Abstract
Lessons Learned
Oraxol, a novel oral formulation of paclitaxel, displayed modest efficacy as second-line chemotherapy for gastric cancer.
Considering its favorable toxicity profiles, further studies are warranted in various solid tumors including gastric cancer.
Background.
Oraxol consists of paclitaxel and HM30181A, a P-glycoprotein inhibitor, to increase the oral bioavailability of paclitaxel. This phase I/II study (HM-OXL-201) was conducted to determine the maximum tolerated dose (MTD) and recommended phase II dose (RP2D) of Oraxol. In addition, we investigated the efficacy and safety of Oraxol as second-line chemotherapy for metastatic or recurrent gastric cancer (GC).
Methods.
In the phase I component, paclitaxel was orally administered at escalating doses (90, 120, or 150 mg/m2 per day) with a fixed dose (15 mg/day) of HM30181A. Oraxol was administrated 6 times per cycle (days 1, 2, 8, 9, 15, and 16) every 4 weeks. In the phase II component, the efficacy and safety of Oraxol were evaluated.
Results.
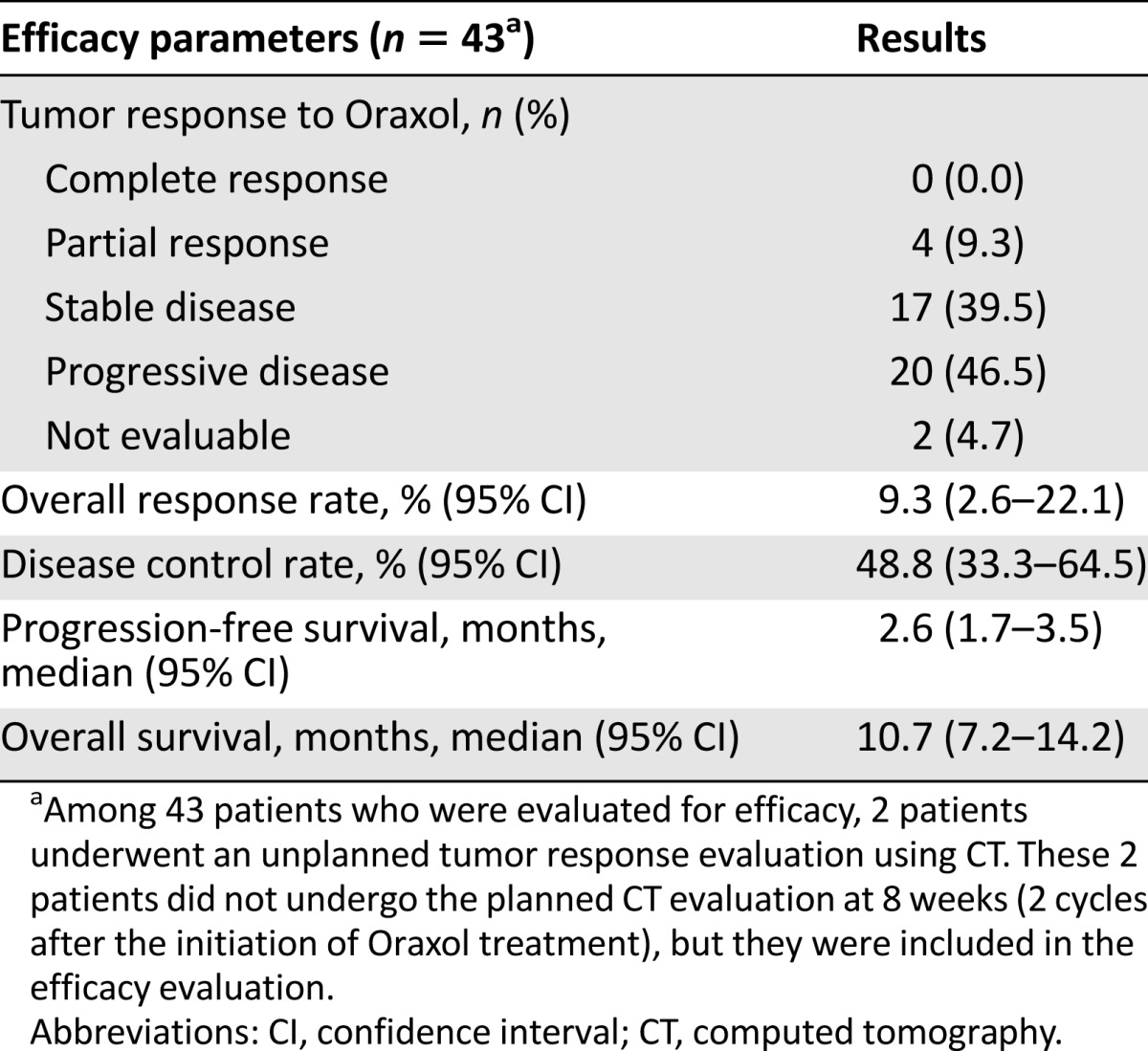
In the phase I component, the MTD could not be determined. Based on toxicity and pharmacokinetic data, the RP2D of oral paclitaxel was determined to be 150 mg/m2. In the phase II component, 4 of 43 patients (9.3%) achieved partial responses. Median progression-free survival and overall survival were 2.6 and 10.7 months, respectively. Toxicity profiles were favorable, and the most common drug-related adverse events (grade ≥3) were neutropenia and diarrhea.
Conclusion.
Oraxol exhibited modest efficacy and favorable toxicity profiles as second-line chemotherapy for GC.
Abstract
摘要
背景. Oraxol由紫杉醇和HM30181A(一种P糖蛋白抑制剂,可增加紫杉醇的口服生物利用度)组成。本项I期/II期研究(HM-OXL-201)旨在确定Oraxol的最大耐受剂量(MTD)和II期推荐研究剂量(RP2D)。此外,我们还对Oraxol用于转移性或复发性胃癌(GC)二线化疗的有效性和安全性进行了研究。
方法. 在I期研究阶段,紫杉醇经口服给药且剂量逐渐提升(90、120和150 mg/m2/天),HM30181A按固定剂量给药(15 mg/天)。Oraxol每周期给药6次(第1、2、8、9、15和16天),每4周为一周期。在II期研究阶段评价Oraxol的有效性和安全性。
结果. I期研究阶段未能确定MTD。依据毒性和药代动力学数据,确定口服紫杉醇的RP2D为150 mg/m2。在II期研究阶段,4/43例患者(9.3%)达到部分缓解。中位无进展生存和总生存分别为2.6个月和10.7个月。毒性特征谱良好,最常见的药物相关不良事件(≥ 3级)为中性粒细胞减少症和腹泻。
结论. Oraxol用于胃癌二线化疗时表现出一定的有效性和良好的毒性特征谱。The Oncologist 2015;20:896–897
Author Summary
Discussion
Paclitaxel has been administrated intravenously because of its poor oral bioavailability. Because paclitaxel is insoluble in water, the original formulation of paclitaxel contains the vehicle Cremophor EL (CrEL); however, the addition of CrEL causes hypersensitivity reactions and exerts an additive effect on paclitaxel-induced neuropathy. The original formulation of paclitaxel inconveniences patients and increases the risk of toxicities. Consequently, there have been many efforts to develop a new formulation of paclitaxel.
Oraxol is composed of a paclitaxel capsule and an HM30181A tablet (Hanmi Pharmaceutical Co. Ltd., Seoul, Republic of Korea, http://www.hanmipharm.com). HM30181A, [2-(2-{4-[2-(6,7-dimeth,oxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-phenyl}-2H-tetrazol-5-yl)-4,5-dimethoxyphenyl]amide, is a novel inhibitor of P-glycoprotein in the gastrointestinal mucosa. In this phase I/II study (HM-OXL-201), both paclitaxel and HM30181A were administered simultaneously on an empty stomach.
In the phase I component of this study (n = 10), no dose-limiting toxicity was observed, and thus the MTD could not be determined. In gastric cancer cell lines, paclitaxel exhibited cytotoxicity at concentrations >0.01 μM. In the pharmacokinetic analysis, the means of T>0.01 (time of plasma concentration of paclitaxel >0.01 μM) at three paclitaxel dose levels were 17.7, 43.2, and 47.5 hours, respectively. The area under the plasma concentration-time curves also increased according to the paclitaxel dose. Based on these toxicity and pharmacokinetic data, dose level 3 (oral paclitaxel 150 mg/m2 per day and HM30181A 15 mg/day, both on days 1, 2, 8, 9, 15, and 16 every 4 weeks) was determined as the RP2D.
In the phase II component (n = 46), this weekly Oraxol regimen displayed favorable toxicity profiles. The incidence of severe neutropenia (grade ≥3) was 30.4%, which was similar to that reported in previous phase III trials of conventional weekly paclitaxel (second line) in metastatic or recurrent GC. Severe nonhematologic toxicities were rare. Particularly, Oraxol appears to cause less peripheral neuropathy than conventional weekly paclitaxel. In our study, weekly Oraxol was associated with a response rate (RR) of 9.3% and progression-free survival (PFS), and overall survival (OS) of 2.6 and 10.7 months, respectively (Table 1). Statistically, our study did not meet the primary endpoint (RR); however, clinically, Oraxol appears to have efficacy similar to other cytotoxic agents commonly used as second-line chemotherapy in metastatic or recurrent GC. Regarding conventional weekly paclitaxel, RRs of 9%–20.9% and PFS and OS of 2.9–4.4 and 7.4–9.5 months, respectively, were reported. Although weekly Oraxol treatment did not meet the primary endpoint in this study, we demonstrated that Oraxol has its own advantages (favorable safety profiles, including less neuropathy and no hypersensitivity reactions, and the convenience of oral administration) over conventional paclitaxel. Consequently, we believe that Oraxol is worthy of further investigation. In particular, the combination of Oraxol with various chemotherapeutic agents is expected to be very promising because Oraxol displayed favorable toxicity profiles.
Table 1.
Efficacy results of the phase II component
Supplementary Material
Footnotes
Access the full results at: Bang-15-202.theoncologist.com
ClinicalTrials.gov Identifier: NCT01491217
Sponsor(s): Hanmi Pharmaceutical Co., Ltd., Seoul, Republic of Korea
Principal Investigator: Yung-Jue Bang
IRB Approved: Yes
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


