Abstract
Intensive chemotherapy for human immunodeficiency virus (HIV)-associated non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) has resulted in durable remissions in a substantial proportion of patients. High-dose chemotherapy and autologous stem cell transplantation (AuSCT), moreover, has resulted in sustained complete remissions in selected patients with recurrent chemosensitive disease. Based on a favorable experience with dose-reduced high-dose busulfan, cyclophosphamide, and AuSCT for older patients with non-HIV–associated aggressive lymphomas, an AIDS Malignancy Consortium multicenter trial was undertaken using the same dose-reduced busulfan and cyclophosphamide preparative regimen with AuSCT for recurrent HIV-associated NHL and HL. Of the 27 patients in the study, 20 received an AuSCT. The median time to achievement of an absolute neutrophil count (ANC) of ≥ 0.5 × 109/L was 11 days (range, 9-16 days). The median time to achievement of an unsupported platelet count of ≥ 20 × 109/L was 13 days (range, 6-57 days). One patient died on day +33 posttransplantation from hepatic veno-occlusive disease (VOD) and multiorgan failure. No other fatal regimen-related toxicity occurred. Ten of 19 patients (53%) were in complete remission at the time of their day +100 post-AuSCT evaluation. Of the 20 patients, 10 were alive and event-free at a median of 23 weeks post-AuSCT. Median overall survival (OS) was not reached by 13 of the 20 patients alive at the time of last follow-up. This multi-institutional trial demonstrates that a regimen of dose-reduced high-dose busulfan, cyclophosphamide, and AuSCT is well tolerated and is associated with favorable disease-free survival (DFS) and OS probabilities for selected patients with HIV-associated NHL and HL.
Keywords: Acquired immunodeficiency syndrome, Autologous stem cell transplantation, Busulfan, Cyclophosphamide, Lymphoma
Introduction
High-dose chemotherapy and autologous stem cell transplantation (AuSCT) is the treatment of choice for relapsed, chemotherapy-sensitive, and aggressive or highly aggressive non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) [1,2]. The prospective randomized Parma trial found improved survival outcomes in patients with recurrent NHL who received an autologous bone marrow transplant (BMT) compared with those who received additional conventional salvage chemotherapy [1]. Two other prospective randomized trials also demonstrated improvements in progression-free survival (PFS) for patients with recurrent HL who received AuSCT compared with those who received chemotherapy alone [3,4].
NHL occurring in association with human immunodeficiency virus (HIV) infection usually has an aggressive or highly aggressive histology [5-7]. Historically, patients with HIV-associated NHL have fared poorly in terms of both insufficient control of lymphoma and substantial comorbidity compromising their ability to receive optimal chemotherapy. With the advent of highly active antiretroviral therapy (HAART) and the increasing diagnosis of HIV-associated NHL in patients without other AIDS-defining illnesses, more aggressive antilymphoma treatment, including AuSCT for patients in relapse, has been evaluated [8-12]. Selected patients with a relatively low HIV RNA viral load (HIV VL) but no history of recent opportunistic infections may achieve durable disease control after either primary therapy or AuSCT for recurrent disease [13-16].
Although HL is not an AIDS-defining malignancy, the incidence is increased in patients with HIV infection [11,17]. Durable PFS is also possible with aggressive multiagent chemotherapy for HIV-associated HL. Less well studied is the role of AuSCT for treating recurrent HIV-associated HL [13].
The optimal preparative therapy for AuSCT for NHL and HL is uncertain. Given the likely increased risk of transplantation-related complications in older patients and patients with significant comorbidity, such as HIV infection, regimens have been designed to minimize regimen-related toxicity (RRT), whereas still delivering adequate cytoreductive therapy. In an effort to reduce RRT for AuSCT and expand autologous transplantation applications to older patients than have historically been treated, we designed a dose-reduced busulfan (BU) and cyclophosphamide (CY) preparative regimen for AuSCT. This regimen consisted of the administration of 14 mg/kg of oral or, more recently, 11.2 mg/kg of intravenous (IV) BU with 120 mg/kg of CY for patients age 50 years and older [18]. This regimen has been well tolerated in patients up to age 78, and favorable PFS and overall survival (OS) outcomes have been observed compared with patients under age 50 who received 16 mg/kg of oral BU. Based on this encouraging experience, we designed a pilot evaluation of dose-reduced BU and CY as preparative therapy for AuSCT for treating HIV-associated NHL and HL.
Patients and Methods
Twenty-seven patients with HIV-associated NHL or HL were enrolled in a multicenter AIDS Malignancy Consortium (AMC) trial sponsored by the National Cancer Institute. Institutional review board approval was obtained at each participating institution for the conduct of the trial. Written informed consent was obtained from all patients.
Eligibility criteria for transplantation included HIV-1 infection confirmed by Western blot or a detectable HIV VL, a CD4+ count > 50 cells/μL, an HIV VL < 110,000 copies/mL, a diagnosis intermediate or high-grade NHL or HL, and failure to achieve complete remission (CR) with initial therapy or relapse after initial therapy. Patients were required to have a Karnofsky performance status > 60% and to have lymphoma that was sensitive to their most recent chemotherapy regimen, as defined by an improvement in bidimensional tumor measurements of at least 25% or an improvement in evaluable disease sustained over 4 weeks. Exclusion criteria included the presence of an active opportunistic infection, serum creatinine of > 2.0 mg/dL and significant organ dysfunction (eg, left ventricular ejection fraction < 45% or diffusing capacity of the lung for carbon monoxide < 60% of predicted), AST > 3 times the upper limits of normal, bilirubin > 2.0 mg/dL (unless the patient's hyperbilirubinemia was ascribed to antiretroviral therapy), and active leptomeningeal or parenchymal central nervous system (CNS) lymphoma. The patient's demographic profiles and baseline HIV disease characteristics, including CD4+ cell count, HIV VL, and lymphoma histopathology, are given in Table 1. Individual patient characteristics, including International Prognostic Index (IPI), number of previous chemotherapy regimens, and remission status at transplantation, are given in Table 2. Fifteen patients had NHL; 12 had diffuse large B cell, B cell anaplastic, or immunoblastic lymphoma; and 3 had Burkitt or atypical Burkitt lymphoma, according to the Revised European-American Lymphoma–World Health Organization classification of NHL [19]. Five patients had HL, 3 of whom had mixed cellularity histology. All of the patients who underwent transplantation were male.
Table 1. Patient and baseline disease characteristics.
| Characteristic | n |
|---|---|
| Patients receiving AuSCT | 20 |
| Male:female ratio | 20:0 |
| Race/ethnicity | |
| White | 14 |
| Black | 2 |
| Hispanic | 4 |
| Median age, years (range) | 42 (33-60) |
| CD4+cell count, cells/μL, baseline median (range) | 203 (53-574) |
| HIV viral load, copies/mL, baseline median (range) | 317 (50-5712) |
| Lymphoma histology | |
| NHL | 15 |
| DLBCL | 8 |
| Immunoblastic | 3 |
| Anaplastic B cell | 1 |
| Burkitt/atypical Burkitt | 3 |
| HL | 5 |
| Mixed cellularity | 3 |
| Nodular sclerosis | 1 |
| Not specified | 1 |
DLBCL, diffuse large B cell lymphoma.
Table 2. Baseline patient characteristics.
| Patient | Age, years | Disease | IPI score | Previous chemotherapy regimens | Remission status at transplantation |
|---|---|---|---|---|---|
| 1 | 41 | HD | NA | 3 | CR |
| 2 | 36 | SNCL | 3 | 3 | CR |
| 3 | 34 | HD | NA | 2 | CS |
| 4 | 47 | DLBCL | 1 | 2 | PR |
| 5 | 51 | DLBCL | 2 | 2 | PR |
| 6 | 43 | SNCL | 2 | 3 | CS |
| 7 | 43 | DLBCL | 2 | 2 | CS |
| 8 | 38 | DLBCL | 1 | 2 | CS |
| 9 | 43 | SNCL | 1 | 3 | CS |
| 10 | 50 | B cell immunoblastic | 1 | 3 | CS |
| 11 | 36 | B cell immunoblastic | 3 | 2 | CS |
| 12 | 64 | HD | NA | 3 | CS |
| 13 | 42 | Anaplastic B cell | 4 | 2 | CS |
| 14 | 37 | DLBCL | 3 | 2 | CS |
| 15 | 39 | DLBCL | 1 | 3 | CR |
| 16 | 44 | DLBCL | 1 | 2 | CR |
| 17 | 36 | B cell immunoblastic | 2 | 2 | CS |
| 18 | 33 | HD | NA | 2 | CR |
| 19 | 37 | HD | NA | 2 | CR |
| 20 | 55 | DLBCL | 0 | 2 | CS |
IPI indicates International Prognostic Index; CS, chemotherapy sensitive (as defined in Patients and Methods); DLBCL, diffuse large B cell lymphoma; SNCL, small noncleaved cell (Burkitt/atypical Burkitt) lymphoma; NA, not applicable.
Stem Cell Mobilization
For stem cell mobilization, patients received CY 3000 mg/m2 with hydration and mesna 15 mg/kg before and 3, 6, and 9 hours after CY for uroprotection, or an alternative salvage chemotherapy to which the lymphoma was responding. Granulocyte colony-stimulating factor (G-CSF) was administered at a dose of 10 mg/kg/day beginning 2 days after chemotherapy. Once the white blood count (WBC) was ≥ 0.5 × 109/L, leukapheresis was performed a maximum of 4 times until an intended number of 2 × 106CD34+ cells/kg were collected.
Transplantation Preparative Therapy
Four patients received oral BU at a dose of 1 mg/kg every 6 hours for a total of 14 doses, and 16 patients received IV BU at a dose of 0.8 mg/kg every 6 hours for a total of 14 doses. Phenytoin 400 mg daily was given prophylactically to prevent seizures. CY 60 mg/kg/day was given i.v. on transplantation days −3 and −2. Mesna 15 mg/kg 15 minutes before and 3, 6, and 9 hours after CY was given on days −3 and −2. For patients > 20% above their ideal weight, both the BU and CY doses were calculated based on a weight 50% between the patient's actual weight and ideal body weight.
Stem Cell Infusion
Autologous peripheral stem cells were thawed in a 37°C water bath and infused through the patient's central venous catheter on transplantation day 0. A median infusion of 4.62 × 106/kg CD34+ cells (range, 1.53-6.34 × 106/kg) was administered.
Concurrent Therapies
CNS Prophylaxis. Patients with NHL received a single 50-mg dose of intrathecal cytosine arabinoside before conditioning at the time of diagnostic lumbar puncture.
Anti-Infection Prophylaxis. All patients received tri-methoprim-sulfamethoxazole (TMP-SMX)-DS (double strength) every other day during the preparative therapy. TMP-SMX-DS was held from the day of stem cell infusion (day 0) until engraftment. Patients who were allergic to or intolerant of TMP-SMX received alternative Pneumocystis jiroveci pneumonia (PCP) prophylaxis with dapsone or atovaquone. PCP prophylaxis was continued for a minimum of 3 months and was discontinued only if the CD4+ count exceeded 200 cells/μL. Fluconazole 200 mg daily (or another systemic antifungal agent of the investigator's choice) was begun during preparative therapy and was continued for up to 6 months posttransplantation.
Antiretroviral therapy, including a combination of 2 nucleoside analogs and 1 or 2 protease inhibitors, was continued (or initiated before stem cell harvesting) as tolerated. Ritonavir was not incorporated into the HAART regimen because of its unpredictable hepatic p450 effects on drug metabolism, and zidovudine was not incorporated into the HAART regimen because of its potential myelosuppressive effects.
All blood products were leukocyte-reduced using third-generation leukocyte filters, and irradiated (2500 cGy) from a 137/Cs irradiator to prevent transfusion-associated graft-versus-host disease (GVHD). G-CSF was given at a dose of 5 mg/kg i.v. or subcutaneously, beginning on day +1 and was continued until the WBC count was ≥ 10 × 109/L.
Evaluation of Response
All patients were assessed by physical examination and repeat imaging studies for response at approximately 3 months posttransplantation, then every 3 months during the first year and every 6 months during the second year. Response criteria were according to the established guidelines for lymphoma response evaluation [20].
Measurement of CD4+ Cell Levels and HIV VL
CD4+ cell counts and HIV VL evaluation were scheduled for days +14 and +21 and months 1, 2, 3, 6, 9, and 12 post-AuSCT. The assays were performed at each participating institution.
Statistical Methods
This study was designed to assess the safety and tolerability of intensive chemotherapy and AuSCT for the treatment of HIV-related NHL and HL. A minimum of 4 and a maximum of 25 patients were to be treated on this protocol The study would have been stopped had the number of transplantation-related deaths exceeded the number expected with an underlying mortality rate from transplantation of 20%. All deaths occurring within 30 days of transplantation were considered transplantation-related.
Summary statistics were used to describe the demographic features and baseline characteristics of the study population. The binomial proportion and its 95% confidence interval were used to estimate the response at 100 days posttransplantation. The Kaplan-Meier method was used to describe the distribution of OS and event-free survival.
Results
Of the 27 patients enrolled in the study, 20 received an AuSCT. Seven patients did not receive an AuSCT, because of inadequate stem cell collection (n = 2), early disease progression (n = 4), or cardiac dysfunction (n = 1).
Engraftment
The median time to achievement of an absolute neutrophil count (ANC) ≥ 0.5 × 109/L was 11 days (range, 9-16 days). Among the 18 evaluable patients (1 patient never achieved a platelet count of > 20 × 109/L, and 1 had not recovered his platelet count by the time of his death on day +33), the median time to achievement of an unsupported platelet count of ≥ 20× 109/L was 13 days (range, 6-57 days).
Toxicities
One patient developed rapidly rising transaminase levels post-AuSCT. A liver biopsy on day +12 showed hepatic veno-occlusive disease (VOD). On day +14, defibrotide was initiated, but progressive multiorgan failure ensued. Despite aggressive medical care, including intubation and mechanical ventilation, he expired on day + 33 post-AuSCT.
No other fatal regimen-related toxicity occurred. No patient developed grade III or IV nausea or vomiting. Five patients developed oropharyngeal mucositis (4 grade I-II, 1 grade III). Six patients experienced febrile neutropenia. Four patients had a documented bacteremia ≤ 100 days post-AuSCT (3 coagulase-negative staphylococcus, 1 pleomorphic Gram-negative rod). Other infections occurring within 100 days post-AuSCT included Clostridium difficile colitis (n = 3), cytomegalovirus (CMV) infection (n = 4), herpes simplex virus (HSV) infection (n = 1) and Enterococcus faecalis urinary tract infection (n = 1). Other nonhematologic grade III-IV toxicities (≤ 100 days post-AuSCT) are listed in Table 3.
Table 3. Nonhematologic grade III-IV toxicities (≤ 100 days post-AuSCT).
| Toxicity | n |
|---|---|
| Stomatitis/pharyngitis | 1 |
| Febrile neutropenia | 6 |
| Documented infection* | 12 |
| Metabolic† | 5 |
| Headache/other pain | 3 |
| Thromboembolism | 1 |
| Thrombotic microangiopathy | 1 |
| Hemorrhage | 4 |
| Mental status change | 2 |
Bacteremia (n = 4), Clostridium difficile colitis (n = 3), herpes simplex skin infection (n = 1), cytomegalovirus infection (n = 3), Enterococcus faecalis urinary tract infection (n = 1).
Hyperuricemia (n = 1), hyperkalemia (n = 1), hypermagnesemia (n = 1), hypophosphatemia (n = 1), other (n = 1).
Response Rates and Survival
Of the 20 patients who received an AuSCT, only 1 early transplantation-related death occurred (from hepatic VOD on day +33 post-AuSCT). Ten of the 19 evaluable patients (53%) were in CR at the time of their day + 100 staging evaluation. Because only 3 patients with HL were evaluable for response (1 patient in CR), it was not possible to analyze the HL and NHL patients separately with respect to antitumor efficacy. Nine patients exhibited progression of disease by day + 100 post-AuSCT. Of the 20 patients, 10 were alive and event-free at a median of 23 weeks post-AuSCT (range, 4-120+ weeks) (Figure 1). Median OS had not been reached, with 13 of 20 patients alive at the time of last follow-up (Figure 1). Event-free and OS probabilities at 6 months posttransplantation are 49.5% and 74.4%, respectively.
Figure 1.
Event-free survival (EFS) and OS (median OS not yet reached) at a median of 23 weeks post-AuSCT.
CD4+ Cell Counts and HIV VL after ASCT
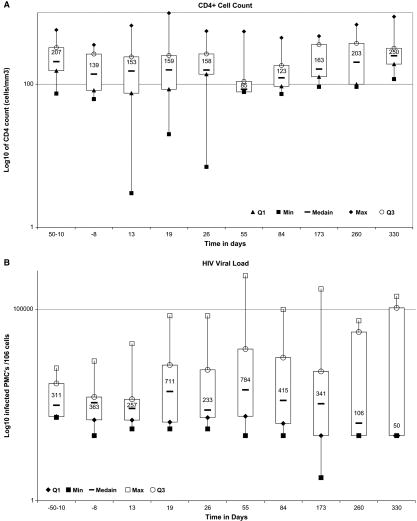
No consistent temporal trend can be seen with respect to the available CD4+ counts and HIV VLs, and significant interpatient variability exists with respect to these measures (Figure 2). Thus, the conditioning regimen did not appear to have a consistent effect on these measures of HIV activity. The variability in these measures may reflect the use of different HAART regimens based on the investigator's discretion (ie, not mandated by the protocol) and that there was substantial variability in how well these regimens suppressed HIV viremia.
Figure 2.
Distribution levels of CD4 counts (A) and HIV RNA copies/mL (B) by time interval in 20 patients who received AuSCT for recurrent HIV-associated NHL or HL. The upper and lower bars of each box plot represent the maximum and minimum values, respectively. The horizontal bar at the center of each boxplot represents the median values, and the top (Q3) and bottom (Q1) of each box plot represent the 75th and 25th percentiles, respectively.
Discussion
The indications for AuSCT for treating HIV-infected patients with relapsed NHL and HL but with HAART options and no active opportunistic infection are becoming increasingly clear. The present multi-institutional clinical trial demonstrates that AuSCT in this patent population is safe and is associated with potent antitumor effects. Several pilot studies using high-dose chemotherapy followed by AuSCT for recurrent or refractory NHL or HL also have evaluated the safety and efficacy of AuSCT for HIV-associated lymphoma (Table 4). Krishnan et al. [13] from City of Hope described 20 patients who received AuSCT for recurrent or refractory HIV-associated NHL or HL. Conditioning therapy consisted of CY, carmustine, and etoposide, or, for patients under age 55 without previous radiation therapy, total body irradiation (TBI), CY, and etoposide. The PFS and OS probabilities were 85% at a median posttransplantation follow-up of 31.8 months. Gabarre et al. [15] described the outcomes after AuSCT in 14 patients with HIV-associated NHL or HL. Five patients were alive, 4 of whom were disease-free at 14-49 months posttransplantation. No transplantation-related deaths occurred. Re et al. [16] treated 16 patients with HIV-associated NHL or HL with high-dose BCNU, etoposide, cytosine arabinoside, and melphalan (BEAM) chemotherapy and AuSCT. No transplantation-related deaths occurred. Six patients were alive and disease-free at a median of 8 months posttransplantation. Serrano et al. [14] described the outcomes of 11 patients with HIV-associated lymphoma who received high-dose chemotherapy and AuSCT. Eight patients were alive in CR at a median of 30 months after AuSCT. CD4+ cell count reconstitution while patients continued to receive HAART was well demonstrated, and there was no clear evidence of worsening underlying HIV infection. Moreover, in vitro studies of immune reconstitution by Benicchi et al. [21], including analysis of interleukin-7 levels, T cell subsets, and T cell V-beta repertoires, in 4 patients who underwent AuSCT demonstrated no worsening of immune deficits in this patient population.
Table 4. Clinical trials of AuSCT for HIV-asssociated lymphoma.
| Investigator | Disease (patients receiving AuSCT) | Preparative regimen | PBSC, median number (CD34+cells/kg) | ANC > 0.5, days (range) | TRM | PFS/EFS* | OS* |
|---|---|---|---|---|---|---|---|
| Krishnan et al. [13] | NHL (n=18) | CBV (n=17) | 10.6 × 106 | 11 (9-23) | 1/20 (5%) | 85% at 32 months | 85% at 32 months |
| HL (n = 2) | fTBI/CY/VP-16 (n=3) | ||||||
| Serrano et al. [14] | NHL (n=7) | BEAM (n=10) | 4.7 × 106 | 16 (9-33) | 0/11 (0%) | 65% at 30 months | 65% at 30 months |
| HL (n=4) | BEAC (n=1) | ||||||
| Gabarre et al. [15] | NHL (n=8) | BEAM (n=5) | 5.8 × 106 | 12 (7-14) | 0/14 (0%) | 4/14 in CCR at 26 months | 5/14 at 32 months |
| HL (n=6) | TBI/CY (n=5) | ||||||
| Other (n=4) | |||||||
| Re etal. [16] | NHL (n=8) | BEAM | 6.8 × 106 | 10 (8-10) | 0/16 (0%) | 6/10 in CCR at 8 months | 6/10 at 8 months |
| HL (n=8) |
BEAC indicates BCNU, etoposide, cytarabine; cyclophosphamide; fTBI, fractionated total body irradiation; CBV, cyclophosphamide, BCNU, etoposide (VP-16); PBSC, peripheral blood stem cells; TRM, transplantation-related mortality; CCR, continuous complete remission.
PFS/OS of patients receiving AuSCT (median follow-up)
High-dose BU and CY as preparative therapy for AuSCT has been most widely evaluated in the treatment of myeloid malignancies [22,23]. Originally administered in its oral form, BU has highly variable absorption and pharmacokinetics, making the severity of RRT difficult to predict. With pharmacokinetic analyses designed to limit exposure to excessively high drug concentrations, a decreased incidence of severe RRT has been observed [24]. Moreover, achieving targeted area under the curve (AVC) drug concentration has been shown to correlate with a reduced incidence of recurrence of some hematologic malignancies (eg, chronic myeloid leukemia) [25]. IV BU is associated with a much more narrow and predictable pharmacokinetic profile and has been associated with a lower incidence of hepatic VOD and other severe RRTs compared with preparative regimens using oral BU [26-29]. BU and CY as preparative therapy for AuSCT for NHL and HL is an active (albeit not as well-studied) regimen [30-32]. There are no convincing data to support a correlation between BU levels and toxicity or freedom from relapse in this population.
The choice of reduced-dose BU and CY as preparative therapy for AuSCT was based on a favorable experience with this regimen in older patients with non-HIV–related malignancies undergoing transplantation [18]. The apparent lack of need for monitoring BU levels when the oral dose was reduced to 14 mg/kg (or the i.v. dose was reduced to 11.2 mg/kg) in this population, as well as the apparent preservation of a potent antilymphoma effect with this regimen, suggest that this was a reasonable regimen to study in patients with relapsed HIV-related lymphoma. This AIDS Malignancy Consortium–coordinated trial demonstrates that this regimen is feasible and remarkably well tolerated by patients in a multicenter setting. Comparable PFS probabilities to those reported by Serrano et al. [14], Gabarre et al. [15], and Re et al. [16] demonstrate that the dose-adjusted BU and Cy preparative regimen is an effective strategy in the management of relapsed HIV-associated lymphoma. The apparently superior survival outcomes reported by Krishnan et al. [13] may reflect a different patient population in terms of disease status, previous treatment, HAART regimen, or other factors. Given the similar survival outcomes in our study to those of the other transplantation studies, it is not clear that the preparative regimen is a determining factor in terms of relapse probability and survival.
The fact that only 20 of the 27 patients enrolled in this trial received an AuSCT (because in 4 cases of early disease progression) underscores the tenuous remission status of many patients with HIV-associated lymphoma and highlights the importance of carefully selecting patients whose HIV infection is under reasonable control and whose disease is responding to salvage therapy. The exclusion of patients primarily from early disease progression also was noted by Re et al. [16] and Serrano et al. [14]; only 10 of 16 (63%) and 11 of 14 (79%) patients, respectively, in those 2 series received an AuSCT. These statistics also likely underestimate the number of patients who would not receive a transplant if the trials enrolled patients at the time of relapse and before salvage therapy.
Dose-reduced BU and CY and AuSCT for HIV-associated lymphoma is associated with an acceptable safety profile. Among the 20 patients who received an AuSCT, only 1 death (5%) was attributable to the regimen (hepatic VOD and multiorgan failure). Aside from the expected grade IV hematologic toxicities and febrile neutropenia in 6 of the 20 patients (30%), only 4 other instances of grade IV toxicity occurred (Table 2). Immune reconstitution in this population of relatively older individuals (median age 42; range, up to 60 years) also was favorable. No deaths attributable to infection occurred. CD4+ cell counts reached a nadir at approximately 2 months posttransplantation, then recovered to baseline levels (on average in the group as a whole) by day +260.
The antitumor efficacy of the BU and CY regimen was well demonstrated. Ten of the 20 patients were in CR at a median of 23 weeks (range, 4-120+ weeks) posttransplantation. The event-free and OS probabilities of 49.5% and 74.4% at 6 months are similar to those in patients receiving AuSCT for recurrent non-HIV–associated, chemosensitive, aggressive NHL or HL [1,2,33,34]. Based on the small number of patients in this and other published series and the lack of data from a prospective randomized trial, a conclusion cannot be reached regarding the optimal preparative regimen for AuSCT. Nonetheless, dose-reduced BU and CY is clearly well tolerated and has substantial antitumor activity in patients with HIV-associated aggressive NHL and HL, and should be further evaluated in a larger prospective clinical trial. Larger studies would also provide more insight into various parameters that are likely important in influencing patient outcome, such as type of HAART, CD4+ cell count and HIV VL before AuSCT, Epstein-Barr virus status, and tumor histology.
Acknowledgments
This work was supported by the following AIDS Malignancy Consortium grants from the National Cancer Institute: U01CA071375, U01CA070062, U01CA083035, U01CA070054, U01CA070047, U01CA083118, U01CA083216, and U01CA070019. We thank our patients for participating in this clinical trial and Dr Christine Colby for her helpful assistance in preparing the manuscript.
References
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;323:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Lin TS, Copelan EA. Autologous stem cell transplantation for non-Hodgkin's lymphoma. Curr Hematol Rep. 2003;2:310–315. [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 4.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 5.Navarro WH, Kaplan LD. AIDS-related lymphoproliferative disease. Blood. 2006;107:13–20. doi: 10.1182/blood-2004-11-4278. [DOI] [PubMed] [Google Scholar]
- 6.Clayton A, Mughal T. The changing face of HIV-associated lymphoma: what can we learn about optimal therapy in the post–highly active antiretroviral therapy era? Hematol Oncol. 2004;22:111–120. doi: 10.1002/hon.735. [DOI] [PubMed] [Google Scholar]
- 7.Bower M, Gazzard B, Mandalia S, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann Intern Med. 2005;143:265–273. doi: 10.7326/0003-4819-143-4-200508160-00007. [DOI] [PubMed] [Google Scholar]
- 8.Mounier N, Spina M, Gabarre J, et al. AIDS-related non-Hodgkin's lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107:3832–3840. doi: 10.1182/blood-2005-09-3600. [DOI] [PubMed] [Google Scholar]
- 9.Spina M, Gabarre J, Rossi G, et al. Stanford V regimen and concomitant HAART in 59 patients with Hodgkin disease and HIV infection. Blood. 2002;100:1984–1988. doi: 10.1182/blood-2002-03-0989. [DOI] [PubMed] [Google Scholar]
- 10.Weiss R, Mitrou P, Arasteh K, et al. Acquired immunodeficiency syndrome-related lymphoma: simultaneous treatment with combined cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy and highly active antiretroviral therapy is safe and improves survival. Results of the German Multicenter Trial. Cancer. 2006;106:1560–1568. doi: 10.1002/cncr.21759. [DOI] [PubMed] [Google Scholar]
- 11.Lim ST, Levine AM. Non-AIDS–defining cancers and HIV infection. Curr HIV/AIDS Rep. 2005;2:146–153. doi: 10.1007/s11904-005-0008-4. [DOI] [PubMed] [Google Scholar]
- 12.Straus DJ. HIV-associated lymphoma: promising new results, but with toxicity. Blood. 2005;105:1842. doi: 10.1182/blood-2004-12-4729. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan A, Molina A, Zaia J, et al. Durable remissions with autologous stem cell transplantation for high-risk HIV-associated lymphomas. Blood. 2005;105:874–878. doi: 10.1182/blood-2004-04-1532. [DOI] [PubMed] [Google Scholar]
- 14.Serrano D, Carrion R, Balsalobre P, et al. HIV-associated lymphoma successfully treated with peripheral blood stem cell transplantation. Exp Hematol. 2005;33:487–494. doi: 10.1016/j.exphem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Gabarre J, Marcelin AG, Azar N, et al. High-dose therapy plus autologous hematopoietic stem cell transplantation for human immunodeficiency virus (HIV)-related lymphoma: results and impact on HIV disease. Haematologica. 2004;89:1100–1108. [PubMed] [Google Scholar]
- 16.Re A, Cattaneo C, Michieli M, et al. High-dose therapy and autologous peripheral blood stem cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J Clin Oncol. 2003;21:4423–4427. doi: 10.1200/JCO.2003.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Wood C, Harrington W., Jr AIDS and associated malignancies. Cell Res. 2005;15:947–952. doi: 10.1038/sj.cr.7290372. [DOI] [PubMed] [Google Scholar]
- 18.Spitzer TR, Cox B, Sepe P, et al. Dose adjusted busulfan/cyclophosphamide and autologous stem cell transplantation for recurrent lymphoma. Blood. 2004;104:522a. [Google Scholar]
- 19.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. J Clin Oncol; Report of the Clinical Advisory Committee meeting; Airlie House, Virginia. November 1997; 1999. pp. 3835–3849. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Benicchi T, Ghidini C, Re A, et al. T-cell immune reconstitution after hematopoietic stem cell transplantation for HIV-associated lymphoma. Transplantation. 2005;80:673–682. doi: 10.1097/01.tp.0000168490.29862.b8. [DOI] [PubMed] [Google Scholar]
- 22.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 23.Santos GW. The development of busulfan/cyclophosphamide preparative regimens. Semin Oncol. 1993;20:12–17. [PubMed] [Google Scholar]
- 24.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25:55–61. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 25.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–3060. [PubMed] [Google Scholar]
- 26.Olavarria E, Hassan M, Eades A, et al. A phase I/II study of multiple-dose intravenous busulfan as myeloablation prior to stem cell transplantation. Leukemia. 2000;14:1954–1959. doi: 10.1038/sj.leu.2401921. [DOI] [PubMed] [Google Scholar]
- 27.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Choi SJ, Kim SE, et al. Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol. 2005;84:321–330. doi: 10.1007/s00277-004-0982-4. [DOI] [PubMed] [Google Scholar]
- 29.Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6:548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 30.Kiss TL, Panzarella T, Messner HA, et al. Busulfan and cyclophosphamide as a preparative regimen for allogeneic blood and marrow transplantation in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2003;31:73–78. doi: 10.1038/sj.bmt.1703790. [DOI] [PubMed] [Google Scholar]
- 31.de Magalhaes-Silverman M, Lister J, Rybka W, et al. Busulfan and cyclophosphamide (BU/CY2) as preparative regimen for patients with lymphoma. Bone Marrow Transplant. 1997;19:777–781. doi: 10.1038/sj.bmt.1700733. [DOI] [PubMed] [Google Scholar]
- 32.Avalos BR, Klein JL, Kapoor N, et al. Preparation for marrow transplantation in Hodgkin's and non-Hodgkin's lymphoma using Bu/CY. Bone Marrow Transplant. 1993;12:133–138. [PubMed] [Google Scholar]
- 33.Vose JM, Rizzo DJ, Tao-Wu J, et al. Autologous transplantation for diffuse aggressive non-Hodgkin lymphoma in first relapse or second remission. Biol Blood Marrow Transplant. 2004;10:116–127. doi: 10.1016/j.bbmt.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin's disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]