This study examined the impact of the 21-gene Oncotype DX Recurrence Score assay in a French clinical setting in terms of treatment recommendations, physicians’ confidence before and after knowing the Recurrence Score value, and physicians’ perception of the assay. Using the assay was associated with a significant change in treatment decisions and an overall reduction in chemotherapy use.
Keywords: Adjuvant, Chemotherapy, Early breast cancer, Estrogen receptor-positive
Abstract
Background.
The 21-gene Oncotype DX Recurrence Score assay is a validated assay to help decide the appropriate treatment for estrogen receptor-positive (ER+), early-stage breast cancer (EBC) in the adjuvant setting. The choice of adjuvant treatments might vary considerably in different countries according to various treatment guidelines. This prospective multicenter study is the first to assess the impact of the Oncotype DX assay in the French clinical setting.
Methods.
A total of 100 patients with ER+, human epidermal growth factor receptor 2-negative EBC, and node-negative (pN0) disease or micrometastases in up to 3 lymph nodes (pN1mi) were enrolled. Treatment recommendations, physicians’ confidence before and after knowing the Recurrence Score value, and physicians’ perception of the assay were recorded.
Results.
Of the 100 patients, 95 were evaluable (83 pN0, 12 pN1mi). Treatment recommendations changed in 37% of patients, predominantly from chemoendocrine to endocrine treatment alone. The proportion of patients recommended chemotherapy decreased from 52% pretest to 25% post-test. Of patients originally recommended chemotherapy, 61% were recommended endocrine treatment alone after receiving the Recurrence Score result. For both pN0 and pN1mi patients, post-test recommendations appeared to follow the Recurrence Score result for low and high values. Physicians’ confidence improved significantly.
Conclusion.
These are the first prospective data on the impact of the Oncotype DX assay on adjuvant treatment decisions in France. Using the assay was associated with a significant change in treatment decisions and an overall reduction in chemotherapy use. These data are consistent with those presented from European and non-European studies.
Implications for Practice:
This study shows that in estrogen receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer (either node-negative or with micrometastases in up to 3 lymph nodes), Oncotype DX testing is associated with a treatment recommendation change in more than a third of patients (primarily from chemoendocrine treatment to endocrine treatment alone but also in the opposite direction) and an overall reduction in chemotherapy use. These results are consistent with those from other decision impact studies worldwide and further emphasize the role of Oncotype DX testing in management of early breast cancer, as reflected in international treatment guidelines.
Introduction
Only a small proportion of patients with hormone receptor-positive (HR+) invasive early breast cancer (EBC) benefit from chemotherapy [1, 2]. However, a relatively high proportion of women are recommended chemotherapy in France [3].
A key challenge in making treatment decisions for patients with estrogen receptor-positive (ER+), human epidermal growth factor receptor 2 (HER2)-negative EBC is that the traditional markers used are mainly prognostic and not predictive of chemotherapy benefit. A relatively high proportion of patients is also classified as intermediate risk by most classical markers.
The Oncotype DX Recurrence Score assay is a real-time reverse transcriptase-polymerase chain reaction-based assay specifically developed and optimized for use in archival formalin-fixed, paraffin-embedded tumor tissue [4, 5]. It determines the expression of 5 reference genes and 16 cancer-related genes selected based on the correlation of gene expression and the risk of distant recurrence [6–8]. The Oncotype DX breast cancer assay has been validated as a prognostic marker in both node-negative and node-positive HR+ disease [2, 9–11]. The Recurrence Score has also been validated as a predictive marker for the magnitude of adjuvant chemotherapy benefit [2, 11] with a significant benefit in patients with high Recurrence Score values (28% absolute benefit in 10-year risk of distant recurrence) and minimal, if any, benefit from chemotherapy in patients with low values. In patients with intermediate values (Recurrence Scores of 18–30), the available data cannot rule out that some patients may benefit from chemotherapy [2, 11].
The use of this assay to guide clinical treatment decisions has been addressed within clinical guidelines [12–15]. The 2013 St. Gallen Consensus acknowledges the assay not only as a prognostic test but also as a marker predictive of chemotherapy responsiveness for patients with luminal disease [15].
Several prospective studies showed that knowledge of the Recurrence Score result affects management of patients [16–22]. Results are very consistent across different health care systems, with treatment recommendations changing in approximately one third of patients. Use of the assay has been found to be cost-effective or even cost-saving in different national health care systems [22–32]. This study was performed as a prospective clinical study to evaluate the impact of the Oncotype DX breast cancer assay on adjuvant decision making in French clinical practice for patients with ER+ EBC.
Materials and Methods
The SWITCH study was a prospective study involving seven French centers (ClinicalTrials.gov identifier NCT01446185). It was approved by the national ethics committee and the local research committees of all participating institutions. All patients provided written informed consent.
Study Objectives
The primary objective was to assess the impact on adjuvant treatment decisions when using the Recurrence Score result in patients with ER+, HER2-negative EBC with node-negative disease (pN0) or with micrometastasis in up to 3 lymph nodes (pN1mi). The secondary objectives were to assess (a) the participating physicians’ level of confidence in their treatment decision before (pretest) and after (post-test) receiving the Recurrence Score result and (b) their perceptions regarding the utility of the assay.
Patients
Enrollment was offered consecutively to eligible women who had operable invasive EBC, ER+ (defined by >10% of cells stained [33]), HER2-negative pN0 or histological proof of micrometastasis in regional lymph nodes (pN1mi). Other inclusion criteria were: potential candidate for systemic chemotherapy, good performance status, age of ≥18 years, and signed informed consent for the study.
Adjuvant Treatment Recommendations
Investigators had to document their treatment recommendations before and after knowing the Recurrence Score result. Each case was discussed twice within the respective institution’s multidisciplinary tumor board. In the first board meeting, adjuvant treatment was recommended according to the valid French guidelines [3] based on clinical and histopathological information. An improvement in disease-free survival of >5% was usually regarded as a cutoff for recommending chemotherapy. Each case was rediscussed in a second meeting, and treatment was recommended considering the Recurrence Score result.
Physician’s Questionnaires
A baseline questionnaire captured physicians’ initial treatment recommendations and answers to queries regarding their confidence in their treatment recommendations before the assay had been performed. A follow-up questionnaire recorded treatment recommendations, as well as physicians’ confidence in their recommendations and their perceptions of the assay post-test. Answers could be chosen from a Likert scale with the following options: strongly disagree, disagree, neither disagree nor agree, agree, strongly agree, and do not know.
Statistics
Sample size was determined based on the assumption of an overall treatment recommendation change rate (from an initial recommendation for chemoendocrine to endocrine treatment and vice versa) of 18%. A sample size of 100 patients allowed determination of this switch rate with a 95% confidence interval (CI) of 11.0%–26.9% (i.e., with a CI width of 15.9%).
Descriptive statistics were used to summarize patient and tumor characteristics. The proportion of patients for whom treatment recommendations changed from before to after knowledge of the assay result was calculated for all patients by nodal status and by Recurrence Score group (low indicates Recurrence Score results of <18, intermediate indicates Recurrence Score results between 18 and 30, and high indicates Recurrence Score results of ≥31). The chemotherapy alone and chemoendocrine therapy options were combined, as were the observation and endocrine therapy options. Change rates were reported with 95% CI calculated using the Clopper-Pearson method. McNemar’s test was used to assess whether the proportion of patients recommended chemotherapy changed from pretest to post-test.
The physicians’ level of confidence in the adjuvant treatment recommendation was measured both pretest and post-test in the response to the question: “I am confident in my treatment recommendation.” The physicians’ post-test perceptions of the utility of the assay were assessed by the answers to the question: “The Oncotype DX assay results provided additional information.” Answers were assigned numeric values from 1 to 5, respectively. Evolution of the level of confidence pretest to post-test was derived by subtracting the pretest value from the post-test value (resulting negative values were reported as decreases in confidence, and resulting positive values were reported as increases in confidence) and assessed using the signed rank test.
Results
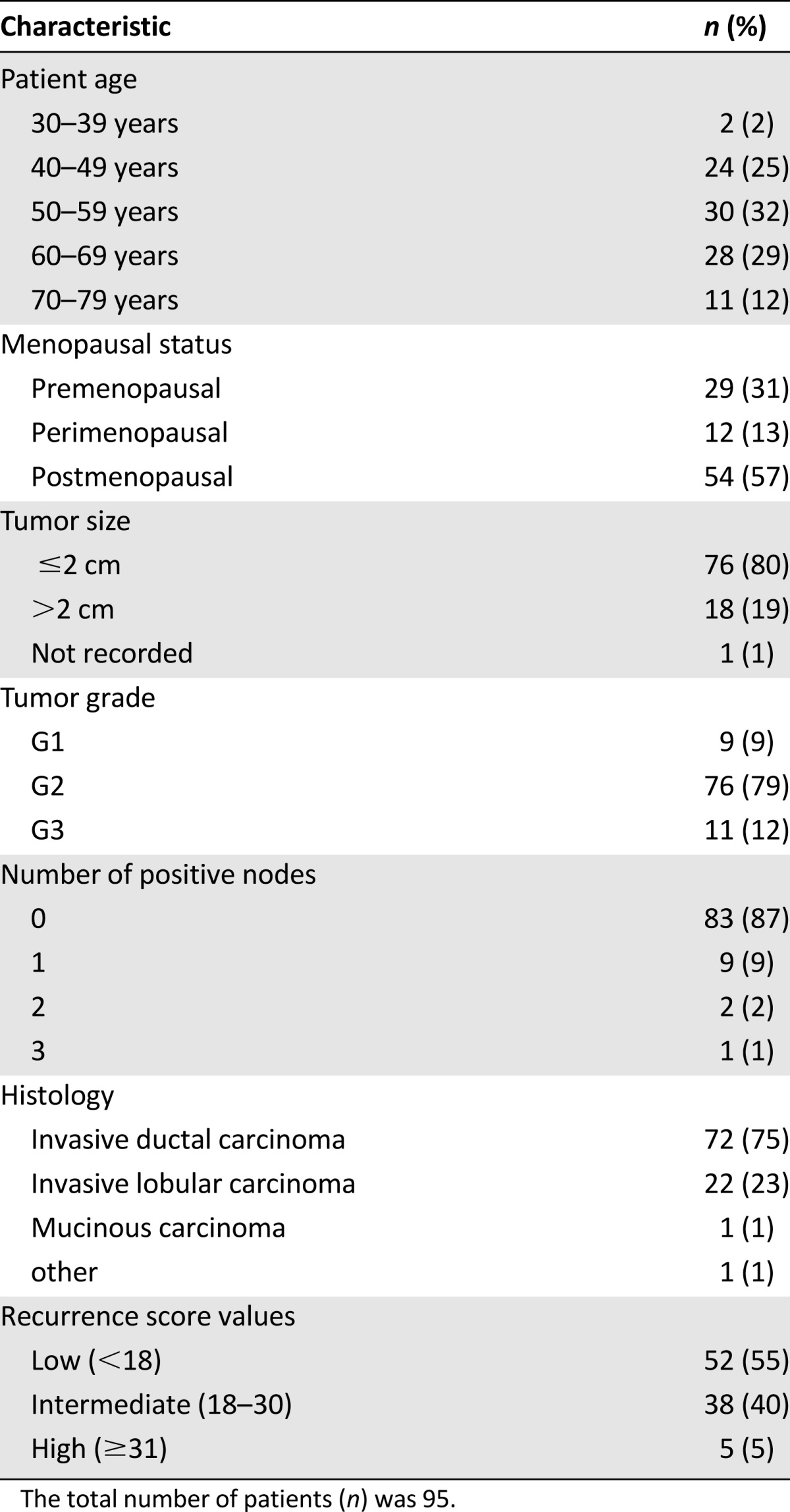
Patient and Tumor Characteristics
In total, 100 patients were enrolled between January 2011 and December 2011. Of those 100 patients, 5 were excluded from the analysis (2 inadequate tissue sample, 2 had a treatment decision before receiving the test result, and 1 had no post-test recommendation available) leaving 95 evaluable patients. Complete patient and tumor characteristics and the distribution of the Recurrence Score values are listed in Table 1. Overall, 52 (54%) patients were in the low, 38 (40%) in the intermediate, and 5 (5%) in the high Recurrence Score groups.
Table 1.
Patient characteristics
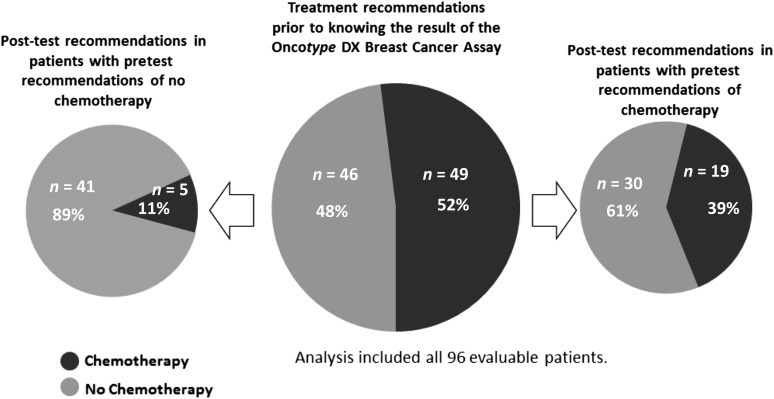
Shift in Treatment Recommendations
Treatment recommendations before and after the assay are listed in Table 2. Overall, the shift in treatment recommendations was predominantly from chemotherapy to no chemotherapy. Of the 95 patients, 35 (37% [95% CI: 27%–47%]) had a change in adjuvant treatment recommendation, with 30 (32%) omitting chemotherapy and 5 (5%) adding chemotherapy. Of 49 patients initially recommended chemotherapy, 30 (61%) were not recommended chemotherapy after receiving the Recurrence Score result (Fig. 1). Of 46 patients initially recommended no chemotherapy, 5 (11%) were recommended chemotherapy post-test. The proportion of patients recommended chemotherapy decreased from 52% pretest to 25% post-test (p < .001; McNemar’s test).
Table 2.
Treatment recommendations before and after Oncotype DX testing
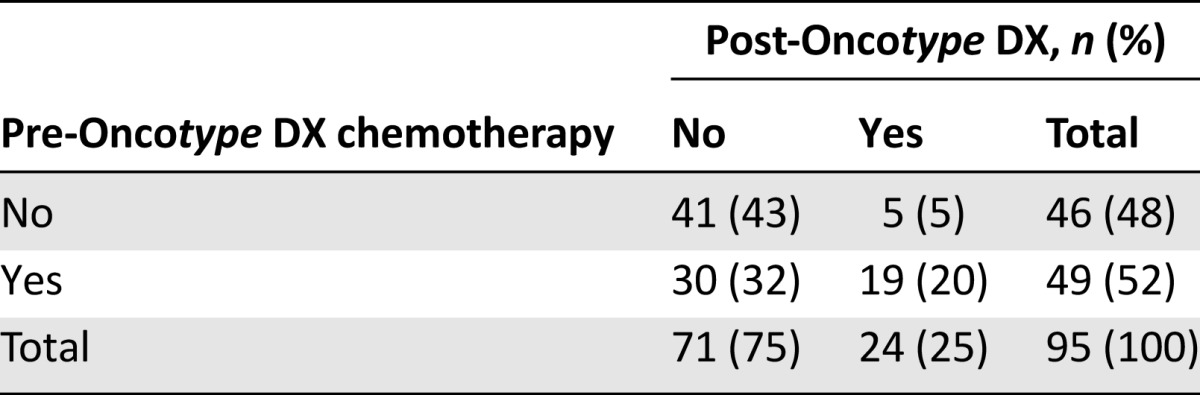
Figure 1.
Shift in treatment recommendations by pretest treatment recommendation.
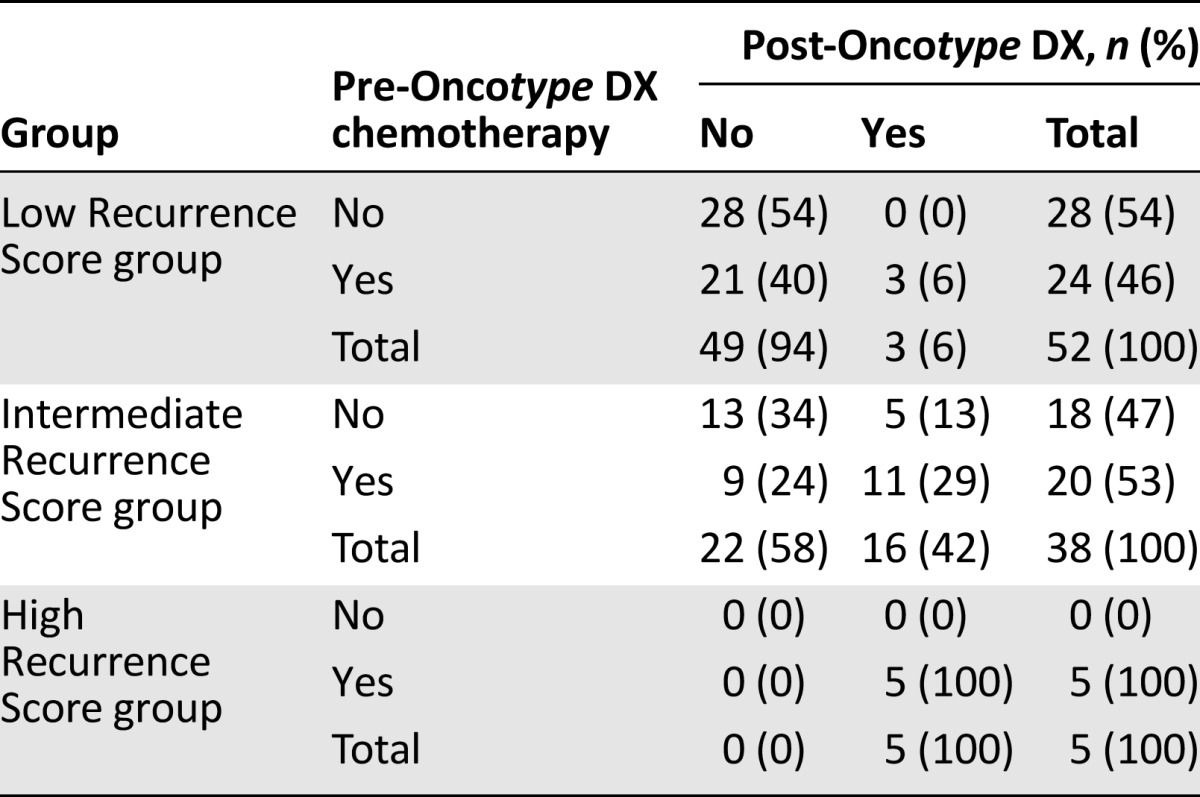
Changes in recommendations by Recurrence Score group are shown in Table 3. Post-test treatment decisions seemed to follow the Recurrence Score result for low and high values. In the low Recurrence Score group, 21 of 24 patients (88%) initially recommended chemotherapy had chemotherapy omitted in their post-test treatment recommendation. All of the 5 patients in the high Recurrence Score group had a post-test recommendation for chemotherapy.
Table 3.
Chemotherapy recommendations pre- and post-testing by Recurrence Score group
Changes in recommendations by nodal status are shown in Table 4. A statistically significant reduction in recommendations for chemotherapy was observed in pN0 patients (p < .001). Of the 42 patients who had no pretest recommendation for chemotherapy, 5 were shifted to chemotherapy post-test. Of the 41 patients with a prior recommendation for chemotherapy, 25 were shifted to no chemotherapy. This corresponded to 6% and 30% of all pN0 patients, respectively. The reduction in chemotherapy recommendations in the 12 pN1mi patients was of borderline statistical significance (p = .063), which may be the consequence of the small number of patients. No patient changed from no chemotherapy pretest to chemotherapy post-test, whereas 5 of 8 patients with a pretest chemotherapy recommendation changed to no chemotherapy post-test, corresponding to 42% of all pN1mi patients.
Table 4.
Chemotherapy recommendations pre- and post-testing by nodal status
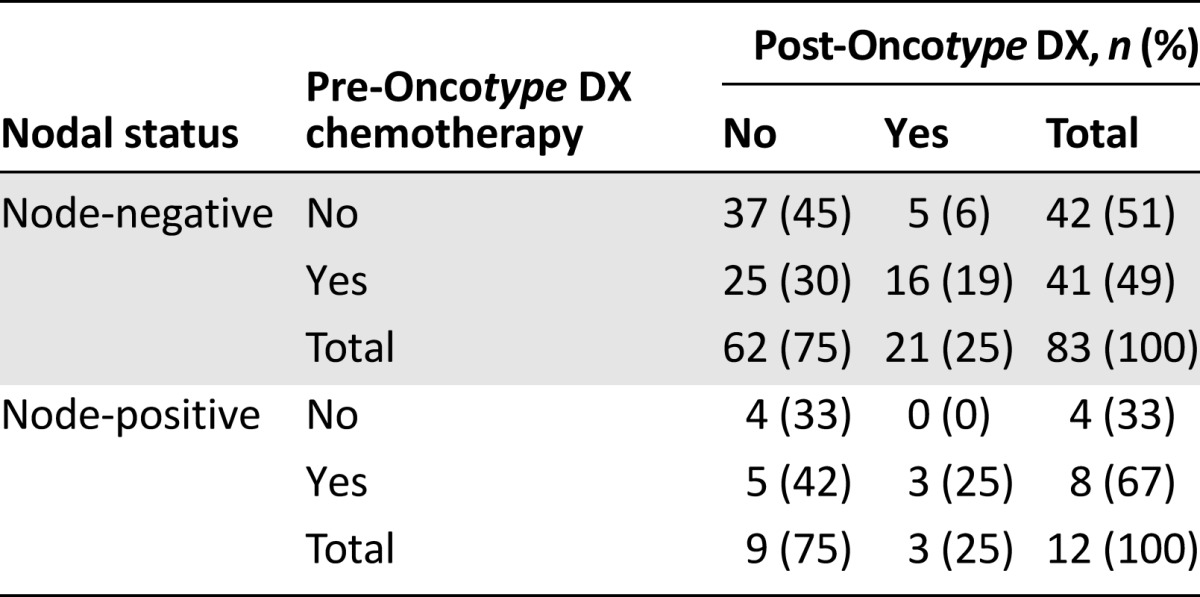
Physicians’ Confidence in Treatment Recommendation Before and After Oncotype DX Testing
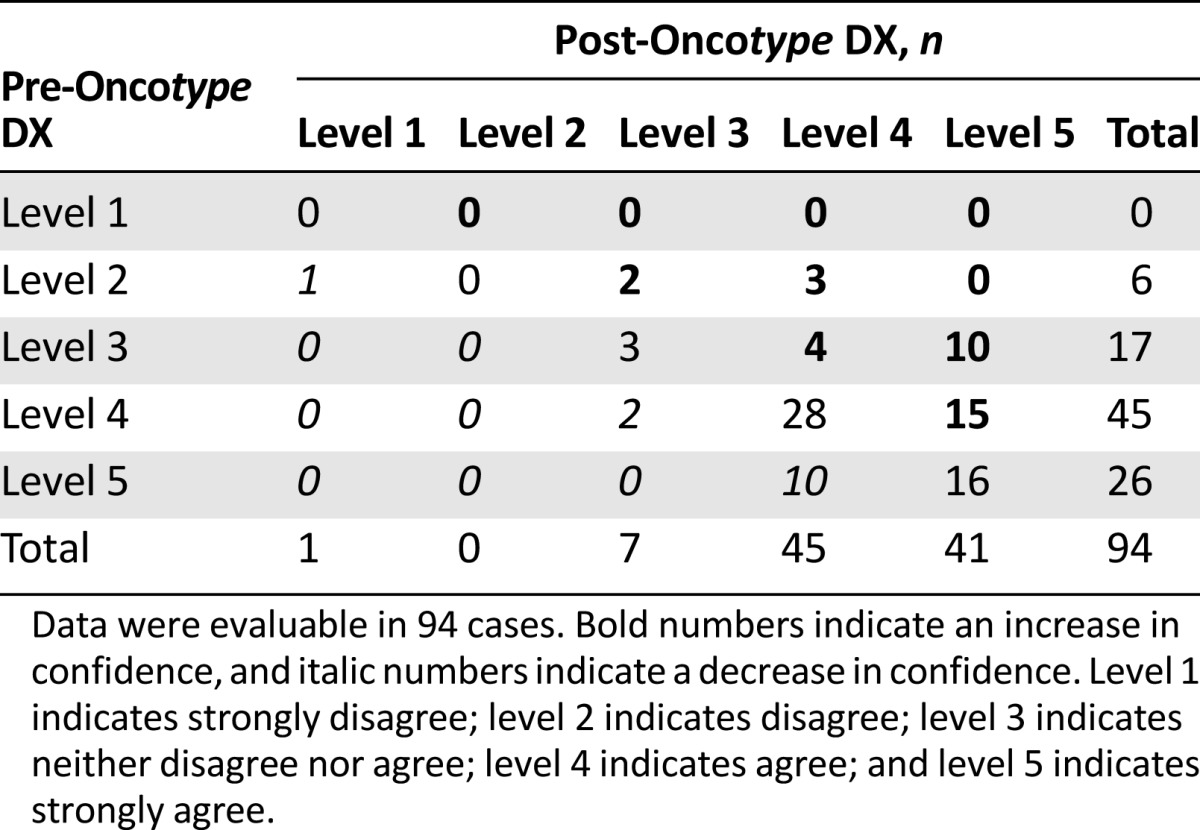
Among 94 cases in which a measure of physicians’ confidence was available, there was an overall significant improvement in physicians’ confidence (p < .001; signed rank test). There were increases by 2 levels in 13 physicians (14%), increases by 1 level in 21 physicians (22%), no change in 47 physicians (50%), and decreases by 1 level in 13 physicians (14%) (Table 5).
Table 5.
Changes in physicians’ confidence from pre- to post-Oncotype DX testing
Perception of the Clinical Utility of the Test
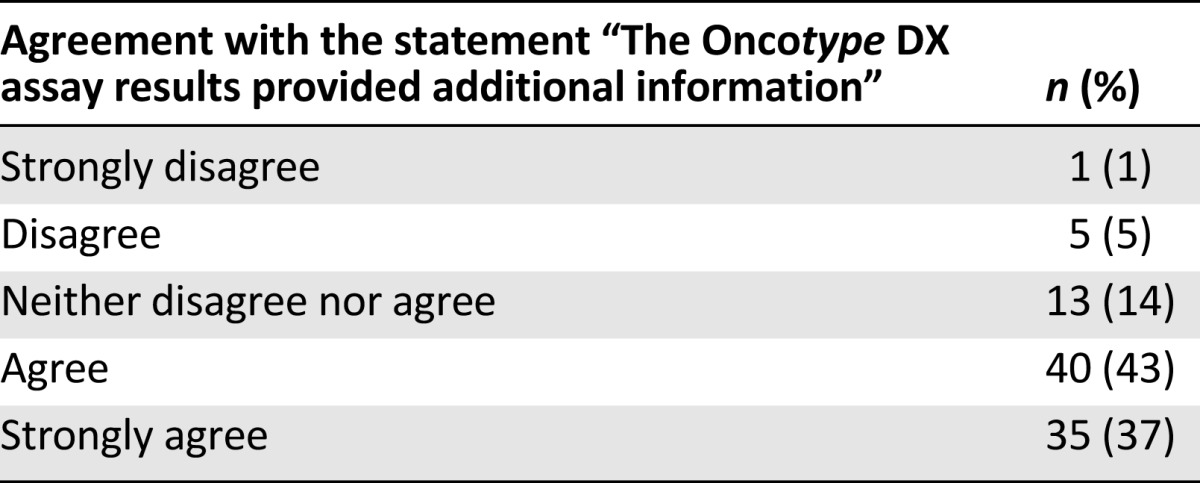
Physicians agreed or strongly agreed that the Oncotype DX assay results provided additional information in 75 of 94 cases (80% [95% CI: 70%–87%]) in which the physician’s response was obtained (Table 6).
Table 6.
Perception of the clinical utility of the Oncotype DX breast cancer assay
Discussion
Here we report the results of the first clinical utility study assessing the impact of integrating the Oncotype DX breast cancer assay into the decision-making process for adjuvant treatment of patients with ER+ EBC in the French clinical setting. Studies with a similar design have been reported from other countries [16–22]. They showed that the initial treatment recommendation is heterogeneous with the initial proportion of patients recommended chemotherapy ranging from 30% to 59%.
Based on traditional clinical and histopathological information, 49% of pN0 patients in our study would have received adjuvant chemotherapy according to our guidelines. This compares with 59% of pN0 patients in the Japanese study, 47% in the U.S. and the German studies, 36% in the Spanish study, and 30% in the Australian study [16, 18–21]. Different treatment traditions but also differences in patient population may affect the differences seen in these studies. One limitation of our study should be noted. Our patient population was primarily low and intermediate risk by classical parameters, and the proportion of tumors with lobular histology was also somewhat higher than in other studies. The distribution of the Recurrence Score results also reflected a low- and intermediate-risk patient population with a low proportion of high values compared with many other studies. Despite the consecutive enrollment asked for in the protocol, we could not preclude some degree of selection. This may have been caused by conventionally high-risk patients not consenting to take the test as a result of their physicians' conviction of the necessity of adjuvant chemotherapy in their respective cases.
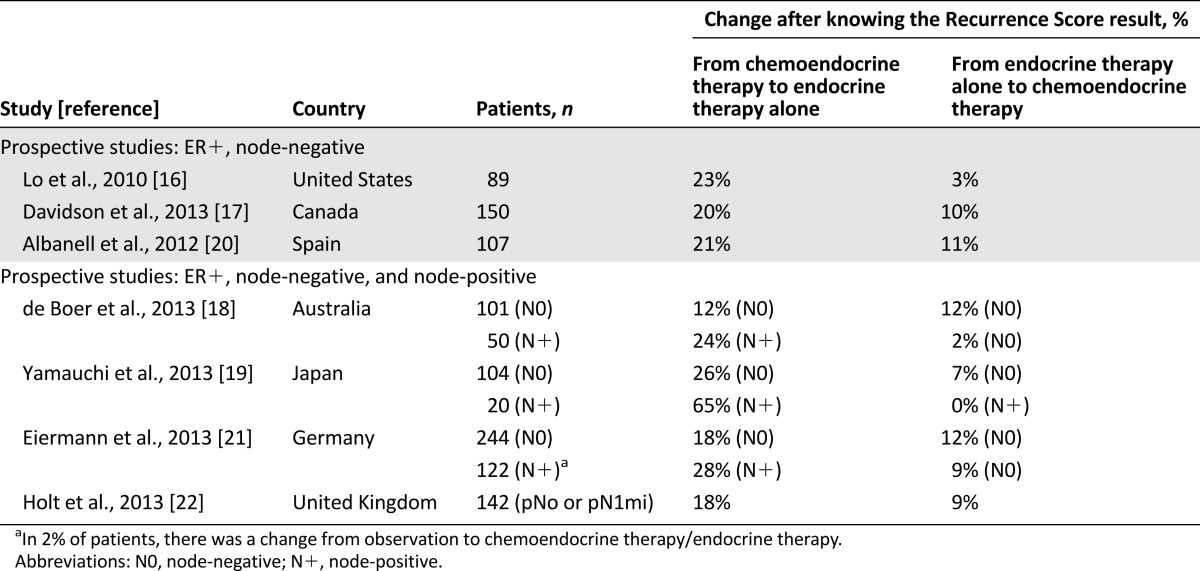
Overall, treatment recommendations in our study changed in 37% of pN0 cases when Recurrence Score results were available as additional information. These results are consistent with those obtained in studies conducted in the U.S., Canada, Japan, Germany, and Spain (Table 7) [16, 17, 19–21]. The change rate in an Australian study was slightly lower (25%), but the proportion of patients originally recommended chemotherapy was also lower than in our study [18]. In our study, of the women with a pretest chemotherapy recommendation, 61% were recommended a less intensive treatment without chemotherapy after knowing the test results. This compares with 39% for the German study, 40% for the Australian study, 48% for the U.S. study, 51% for the Japanese study, and 56% for the Spanish study [16, 18–21]. In the U.K. study, the overall shift in treatment recommendations was 27% for a mixed population of pN0 and pN1mi patients, and 46% of patients with a prior recommendation of chemotherapy were recommended only adjuvant endocrine treatment after the test [22]. We also found a 42% change rate in pN1mi patients, which included only a shift from chemotherapy to no chemotherapy. However, the small number of patients with node-positive disease in our study limits the generalizability of this finding. Also, we had restricted enrollment to patients with micrometastasis in the regional lymph nodes as opposed to four other studies that reported results on the impact of the Recurrence Score in patients with ER+ EBC and 1–3 positive nodes. A U.S. web-based physician survey reported a change rate of 51% in 138 patients with a 33% change rate from chemoendocrine to endocrine therapy [34]. The Australian study found a 26% change in treatment recommendations in 50 patients (Table 7): 12 patients changed to endocrine therapy, and 1 changed to chemoendocrine therapy [18]. In the German study, there was a 39% change rate in 122 patients with a predominant change from chemotherapy to no chemotherapy in 28% [21]. The Japanese study reported a 65% shift in treatment recommendations in 20 patients exclusively from chemotherapy to no chemotherapy [19]. A large retrospective study from Israel in 951 patients with node-positive ER+ EBC found that 24.1% of all patients tested with the Oncotype DX assay (n = 282) received chemotherapy compared with 70.1% of patients with similar baseline characteristics who did not (n = 669) [35].
Table 7.
Summary of findings from prospective decision impact studies with Oncotype DX
The predominant and statistically significant change in our study, as in the other studies, was from a pretest recommendation for chemotherapy to a post-test recommendation without chemotherapy, with a decrease from 52% to 25%. Reassuringly, post-test treatment decisions seemed to follow the Recurrence Score result for low and high Recurrence Score values as was shown for the U.S., Spanish, and German studies [16, 20, 21]. As expected, the reduction in recommendations for chemotherapy was highest in the low Recurrence Score group with a net decrease of 40%. All patients with high Recurrence Score results were treated with chemotherapy. Although there was an overall net reduction of chemotherapy recommendations in the intermediate Recurrence Score group, a high proportion (28%) of patients in this group who were originally recommended endocrine therapy only were recommended chemotherapy post-test. There was no clear cutoff for Recurrence Score values within the intermediate risk group for chemotherapy recommendations. However, numbers in the intermediate subgroup were too small to explore this sufficiently.
We found a significant increase in physicians’ confidence in their treatment decisions (36% of cases) after having the test results. The increase is relatively modest compared with that observed in studies in other countries: Japan (86%), U.S. (76%), Canada (59%), Spain (60%), and Germany (45%) [16, 17, 19–21]. However, physicians in our study agreed or strongly agreed that the Oncotype DX assay results provided additional information in 75 of 94 cases (80% [95% CI: 69%–87%]). It should be noted that the assay was not frequently used in France and that the physicians in our study had limited, if any, experience with using it prior to the study. Results reporting physicians’ confidence may thus also reflect cultural differences.
Our study did not include a pharmacoeconomic analysis to evaluate whether the net decrease in chemotherapy recommendations actually translated into economic savings. However, a recently presented analysis of the cost-effectiveness of using the Oncotype DX breast cancer assay in France compared assignment of adjuvant chemotherapy based on the conventional approach in France and a meta-analysis from nine decision impact studies and projected the use of the test to be cost-saving in French clinical practice because of a decrease in overall chemotherapy costs alongside an increase in overall life years [31]. Findings from pharmacoeconomic analyses of using the assay in decision making in ER+ EBC in various health care systems showed that this approach was, at minimum, cost-effective [22–32].
Conclusion
Because it is not possible to extrapolate data from one country to another, we considered generating specific data from French oncology centers to be highly relevant for making more accurate estimates of the health and economic impact of Oncotype DX use in the French health care system. The results from the SWITCH trial are consistent with those found in other European and non-European countries, confirming that the Oncotype DX breast cancer assay does impact adjuvant decision making in ER+ EBC in clinical practice resulting in a significant, meaningful net reduction in adjuvant chemotherapy recommendations.
Acknowledgments
We thank all the patients participating in the study and Alliance Pour la Recherche en Cancérologie for technical support. Genomic Health, Inc., was the sponsor of the study and was given an opportunity to review and comment on the manuscript. The authors are solely responsible for the content of the manuscript with no restrictions set by the sponsor. Avital Bareket-Samish, Ph.D., provided medical editing assistance.
Author Contributions
Conception/Design: Joseph Gligorov, Roman Rouzier, Frederique M. Penault-Llorca
Provision of study material or patients: Joseph Gligorov, Xavier B. Pivot, William Jacot, Hervé L. Naman, Dominique Spaeth, Jean-Louis Misset, Rémy Largillier, Jean-Loup Sautiere, Anne de Roquancourt, Christophe Pomel, Philippe Rouanet, Roman Rouzier, Frederique M. Penault-Llorca
Collection and/or assembly of data: Joseph Gligorov, Xavier B. Pivot, William Jacot, Hervé L. Naman, Dominique Spaeth, Jean-Louis Misset, Rémy Largillier, Jean-Loup Sautiere, Anne de Roquancourt, Christophe Pomel, Philippe Rouanet, Roman Rouzier, Frederique M. Penault-Llorca
Data analysis and interpretation: Joseph Gligorov, Xavier B. Pivot, Roman Rouzier, Frederique M. Penault-Llorca
Manuscript writing: Joseph Gligorov, Xavier B. Pivot, Roman Rouzier, Frederique M. Penault-Llorca
Final approval of manuscript: Joseph Gligorov, Xavier B. Pivot, William Jacot, Hervé L. Naman, Dominique Spaeth, Jean-Louis Misset, Rémy Largillier, Jean-Loup Sautiere, Anne de Roquancourt, Christophe Pomel, Philippe Rouanet, Roman Rouzier, Frederique M. Penault-Llorca
Disclosures
Joseph Gligorov: Genomic Health (C/A); Xavier B. Pivot: Roche, TEVA, Amgen (C/A), Pierre Fabre, Genomic Health (H); Jean-Louis Misset: ABscience (C/A); Roman Rouzier: Genomic Health (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 3.Bachelot T, Bouzid K, Delozier T, et al. RPC Nice St. Paul de Vence, État des lieux et mise à jour de traitements systémiques adjuvants. Oncologie. 2011;13:698–702. [Google Scholar]
- 4.Cronin M, Pho M, Dutta D, et al. Measurement of gene expression in archival paraffin-embedded tissues: Development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol. 2004;164:35–42. doi: 10.1016/S0002-9440(10)63093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53:1084–1091. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- 6.Esteban J, Baker J, Cronin M, et al. Tumor gene expression and prognosis in breast cancer: Multi-gene RT-PCR assay of paraffin-embedded tissue. Prog Proc Am Soc Clin Oncol. 2003;22:850a. [Google Scholar]
- 7.Cobleigh MA, Tabesh B, Bitterman P, et al. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin Cancer Res. 2005;11:8623–8631. doi: 10.1158/1078-0432.CCR-05-0735. [DOI] [PubMed] [Google Scholar]
- 8.Paik S, Shak S, Tang G, et al. Multi-gene RT-PCR assay for predicting recurrence in node negative breast cancer patients: NSABP studies B-20 and B-14. 2003;82:A16a. [Google Scholar]
- 9.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 10.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network Practice Guidelines in Oncology. Breast Cancer (version v3.2014). Available at http://www.NCCN.org. Accessed September 10, 2014.
- 14.Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi7–vi23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 17.Davidson JA, Cromwell I, Ellard SL, et al. A prospective clinical utility and pharmacoeconomic study of the impact of the 21-gene Recurrence Score® assay in oestrogen receptor positive node negative breast cancer. Eur J Cancer. 2013;49:2469–2475. doi: 10.1016/j.ejca.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 18.de Boer RH, Baker C, Speakman D, et al. The impact of a genomic assay (Oncotype DX) on adjuvant treatment recommendations in early breast cancer. Med J Aust. 2013;199:205–208. doi: 10.5694/mja12.11334. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi H, Nakagawa C, Takei H, et al. Prospective study of the effect of the 21-gene assay on adjuvant clinical decision-making in Japanese women with estrogen receptor-positive, node-negative, and node-positive breast cancer. Clin Breast Cancer. 2014;14:191–197. doi: 10.1016/j.clbc.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Albanell J, Gonzales A, Ruiz-Borrego M, et al. Prospective transGEICAM Study of Oncotype DX in clinical decision making in women with estrogen receptor-positive, node-negative breast cancer. Ann Oncol. 2012;23:625–631. doi: 10.1093/annonc/mdr278. [DOI] [PubMed] [Google Scholar]
- 21.Eiermann W, Rezai M, Kümmel S, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol. 2013;24:618–624. doi: 10.1093/annonc/mds512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt S, Bertelli G, Humphreys I, et al. A decision impact, decision conflict and economic assessment of routine Oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer. 2013;108:2250–2258. doi: 10.1038/bjc.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 24.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: An economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 25.Tsoi DT, Inoue M, Kelly CM, et al. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. The Oncologist. 2010;15:457–465. doi: 10.1634/theoncologist.2009-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klang SH, Hammerman A, Liebermann N, et al. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health. 2010;13:381–387. doi: 10.1111/j.1524-4733.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 27.Kondo M, Hoshi SL, Yamanaka T, et al. Economic evaluation of the 21-gene signature (Oncotype DX) in lymph node-negative/positive, hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03) Breast Cancer Res Treat. 2011;127:739–749. doi: 10.1007/s10549-010-1243-y. [DOI] [PubMed] [Google Scholar]
- 28.de Lima Lopes G, Chien R, Hornberger J. Cost-benefit of the 21-gene breast cancer recurrence score assay for patients in Singapore. Breast J. 2013;19:220–221. doi: 10.1111/tbj.12092. [DOI] [PubMed] [Google Scholar]
- 29.Madaras B, Rózsa P, Gerencsér Z et al. Evaluation of the cost-effectiveness of Oncotype-DX multigene assay in Hungary. Paper presented at: International St. Gallen Breast Cancer Conference; March 16–19, 2011; St. Gallen, Switzerland. [Google Scholar]
- 30.Pronzato P, Plun-Favreau J. Is the 21-gene breast cancer test (Oncotype DX) cost-effective? Paper presented at: San Antonio Breast Cancer Symposium; December 6–10, 2011; San Antonio, TX. [Google Scholar]
- 31.Holt S, Bertelli G, Humphreys I, et al. A decision impact, decision conflict and economic assessment of routine Oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer. 2013;108:2250–2258. doi: 10.1038/bjc.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vataire AL, Laas E, Aballéa S, et al. Cost-effectiveness of a chemotherapy predictive test [in French] Bull Cancer. 2012;99:907–914. doi: 10.1684/bdc.2012.1652. [DOI] [PubMed] [Google Scholar]
- 33.Blohmer JU, Rezai M, Kümmel S, et al. Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early-stage breast cancer: A cost-effectiveness evaluation in the German setting. J Med Econ. 2013;16:30–40. doi: 10.3111/13696998.2012.722572. [DOI] [PubMed] [Google Scholar]
- 34.Gligorov J. RPC Saint-Paul-de-Vence: Deuxième [in French] Oncologie. 2007;9:1–96. [Google Scholar]
- 35.Oratz R, Kim B, Chao C, et al. Physician survey of the effect of the 21-gene recurrence score assay results on treatment recommendations for patients with lymph node-positive, estrogen receptor-positive breast cancer. J Oncol Pract. 2011;7:94–99. doi: 10.1200/JOP.2010.000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stemmer SM, Klang SH, Ben-Baruch N, et al. The impact of the 21-gene Recurrence Score assay on clinical decision-making in node-positive (up to 3 positive nodes) estrogen receptor-positive breast cancer patients. Breast Cancer Res Treat. 2013;140:83–92. doi: 10.1007/s10549-013-2603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]