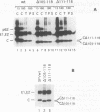
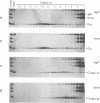
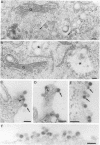
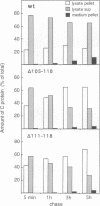
Abstract
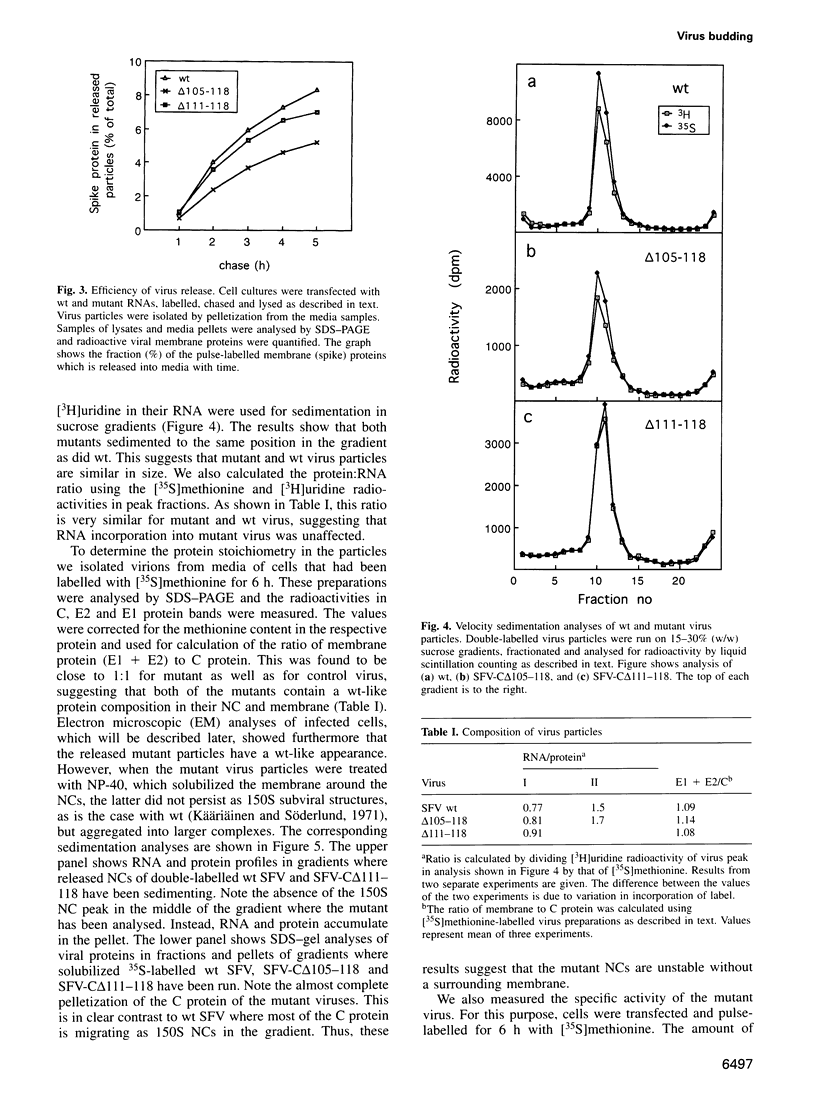
According to the present model for assembly of alphaviruses, e.g. Semliki Forest virus (SFV), the viral genome is first encapsidated into a nucleocapsid (NC) in cytoplasm and this is then used for budding at plasma membrane (PM). The preformed NC is thought to act as a template on which the viral envelope can be organized. In the present work we have characterized two SFV deletion mutants which did not assemble NCs in the cytoplasm but which instead appeared to form NCs at the PM simultaneously with virus budding. The deletions were introduced in a conserved 14 residue long linker peptide that joins the amino-terminal RNA-binding domain with the carboxy-terminal serine-protease domain of the capsid protein. Despite the deletions and the change in morphogenesis, wild-type (wt)-like particles were produced with almost wt efficiency. It is suggested that the NC assembly defect of the mutants is rescued through spike-capsid interactions at PM. The results show that the preassembly of NCs in the cytoplasm is not a prerequisite for alphavirus budding. The apparent similarities of the morphogenesis pathways of wt and mutant SFV with those of type D and type C retroviruses are discussed.
Full text
PDF

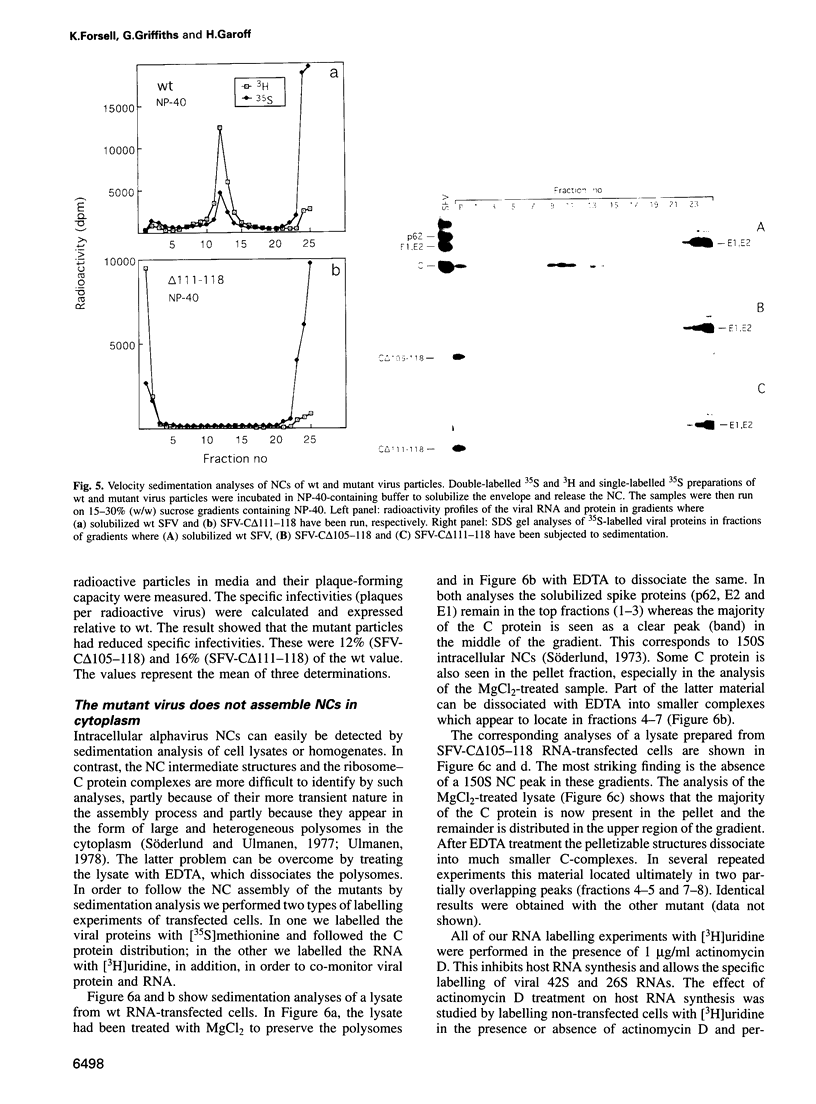
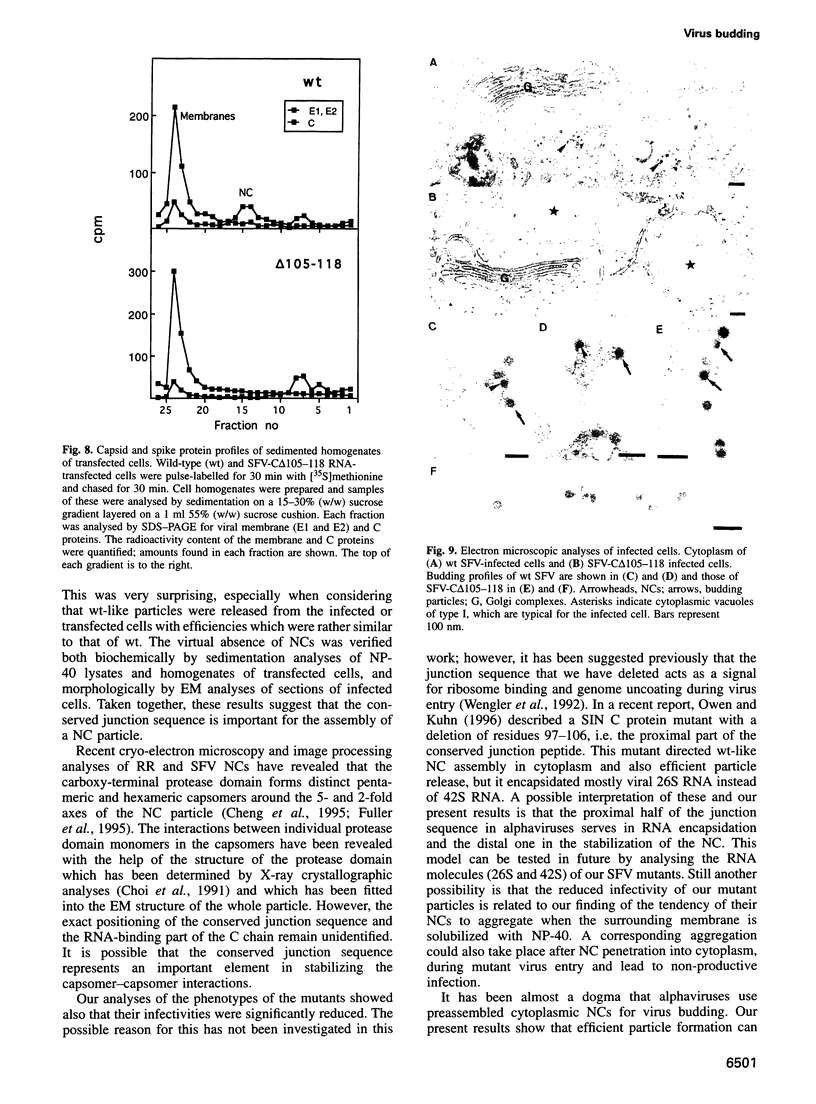
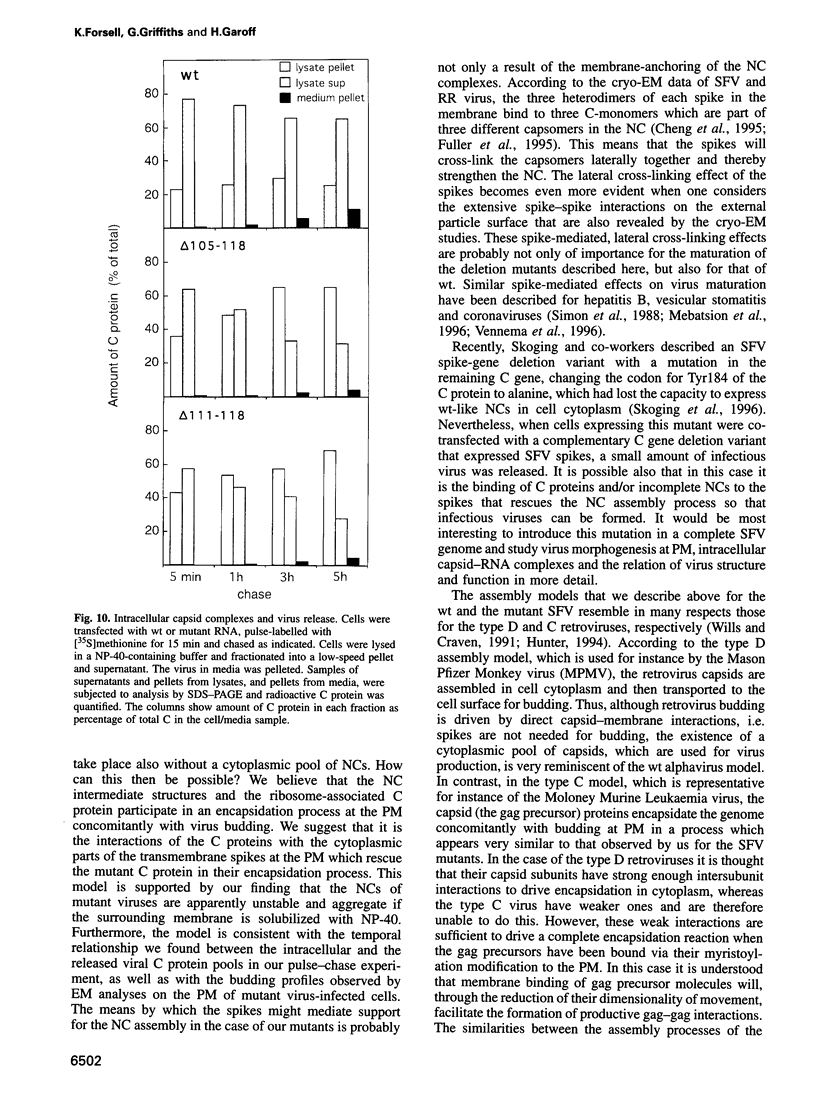



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
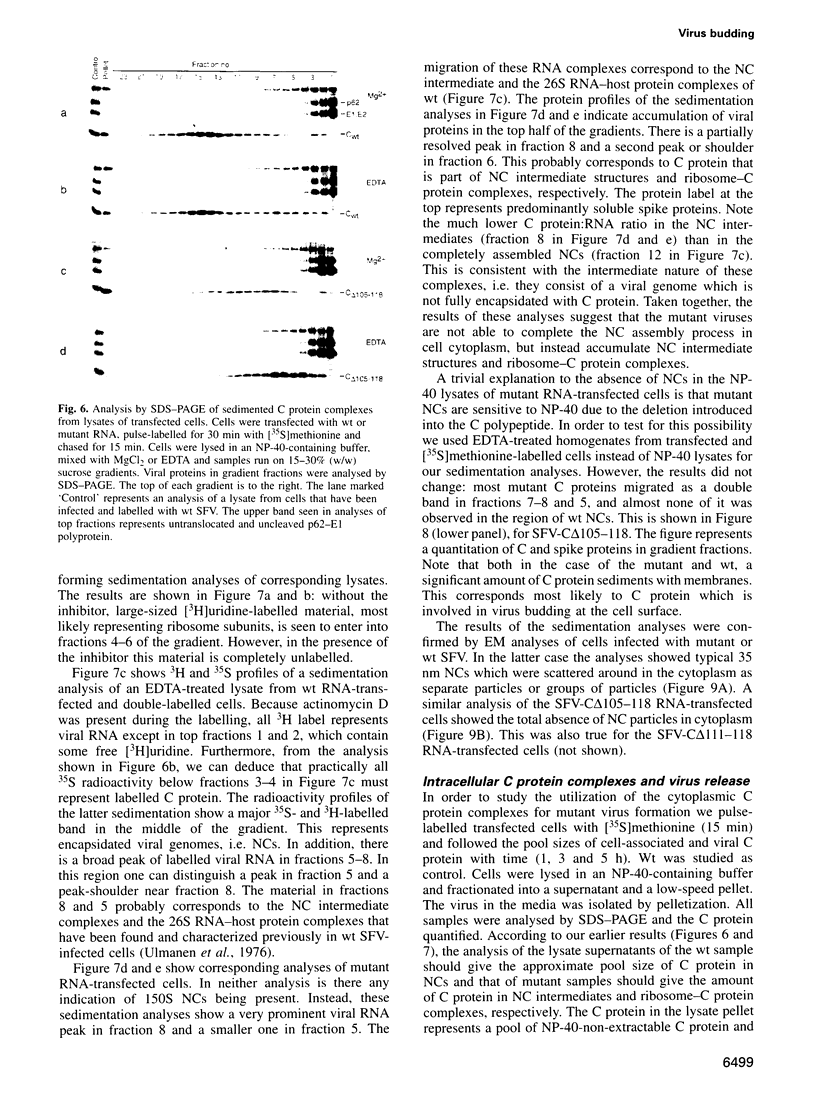
- Chang G. J., Trent D. W. Nucleotide sequence of the genome region encoding the 26S mRNA of eastern equine encephalomyelitis virus and the deduced amino acid sequence of the viral structural proteins. J Gen Virol. 1987 Aug;68(Pt 8):2129–2142. doi: 10.1099/0022-1317-68-8-2129. [DOI] [PubMed] [Google Scholar]
- Cheng R. H., Kuhn R. J., Olson N. H., Rossmann M. G., Choi H. K., Smith T. J., Baker T. S. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995 Feb 24;80(4):621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. K., Tong L., Minor W., Dumas P., Boege U., Rossmann M. G., Wengler G. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature. 1991 Nov 7;354(6348):37–43. doi: 10.1038/354037a0. [DOI] [PubMed] [Google Scholar]
- Chong L. D., Rose J. K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993 Jan;67(1):407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno L., Rice C. M., Strauss J. H. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology. 1983 Aug;129(1):170–187. doi: 10.1016/0042-6822(83)90404-x. [DOI] [PubMed] [Google Scholar]
- Forsell K., Suomalainen M., Garoff H. Structure-function relation of the NH2-terminal domain of the Semliki Forest virus capsid protein. J Virol. 1995 Mar;69(3):1556–1563. doi: 10.1128/jvi.69.3.1556-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., Berriman J. A., Butcher S. J., Gowen B. E. Low pH induces swiveling of the glycoprotein heterodimers in the Semliki Forest virus spike complex. Cell. 1995 Jun 2;81(5):715–725. doi: 10.1016/0092-8674(95)90533-2. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Kondor-Koch C., Riedel H. Structure and assembly of alphaviruses. Curr Top Microbiol Immunol. 1982;99:1–50. doi: 10.1007/978-3-642-68528-6_1. [DOI] [PubMed] [Google Scholar]
- Geigenmüller-Gnirke U., Nitschko H., Schlesinger S. Deletion analysis of the capsid protein of Sindbis virus: identification of the RNA binding region. J Virol. 1993 Mar;67(3):1620–1626. doi: 10.1128/jvi.67.3.1620-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Lustig S., Strauss E. G., Strauss J. H. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5997–6001. doi: 10.1073/pnas.85.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney R. M., Johnson B. J., Brown V. L., Trent D. W. Nucleotide sequence of the 26 S mRNA of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and deduced sequence of the encoded structural proteins. Virology. 1986 Jul 30;152(2):400–413. doi: 10.1016/0042-6822(86)90142-x. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Properties of Semliki Forest virus nucleocapsid. 1. Sensitivity to pancreatic ribonuclease. Virology. 1971 Jan;43(1):291–299. doi: 10.1016/0042-6822(71)90246-7. [DOI] [PubMed] [Google Scholar]
- Laine R., Söderlund H., Renkonen O. Chemical composition of Semliki forest virus. Intervirology. 1973;1(2):110–118. doi: 10.1159/000148837. [DOI] [PubMed] [Google Scholar]
- Levinson R. S., Strauss J. H., Strauss E. G. Complete sequence of the genomic RNA of O'nyong-nyong virus and its use in the construction of alphavirus phylogenetic trees. Virology. 1990 Mar;175(1):110–123. doi: 10.1016/0042-6822(90)90191-s. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Lusa S., Huylebroeck D., Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991 Aug;65(8):4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
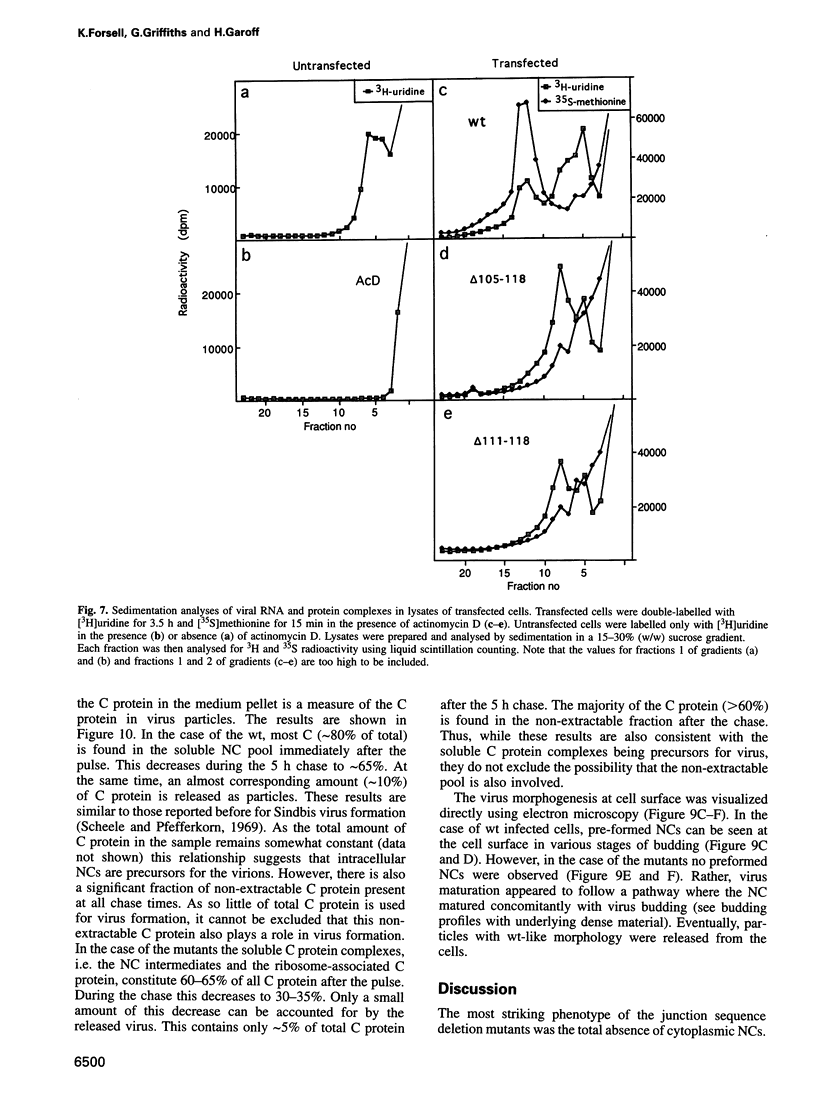
- Mebatsion T., Konig M., Conzelmann K. K. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996 Mar 22;84(6):941–951. doi: 10.1016/s0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Owen K. E., Kuhn R. J. Identification of a region in the Sindbis virus nucleocapsid protein that is involved in specificity of RNA encapsidation. J Virol. 1996 May;70(5):2757–2763. doi: 10.1128/jvi.70.5.2757-2763.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A. M., Brown D. T., Rothnagel R., Chiu W., Schoepp R. J., Johnston R. E., Prasad B. V. Three-dimensional structure of a membrane-containing virus. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9095–9099. doi: 10.1073/pnas.90.19.9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell. 1990 Oct 5;63(1):77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümenapf T., Strauss E. G., Strauss J. H. Aura virus is a New World representative of Sindbis-like viruses. Virology. 1995 Apr 20;208(2):621–633. doi: 10.1006/viro.1995.1193. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Kinetics of incorporation of structural proteins into Sindbis virions. J Virol. 1969 Apr;3(4):369–375. doi: 10.1128/jvi.3.4.369-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., Lingappa V. R., Ganem D. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J Cell Biol. 1988 Dec;107(6 Pt 1):2163–2168. doi: 10.1083/jcb.107.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoging U., Vihinen M., Nilsson L., Liljeström P. Aromatic interactions define the binding of the alphavirus spike to its nucleocapsid. Structure. 1996 May 15;4(5):519–529. doi: 10.1016/s0969-2126(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Strauss E. G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994 Sep;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Garoff H., Baron M. D. The E2 signal sequence of rubella virus remains part of the capsid protein and confers membrane association in vitro. J Virol. 1990 Nov;64(11):5500–5509. doi: 10.1128/jvi.64.11.5500-5509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Garoff H. Incorporation of homologous and heterologous proteins into the envelope of Moloney murine leukemia virus. J Virol. 1994 Aug;68(8):4879–4889. doi: 10.1128/jvi.68.8.4879-4889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Liljeström P., Garoff H. Spike protein-nucleocapsid interactions drive the budding of alphaviruses. J Virol. 1992 Aug;66(8):4737–4747. doi: 10.1128/jvi.66.8.4737-4747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H. Kinetics of formation of the Semliki Forest virus nucleocapsid. Intervirology. 1973;1(5-6):354–361. doi: 10.1159/000148864. [DOI] [PubMed] [Google Scholar]
- Söderlund H., Ulmanen I. Transient association of Semliki Forest virus capsid protein with ribosomes. J Virol. 1977 Dec;24(3):907–909. doi: 10.1128/jvi.24.3.907-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I. Assembly of Semliki Forest virus nucleocapsid: detection of a precursor in infected cells. J Gen Virol. 1978 Nov;41(2):353–365. doi: 10.1099/0022-1317-41-2-353. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Söderlund H., Käriäinen L. Semliki Forest virus capsid protein associates with the 60S ribosomal subunit in infected cells. J Virol. 1976 Oct;20(1):203–210. doi: 10.1128/jvi.20.1.203-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Godeke G. J., Rossen J. W., Voorhout W. F., Horzinek M. C., Opstelten D. J., Rottier P. J. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 1996 Apr 15;15(8):2020–2028. doi: 10.1002/j.1460-2075.1996.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. M., Boere W. A., Garoff H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J Virol. 1989 Dec;63(12):4991–4997. doi: 10.1128/jvi.63.12.4991-4997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J. M., Garoff H. Membrane fusion process of Semliki Forest virus. I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992 Jan;116(2):339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Nitschko H., Ghattas I., Wright R., Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J Virol. 1989 Dec;63(12):5310–5318. doi: 10.1128/jvi.63.12.5310-5318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Würkner D., Wengler G. Identification of a sequence element in the alphavirus core protein which mediates interaction of cores with ribosomes and the disassembly of cores. Virology. 1992 Dec;191(2):880–888. doi: 10.1016/0042-6822(92)90263-o. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]