accepted gold standard being a major obstacle for comparative evaluations of such diagnostic tests. In a recent meta-analysis [44], the authors addressed this problem by using Bayesian latent class analysis, enabling simultaneous estimation of the unknown true prevalence of infection and the sensitivities and specificities of compared diagnostic tests, which was a computational method beyond our means. Another limitation is that we could not evaluate the operational value of using the MM method for the detection of T. trichiura because the prevalence of this STH was low (1.5%) in the study sample. Finally, a fairer comparison between the MM and the KK methods would have been to use a zinc sulphate (ZnSO4(H2O)7 + H2) flotation solution in the MM, which would have enabled the detection of trematode eggs in an MM counting chamber, much like a mini-FLOTAC, which has been positively evaluated for both protozoa and helminth infections in humans, including S. mansoni [38,50,51]. However, the mini- FLOTAC was unavailable to us as the time of our study, and we look forward to a greater availability after it has gone through further validation studies before we can incorporate it into our public health surveys.
Conclusion
Based on the diagnostic sensitivities of the MM and the KK as reported herein, we do not see the value of the extra time, materials, and labor required by the addition of the MM into a diagnostic strategy for STHs in areas where S. mansoni infection is also present, especially resource limited areas such as our study site in Brazil. Initially, we hypothesized that the MM method would improve the sensitivity for the detection of STHs over the use of the KK alone, especially for the diagnosis of hookworm infections. However, as shown here, the inclusion of the MM in our diagnostic workflow did not improve overall sensitivity. Similarly, the MM could not replace the KK in the study area given its lower sensitivity for diagnosing A. lumbricoides and its inability to detect S. mansoni eggs. Nonetheless, when conducting coprological studies in humans, determining which technique to use will depend on the research question, the expected species of interest, the expected prevalence and intensity of each species and finally, the field conditions for implementation—in other words, “finding the right tool for the job”.
Supporting Information
This standard could be used regardless of co-endemnicity.
(TIF)
(DOC)
(DOC)
Acknowledgments
We would like to acknowledge the field team from Americaninhas, including the technicians Adriana Pereira da Silva, Eliane Lopes Conrado and Gisele Alves Batista Nunes, the nurses Luciana Espíndola de Castro and Cássia Ronise Senra Silva and finally all the community members (teacher, parents and students) who made this study possible.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded with Grant#20050-10 from the Sabin Vaccine Institute. The funding was received by RCO, DJD and JMB. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mejia R, Vicuna Y, Broncano N, Sandoval C, Vaca M, et al. (2013) A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg 88: 1041–1047. 10.4269/ajtmh.12-0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verweij JJ (2014) Application of PCR-based methods for diagnosis of intestinal parasitic infections in the clinical laboratory. Parasitology 141: 1863–1872. 10.1017/S0031182014000419 [DOI] [PubMed] [Google Scholar]
- 3. Esteban JG, Munoz-Antoli C, Toledo R, Ash LR (2014) Diagnosis of human trematode infections. Adv Exp Med Biol 766: 293–327. 10.1007/978-1-4939-0915-5_9 [DOI] [PubMed] [Google Scholar]
- 4. Ministry of Agriculture Fisheries and Food (1977) Manual of Veterinary Parasitological Laboratory Techniques. London, UK: Her Majesty’s Stationary Office (HMSO). [Google Scholar]
- 5. Katz N, Chaves A, Pellegrino J (1972) A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14: 397–400. [PubMed] [Google Scholar]
- 6. Komiya Y, Kobayash A (1966) Evaluation of Katos Thick Smear Technic with a Cellophane Cover for Helminth Eggs in Feces. Jpn J Med Sci Biol 19: 59-& [DOI] [PubMed] [Google Scholar]
- 7. Chaves A, de Alcantara OS, Carvalho OS, dos Santos JS (1979) [Comparative study of Lutz, Kato-Katz and modified Faust coprologic methods]. Rev Saude Publica 13: 348–352. [DOI] [PubMed] [Google Scholar]
- 8. Robertson LJ, Crompton DW, Walters DE, Nesheim MC, Sanjur D, et al. (1989) Soil-transmitted helminth infections in school children from Cocle Province, Republic of Panama. Parasitology 99 Pt 2: 287–292. [DOI] [PubMed] [Google Scholar]
- 9. Mangali A, Sasabone P, Syafruddin, Abadi K, Hasegawa H, et al. (1994) Prevalence of intestinal helminthic infections in Kao District, north Halmahera, Indonesia. Southeast Asian J Trop Med Public Health 25: 737–744. [PubMed] [Google Scholar]
- 10. Uga S, Kimura D, Kimura K, Margono SS (2002) Intestinal parasitic infections in Bekasi district, West Java, Indonesia and a comparison of the infection rates determined by different techniques for fecal examination. Southeast Asian J Trop Med Public Health 33: 462–467. [PubMed] [Google Scholar]
- 11. Matthys B, Bobieva M, Karimova G, Mengliboeva Z, Jean-Richard V, et al. (2011) Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasit Vectors 4: 195 10.1186/1756-3305-4-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belizario VY Jr., Liwanag HJ, Naig JR, Chua PL, Madamba MI, et al. (2015) Parasitological and nutritional status of school-age and preschool-age children in four villages in Southern Leyte, Philippines: Lessons for monitoring the outcome of Community-Led Total Sanitation. Acta Trop 141: 16–24. 10.1016/j.actatropica.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 13. Montresor A, Crompton D.W.T., Hall A. Bundy D.A.P. Savioli L. (1998) Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level A guide for managers of control progammes. Geneva: World Health Organization; 1–45 p. [Google Scholar]
- 14. Levecke B, Behnke JM, Ajjampur SS, Albonico M, Ame SM, et al. (2011) A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. PLoS Negl Trop Dis 5: e1201 10.1371/journal.pntd.0001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ash LR, Orihel TC (1991) Parasites: A Guide to Laboratory Procedures and Identification. Chicago, IL, USA: ASCP PRess, American Society for Clinical Pathology; 328 p. [Google Scholar]
- 16. Engels D, Nahimana S, De Vlas SJ, Gryseels B (1997) Variation in weight of stool samples prepared by the Kato-Katz method and its implications. Trop Med Int Health 2: 265–271. [DOI] [PubMed] [Google Scholar]
- 17. Levecke B, Montresor A, Albonico M, Ame SM, Behnke JM, et al. (2014) Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis 8: e3204 10.1371/journal.pntd.0003204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, et al. (2011) Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis 5: e948 10.1371/journal.pntd.0000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pullan RL, Bethony JM, Geiger SM, Correa-Oliveira R, Brooker S, et al. (2010) Human helminth co-infection: no evidence of common genetic control of hookworm and Schistosoma mansoni infection intensity in a Brazilian community. Int J Parasitol 40: 299–306. 10.1016/j.ijpara.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geiger SM, Alexander ND, Fujiwara RT, Brooker S, Cundill B, et al. (2011) Necator americanus and helminth co-infections: further down-modulation of hookworm-specific type 1 immune responses. PLoS Negl Trop Dis 5: e1280 10.1371/journal.pntd.0001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brooker S, Alexander N, Geiger S, Moyeed RA, Stander J, et al. (2006) Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int J Parasitol 36: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jardim-Botelho A, Brooker S, Geiger SM, Fleming F, Souza Lopes AC, et al. (2008) Age patterns in undernutrition and helminth infection in a rural area of Brazil: associations with ascariasis and hookworm. Trop Med Int Health 13: 458–467. 10.1111/j.1365-3156.2008.02022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brooker S, Jardim-Botelho A, Quinnell RJ, Geiger SM, Caldas IR, et al. (2007) Age-related changes in hookworm infection, anaemia and iron deficiency in an area of high Necator americanus hookworm transmission in south-eastern Brazil. Trans R Soc Trop Med Hyg 101: 146–154. [DOI] [PubMed] [Google Scholar]
- 24. Pullan RL, Bethony JM, Geiger SM, Cundill B, Correa-Oliveira R, et al. (2008) Human helminth co-infection: analysis of spatial patterns and risk factors in a Brazilian community. PLoS Negl Trop Dis 2: e352 10.1371/journal.pntd.0000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young KH, Bullock SL, Melvin DM, Spruill CL (1979) Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J Clin Microbiol 10: 852–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WHO Expert Committee on the Control of Schistosomiasis (2001) Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Geneva, Switzerland: WHO Library Cataloguing-in-Publication Data; 57 p. [Google Scholar]
- 27. Goodman D, Haji HJ, Bickle QD, Stoltzfus RJ, Tielsch JM, et al. (2007) A comparison of methods for detecting the eggs of Ascaris, Trichuris, and hookworm in infant stool, and the epidemiology of infection in Zanzibari infants. Am J Trop Med Hyg 76: 725–731. [PubMed] [Google Scholar]
- 28. Glinz D, Silue KD, Knopp S, Lohourignon LK, Yao KP, et al. (2010) Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis 4: e754 10.1371/journal.pntd.0000754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Habtamu K, Degarege A, Ye-Ebiyo Y, Erko B (2011) Comparison of the Kato-Katz and FLOTAC techniques for the diagnosis of soil-transmitted helminth infections. Parasitol Int 60: 398–402. 10.1016/j.parint.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 30. Gualdieri L, Rinaldi L, Petrullo L, Morgoglione ME, Maurelli MP, et al. (2011) Intestinal parasites in immigrants in the city of Naples (southern Italy). Acta Trop 117: 196–201. 10.1016/j.actatropica.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 31. Albonico M, Ame SM, Vercruysse J, Levecke B (2012) Comparison of the Kato-Katz thick smear and McMaster egg counting techniques for monitoring drug efficacy against soil-transmitted helminths in schoolchildren on Pemba Island, Tanzania. Trans R Soc Trop Med Hyg 106: 199–201. 10.1016/j.trstmh.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 32. Barda B, Cajal P, Villagran E, Cimino R, Juarez M, et al. (2014) Mini-FLOTAC, Kato-Katz and McMaster: three methods, one goal; highlights from north Argentina. Parasit Vectors 7: 271 10.1186/1756-3305-7-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knopp S, Rinaldi L, Khamis IS, Stothard JR, Rollinson D, et al. (2009) A single FLOTAC is more sensitive than triplicate Kato-Katz for the diagnosis of low-intensity soil-transmitted helminth infections. Trans R Soc Trop Med Hyg 103: 347–354. 10.1016/j.trstmh.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 34. Ziegelbauer K, Steinmann P, Zhou H, Du ZW, Jiang JY, et al. (2010) Self-rated quality of life and school performance in relation to helminth infections: case study from Yunnan, People's Republic of China. Parasit Vectors 3: 61 10.1186/1756-3305-3-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knopp S, Speich B, Hattendorf J, Rinaldi L, Mohammed KA, et al. (2011) Diagnostic accuracy of Kato-Katz and FLOTAC for assessing anthelmintic drug efficacy. PLoS Negl Trop Dis 5: e1036 10.1371/journal.pntd.0001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inpankaew T, Schar F, Khieu V, Muth S, Dalsgaard A, et al. (2014) Simple fecal flotation is a superior alternative to guadruple Kato Katz smear examination for the detection of hookworm eggs in human stool. PLoS Negl Trop Dis 8: e3313 10.1371/journal.pntd.0003313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sayasone S, Utzinger J, Akkhavong K, Odermatt P (2015) Repeated stool sampling and use of multiple techniques enhance the sensitivity of helminth diagnosis: a cross-sectional survey in southern Lao People's Democratic Republic. Acta Trop 141: 315–321. 10.1016/j.actatropica.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 38. Barda B, Albonico M, Ianniello D, Ame SM, Keiser J, et al. (2015) How Long Can Stool Samples Be Fixed for an Accurate Diagnosis of Soil-Transmitted Helminth Infection Using Mini-FLOTAC? PLoS Negl Trop Dis 9: e0003698 10.1371/journal.pntd.0003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Speich B, Ali SM, Ame SM, Albonico M, Utzinger J, et al. (2015) Quality control in the diagnosis of Trichuris trichiura and Ascaris lumbricoides using the Kato-Katz technique: experience from three randomised controlled trials. Parasit Vectors 8: 82 10.1186/s13071-015-0702-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33: 159–174. [PubMed] [Google Scholar]
- 41. Fleiss JL (1971) Measuring nominal scale agreement among many raters. Psychological Bulletin 76: 378–382. [Google Scholar]
- 42. Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, et al. (2011) Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs Drug Resist 1: 14–27. 10.1016/j.ijpddr.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quinnell RJ, Pullan RL, Breitling LP, Geiger SM, Cundill B, et al. (2010) Genetic and household determinants of predisposition to human hookworm infection in a Brazilian community. J Infect Dis 202: 954–961. 10.1086/655813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikolay B, Brooker SJ, Pullan RL (2014) Sensitivity of diagnostic tests for human soil-transmitted helminth infections: a meta-analysis in the absence of a true gold standard. Int J Parasitol 44: 765–774. 10.1016/j.ijpara.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castilho VLP, Guizelini E, Turri ES, Campos R, Neto VA, et al. (1984) Exame parasitológico quantitativo das fezes: estudo comparativo entre os métodos de McMaster, Stoll-Hausheer e Kato-Katz. Revista da Sociedade Brasileira de Medicina Tropical 17: 209–2012. [Google Scholar]
- 46. David ED, Lindquist WD (1982) Determination of the specific gravity of certain helminth eggs using sucrose density gradient centrifugation. J Parasitol 68: 916–919. [PubMed] [Google Scholar]
- 47.(2013) Molecular Detection of Human Parasitic Pathogens; Liu D, editor. Boca Raton, FL, USA: CRC Press Taylor & Francis Group. [Google Scholar]
- 48. Enoe C, Georgiadis MP, Johnson WO (2000) Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Prev Vet Med 45: 61–81. [DOI] [PubMed] [Google Scholar]
- 49. Ihorst G, Forster J, Petersen G, Werchau H, Rohwedder A, et al. (2007) The use of imperfect diagnostic tests had an impact on prevalence estimation. J Clin Epidemiol 60: 902–910. [DOI] [PubMed] [Google Scholar]
- 50. Barda BD, Rinaldi L, Ianniello D, Zepherine H, Salvo F, et al. (2013) Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl Trop Dis 7: e2344 10.1371/journal.pntd.0002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barda B, Zepherine H, Rinaldi L, Cringoli G, Burioni R, et al. (2013) Mini-FLOTAC and Kato-Katz: helminth eggs watching on the shore of Lake Victoria. Parasit Vectors 6: 220 10.1186/1756-3305-6-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This standard could be used regardless of co-endemnicity.
(TIF)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.