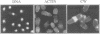Abstract
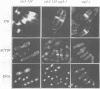
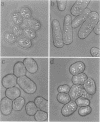
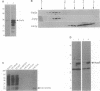
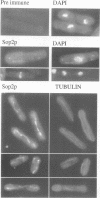
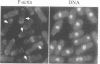
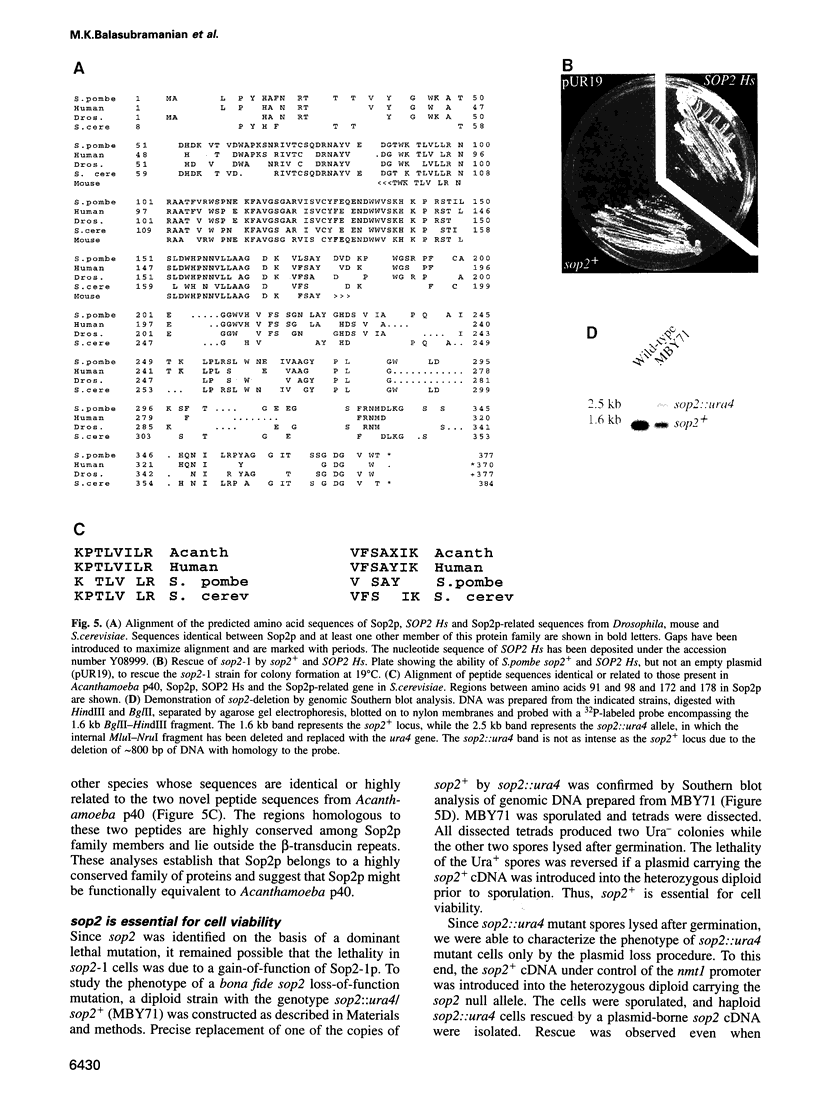
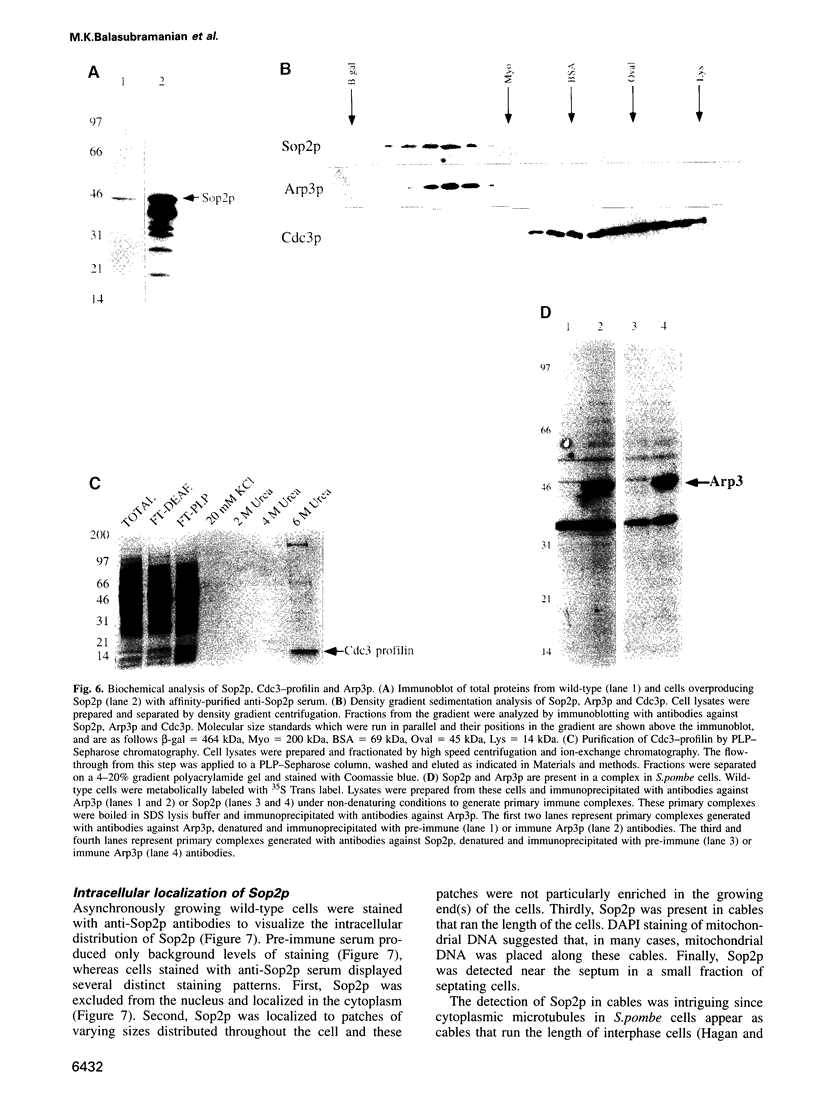
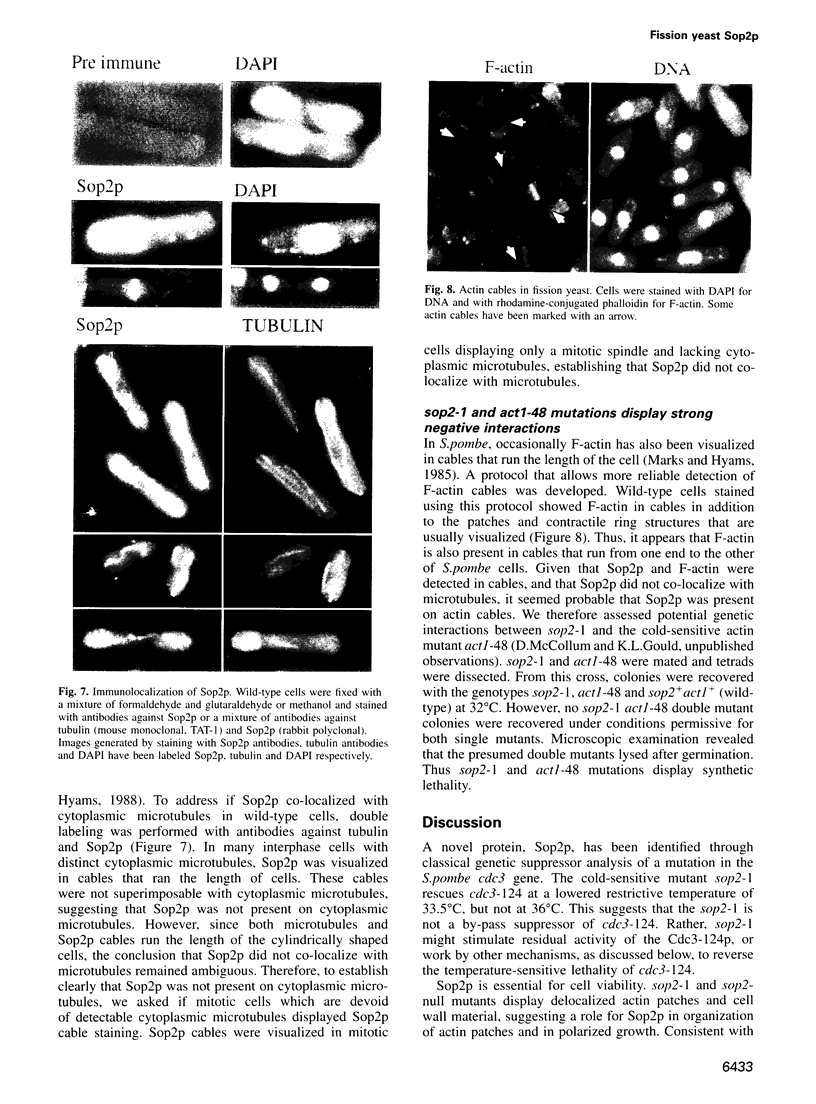
Profilins bind to monomeric actin and also interact with ligands such as phosphoinositide 4,5-bisphosphate, the proline-rich protein VASP and a complex of four to six polypeptides identified in Acanthamoeba that includes two actin-related proteins. Here, we report the identification and characterization of an essential gene from Schizosaccharomyces pombe, sop2+, a mutation in which rescues the temperature-sensitive lethality of a profilin mutation, cdc3-124. The sop2-1 mutant is defective for cell elongation and septation, suggesting that it is involved in multiple cortical actin-requiring processes. Consistent with a role in actin cytoskeletal function, negative interactions have been identified between sop2-1 and act1-48, a mutant allele of actin. Sop2p is a novel 377 amino acid polypeptide with similarity to proteins of the beta-transducin repeat family. Sop2p-related proteins have been identified by sequencing projects in diverse species, and we have isolated a human cDNA highly related to sop2+, SOP2 Hs, which functionally complements the sop2-1 mutation. Sop2p proteins from all species contain peptide sequences identical or highly similar to two peptide sequences from an Acanthamoeba beta-transducin repeat protein present in the profilin binding complex. Biochemical analyses demonstrate that Sop2p is present in a complex which also contains the actin-related protein, Arp3p. Immunofluorescence studies reveal the presence of Sop2p in (i) punctate structures distributed throughout the cell, (ii) cables that extend the length of the cell, and (iii) a medial band in a small percentage of septating cells. Collectively these data demonstrate the interaction of Sop2p with Arp3p, profilin and actin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
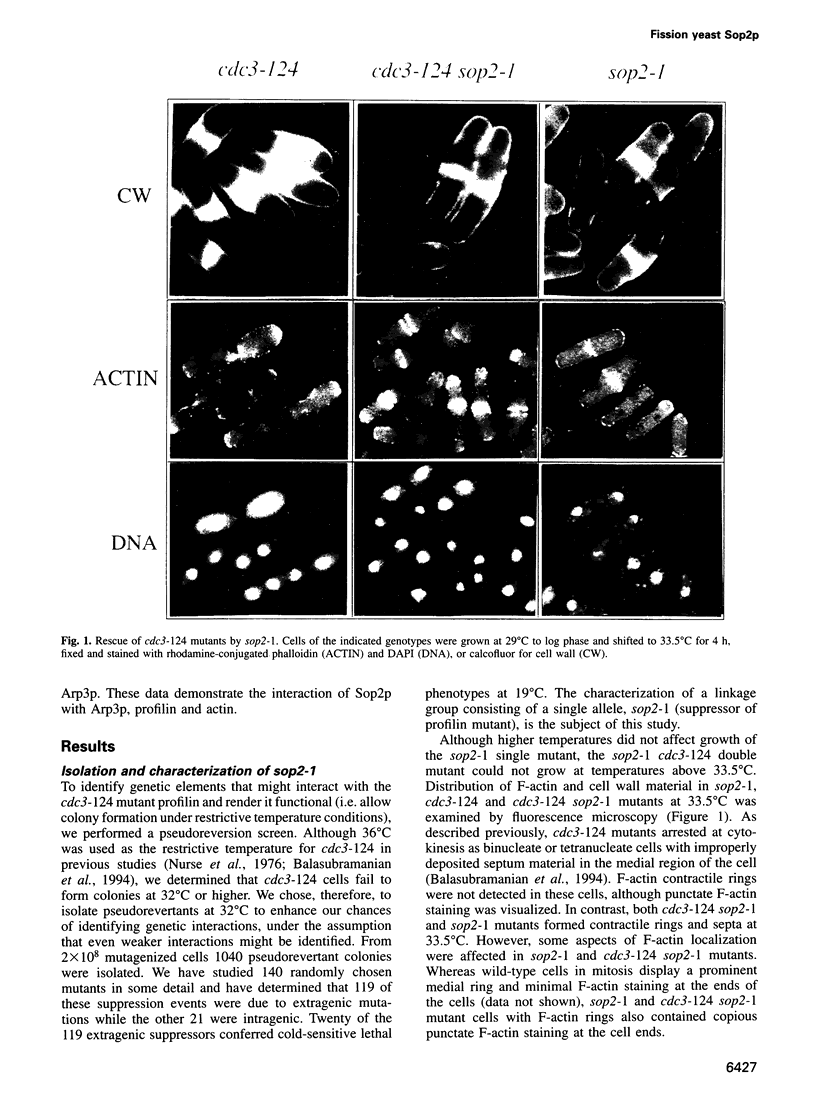
- Balasubramanian M. K., Hirani B. R., Burke J. D., Gould K. L. The Schizosaccharomyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J Cell Biol. 1994 Jun;125(6):1289–1301. doi: 10.1083/jcb.125.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
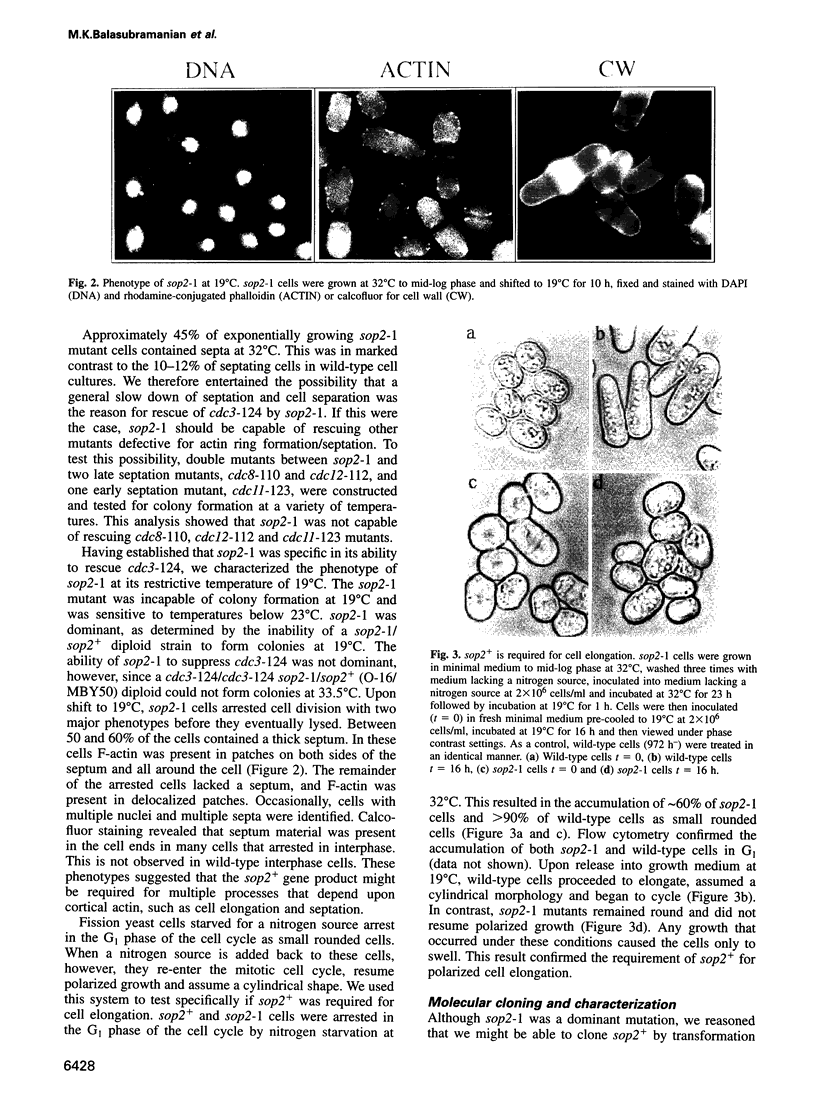
- Barbet N., Muriel W. J., Carr A. M. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene. 1992 May 1;114(1):59–66. doi: 10.1016/0378-1119(92)90707-v. [DOI] [PubMed] [Google Scholar]
- Binette F., Bénard M., Laroche A., Pierron G., Lemieux G., Pallotta D. Cell-specific expression of a profilin gene family. DNA Cell Biol. 1990 Jun;9(5):323–334. doi: 10.1089/dna.1990.9.323. [DOI] [PubMed] [Google Scholar]
- Cooley L., Verheyen E., Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992 Apr 3;69(1):173–184. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Edamatsu M., Hirono M., Watanabe Y. Tetrahymena profilin is localized in the division furrow. J Biochem. 1992 Nov;112(5):637–642. doi: 10.1093/oxfordjournals.jbchem.a123952. [DOI] [PubMed] [Google Scholar]
- Fedorov A. A., Magnus K. A., Graupe M. H., Lattman E. E., Pollard T. D., Almo S. C. X-ray structures of isoforms of the actin-binding protein profilin that differ in their affinity for phosphatidylinositol phosphates. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8636–8640. doi: 10.1073/pnas.91.18.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Becker D. M., Winston F., Guarente L. Striking conservation of TFIID in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Nature. 1990 Jul 19;346(6281):291–294. doi: 10.1038/346291a0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Furman M. I., Wachsstock D., Safer D., Nachmias V. T., Pollard T. D. The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 1992 Sep;3(9):1015–1024. doi: 10.1091/mbc.3.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G., Pollard T. D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991 Mar 8;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Haarer B. K., Lillie S. H., Adams A. E., Magdolen V., Bandlow W., Brown S. S. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol. 1990 Jan;110(1):105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I. M., Hyams J. S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988 Mar;89(Pt 3):343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Haugwitz M., Noegel A. A., Karakesisoglou J., Schleicher M. Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell. 1994 Oct 21;79(2):303–314. doi: 10.1016/0092-8674(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Hoheisel J. D., Maier E., Mott R., McCarthy L., Grigoriev A. V., Schalkwyk L. C., Nizetic D., Francis F., Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. Cell. 1993 Apr 9;73(1):109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Kelleher J. F., Atkinson S. J., Pollard T. D. Sequences, structural models, and cellular localization of the actin-related proteins Arp2 and Arp3 from Acanthamoeba. J Cell Biol. 1995 Oct;131(2):385–397. doi: 10.1083/jcb.131.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Atkinson S. J., Ampe C., Vandekerckhove J., Pollard T. D. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J Cell Biol. 1994 Oct;127(1):107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Poland T. D. Profilin as a potential mediator of membrane-cytoskeleton communication. Trends Cell Biol. 1993 Nov;3(11):381–385. doi: 10.1016/0962-8924(93)90087-h. [DOI] [PubMed] [Google Scholar]
- Markey F., Persson T., Lindberg U. Characterization of platelet extracts before and after stimulation with respect to the possible role of profilactin as microfilament precursor. Cell. 1981 Jan;23(1):145–153. doi: 10.1016/0092-8674(81)90279-8. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993 Jan 15;123(1):127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- McCollum D., Feoktistova A., Morphew M., Balasubramanian M., Gould K. L. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. EMBO J. 1996 Dec 2;15(23):6438–6446. [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Schmidt C. J., Nambudripad R., Smith T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994 Sep 22;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Smith T. F. G protein heterodimers: new structures propel new questions. Cell. 1996 Jan 26;84(2):175–178. doi: 10.1016/s0092-8674(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F. How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell. 1993 Dec 3;75(5):1007–1014. doi: 10.1016/0092-8674(93)90544-z. [DOI] [PubMed] [Google Scholar]
- Prentice H. L. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1992 Feb 11;20(3):621–621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M., Giehl K., Abel K., Haffner C., Jarchau T., Hoppe V., Jockusch B. M., Walter U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995 Apr 18;14(8):1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Shibata H. Poly(L-proline)-binding proteins from chick embryos are a profilin and a profilactin. Eur J Biochem. 1985 Sep 2;151(2):291–297. doi: 10.1111/j.1432-1033.1985.tb09099.x. [DOI] [PubMed] [Google Scholar]
- Verheyen E. M., Cooley L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development. 1994 Apr;120(4):717–728. doi: 10.1242/dev.120.4.717. [DOI] [PubMed] [Google Scholar]
- Vinson V. K., Archer S. J., Lattman E. E., Pollard T. D., Torchia D. A. Three-dimensional solution structure of Acanthamoeba profilin-I. J Cell Biol. 1993 Sep;122(6):1277–1283. doi: 10.1083/jcb.122.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]