This study investigated the use and effectiveness of adjuvant trastuzumab in daily practice compared with the effectiveness in clinical trials to assess the actual use of adjuvant trastuzumab in early human epidermal growth factor receptor 2 (HER2)-positive breast and its efficacy in daily practice. This study shows that in real life, patients treated with trastuzumab in early-stage HER2-positive breast cancer had a 5-year disease-free and overall survival comparable to prior randomized trials.
Keywords: Trastuzumab, Effectiveness, Early breast cancer, Real life
Abstract
Background.
The impact of drug prescriptions in real life as opposed to strict clinical trial prescription is only rarely assessed, although it is well recognized that incorrect use may harm patients and may have a significant impact on health care resources. We investigated the use and effectiveness of adjuvant trastuzumab in daily practice compared with the effectiveness in clinical trials.
Methods.
We included all patients with stage I–III invasive breast cancer, irrespective of human epidermal growth factor receptor 2 (HER2) status, diagnosed in five hospitals in the southeast of The Netherlands in 2005–2007. We aimed to assess the actual use of adjuvant trastuzumab in early HER2-positive breast and its efficacy in daily practice.
Results.
Of 2,684 patients included, 476 (17.7%) had a HER2-positive tumor. Of these, 251 (52.7%) patients had an indication for trastuzumab treatment of which 196 (78.1%) patients actually received it. Of the 225 patients without an indication, 34 (15.1%) received trastuzumab. Five-year disease-free survival was 80.7% for (n = 230) patients treated with versus 68.2% for (n = 246) patients not treated with trastuzumab (p = .0023), and 5-year overall survival rates were 90.7% and 77.4%, respectively (p = .0002). The hazard ratio for disease recurrence was 0.63 (95% confidence interval, 0.37–1.06) for trastuzumab when adjusting for potential confounders.
Conclusion.
This study shows that in real life, patients treated with trastuzumab in early-stage HER2-positive breast cancer had a 5-year disease-free and overall survival comparable to prior randomized trials. For informative decision making, real-life data are of additional value, providing insight on outcome of patients considered ineligible for treatment.
Implications for Practice:
When new drugs are introduced, knowledge about the correct prescription and impact on health care resources is much appreciated. In the first years after the introduction of trastuzumab in The Netherlands, its use was in accordance with the eligibility criteria of the randomized trials. However, currently almost all patients with HER2-positive disease will be offered trastuzumab. This study shows that real-life studies can provide insight into smaller specific patient groups that might benefit from trastuzumab. This may quicken the expansion of indications for drug use, as real life tends to become less strict with growing expertise. Therefore, real-life data are of additional value for informative decision-making, as they provide insight on the outcome of patients considered ineligible for treatment in initial clinical trials.
Introduction
In The Netherlands, approximately 14,000 patients are diagnosed with invasive breast cancer annually [1]. Human epidermal growth factor receptor 2 (HER2) overexpression is present in 15%–20% of new breast cancer cases. Trastuzumab is a monoclonal antibody against the HER2 receptor. Its effectiveness and safety in patients with a HER2-positive tumor in the adjuvant setting has been confirmed in six phase III studies [2–6]. In The Netherlands, the price of trastuzumab is approximately 40,000 Euros per patient per year of treatment [7]. The Dutch Health Care Insurance Board permitted provisional reimbursement of trastuzumab as adjuvant treatment. Continued reimbursement from 2012 and onward depended on study of prospective data on the cost-effective use of trastuzumab in daily practice within The Netherlands. When introducing a new drug, such as trastuzumab, knowledge of correct prescription and impact on health care resources is well-appreciated. Along with prescription derived from the trials, patients with node-negative, small, well-differentiated HER2-positive tumors may also benefit from chemotherapy and trastuzumab, because these patients are known to have a significant risk of relapse when left untreated [8, 9]. In the St. Gallen guidelines of 2011, it was recommended to treat patients with node-negative HER2-positive tumors (pT1bN0 tumors) with adjuvant trastuzumab [10]. In this study, we assessed the use and effectiveness of adjuvant trastuzumab in daily practice compared with the effectiveness in clinical trials.
Materials and Methods
Study Design and Data Collection
We performed an observational cohort study, in which we collected data from all patients with stage I–III breast cancer diagnosed between January 2005 and December 2007 in five Dutch hospitals in the southeast of The Netherlands. Patients were identified by the Netherlands Cancer Registry. Only patients with a HER2-positive tumor were included for this present analysis. Immunohistochemistry was used to determine HER2 status. In case of a 2+ score, in situ hybridization analysis was performed. Once amplification was seen, these patients were also considered HER2-positive.
This study was approved by the medical ethical committee of the Maastricht University Medical Center. The need for obtaining informed consent was waived because of the observational design of the study.
Between 2009 and 2011, data were collected from the patient files by trained data managers. If HER2 status was missing, the HER2 status was centrally reassessed by a breast-dedicated pathologist (B.d.V.). Patients who had a positive HER2 status after central pathology review were also included.
Study Goals
In this study, we first assessed whether adjuvant trastuzumab was given according to the national guidelines at that time. Secondly, we assessed the effectiveness of adjuvant trastuzumab in daily practice and when given according to guidelines and compared this to patient selection for and effectiveness of trastuzumab in clinical trials. Thirdly, we assessed factors that influenced the real-life effectiveness.
Guidelines for Adjuvant Systemic Therapy
In 2005, the criteria in The Netherlands to be a candidate for adjuvant trastuzumab included HER2-positive, node-positive disease present in a patient younger than 60 years of age; 4 or more positive nodes or hormone receptor-negative disease present in a patient between 60 and 70 years of age; a tumor of 10–20 mm and grade III, 20–30 mm and grade II or III, or more than 30 mm of any grade present in a patient with node-negative disease below 60 years; and hormone receptor-negative disease present in a patient below 70 years [11] (supplemental online Table 1).
Definition of Endpoints
Disease-free survival was defined as the interval from the date of diagnosis to the first occurrence of recurrence of breast cancer at any site, second non-breast cancer, or death from any cause, whichever occurred first. Overall survival was defined as the interval from the date of first diagnosis to the date of death. If a patient was still alive and disease-free on October 21, 2011, the date of last follow-up, the patient was censored at that date.
Statistical Analysis
The primary endpoint of our study was 5-year disease-free survival in real life for patients treated with versus without trastuzumab. To compare baseline differences between these two groups, chi-square tests were performed for categorical variables and Mann-Whitney tests for continuous and ordinal variables. Kaplan-Meier estimates for the 5-year disease-free and overall survival rates were calculated, and the Kaplan-Meier curves over time for the two groups were compared with the log-rank test. Cox proportional hazard regression analyses were used to assess the influence of trastuzumab on outcome, correcting for age, comorbidity, and tumor characteristics (which included pathological tumor size, nodal status, and histological grade). Comorbidity was defined by the Charlson Comorbidity Index (CCI) [12], an age-adjusted measure of comorbidity in which different weights were ascribed according to the severity of the comorbidity. Records on comorbidity were kept during data collection. All reported p values were two-sided, and confidence intervals were set at 95%. All analyses were performed using SAS software version 9.2.
Results
Patient Inclusion
We included 2,684 patients. Central pathology review in 106 patients with missing HER2 status revealed that 3 (2.8%) had HER2-positive tumors, and 66 (63%) had HER2-negative tumors. In 37 patients, reassessment of the HER2 status was impossible because of insufficient tumor material. The low HER2-positivity rate on revision may be explained by the high average age (generally 80+) in the patients with missing data and the fact that age shows a strong negative correlation with the risk of a positive HER2 status [13]. Of the 2,684 patients, 476 (17.7%) had a confirmed HER2-positive tumor status.
Guideline Adherence
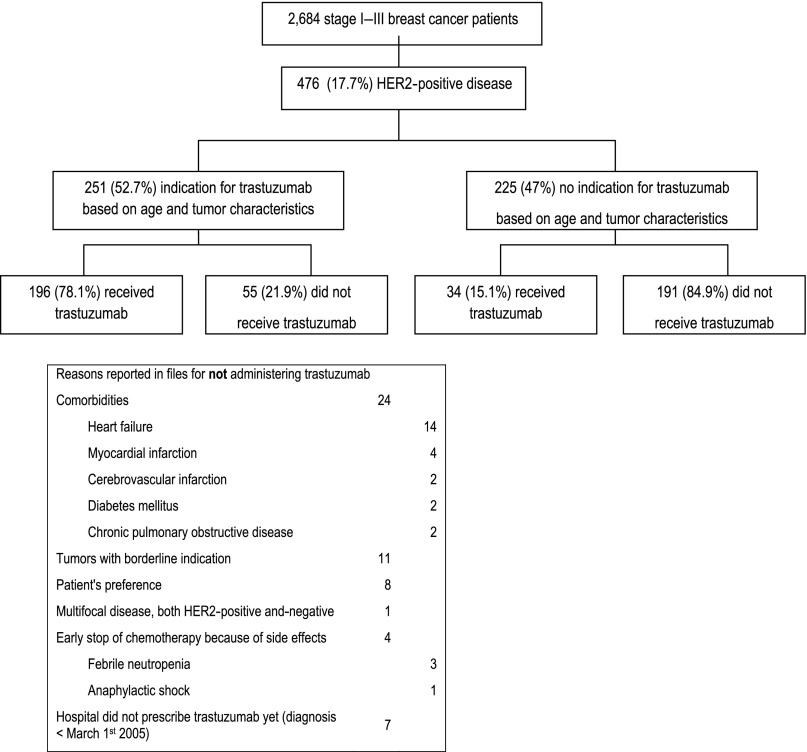
In the HER2-positive group, chemotherapy and trastuzumab were indicated according to the guidelines as described above in 251 (52.7%) patients based on age and tumor characteristics (Table 1; Fig. 1): 196 (78.1%) patients were treated according to indication, and 55 patients (21.9%) did not receive adjuvant trastuzumab. Reasons not to offer trastuzumab included serious comorbidities (24 patients) and borderline indication and/or patient preference (19 patients). Seven patients did not receive trastuzumab because of diagnosis before the introduction of trastuzumab in September 2005.
Table 1.
Patient, tumor, and treatment characteristics of HER2-positive patients with or without indication trastuzumab treatment according to 2005 Dutch guidelines
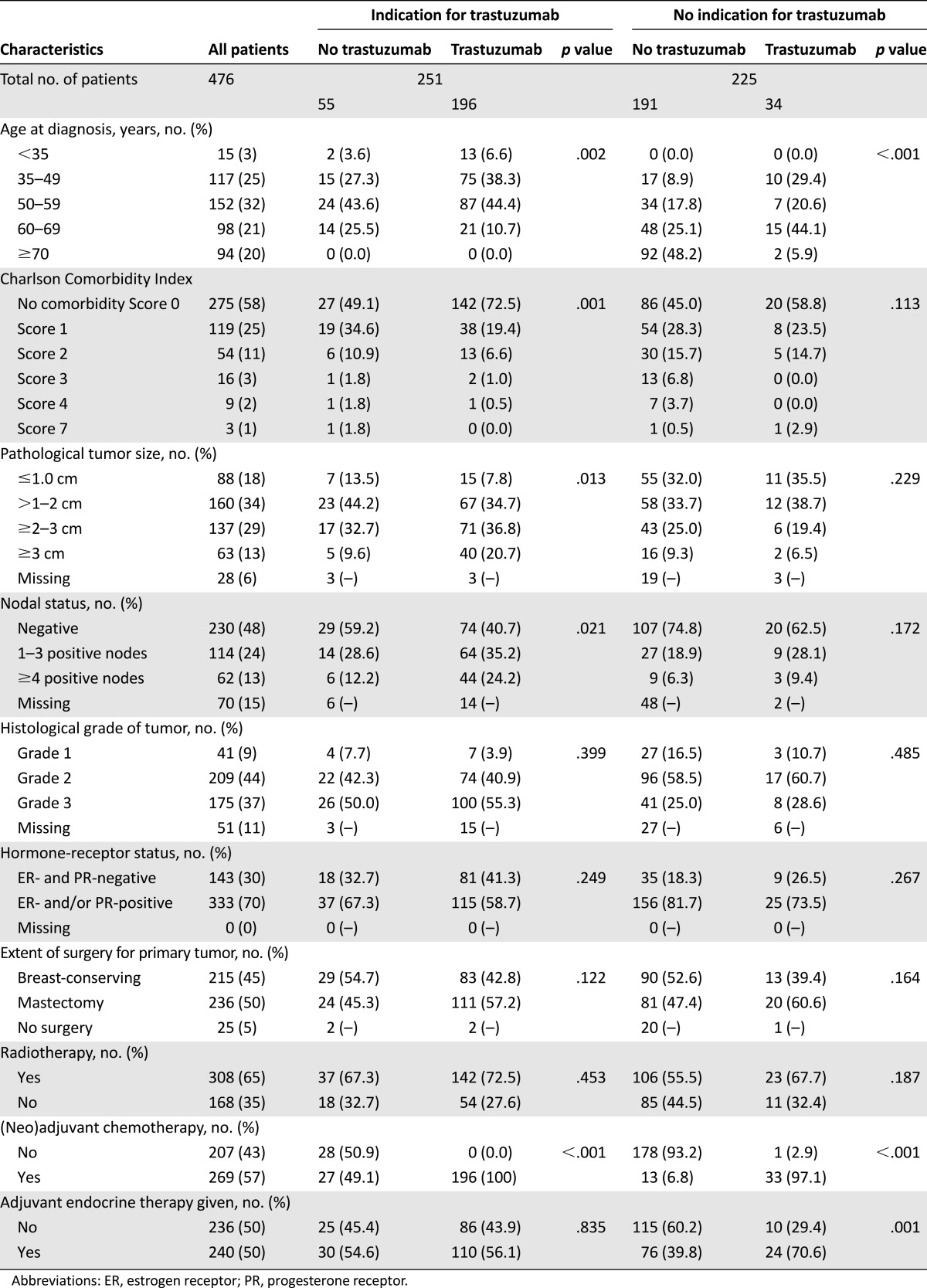
Figure 1.
Actually delivered adjuvant trastuzumab in the years 2005–2007 in the southeast part of The Netherlands in patients consecutively diagnosed with stage I–III breast cancer related to prevailing guidelines at that time.
Abbreviation: HER2, human epidermal growth factor receptor 2.
There were 225 (47.3%) patients without an indication to receive trastuzumab, of whom 191 (84.9%) did not receive trastuzumab. However, 34 patients (15.1%) did receive trastuzumab in spite of the absence of an indication. The majority was of an age or had tumor characteristics that only slightly deviated from the treatment guidelines of 2005. (Table 1) With the current guidelines, all of these 34 patients would qualify for trastuzumab treatment.
Real-Life Patient and Treatment Characteristics
Patients treated with trastuzumab had a median age of 51 years (range 27–72). Median tumor size was 22 mm (range 0–100), with node-positive disease in 56% and a positive hormone-receptor status in 62% of patients. Trastuzumab was delivered for a median 17 cycles (range 2–37).
Baseline patient and tumor characteristics were comparable to those in the HERA [2] trial, NCCTG N9831 [14] and NSABP B-31 [14] trials (supplemental online Table 2). However, in our population, more patients with node-negative tumors received adjuvant systemic therapy.
As a result of selection, the baseline characteristics of patients not treated with trastuzumab, in real life, were different from those who did receive trastuzumab. They were significantly older (median 65 years, range 33–93 years versus median 51 years, range 27–72, p < .0001), were more likely to have comorbidity (54.1% versus 29.6%, p < .0001), and received less adjuvant chemotherapy (16.3% versus 99.6%, p < .0001) compared with those treated with trastuzumab.
In daily practice, treatment with trastuzumab was mostly (n = 187; 80%) initiated after finishing treatment with chemotherapy, in accordance with the protocol of the HERA trial [2]. In 18% (n = 42) of patients, trastuzumab was started concurrently with taxane treatment according to the U.S. pivotal trials [14]. Sequential administration of trastuzumab after chemotherapy was mostly given in 2005 and 2006; in 2007, concurrent treatment was given more often. One patient (0.4%) received trastuzumab without adjuvant chemotherapy. In 52 of 230 (23%) patients, neoadjuvant chemotherapy and trastuzumab were administered. The chemotherapy regimes in patients receiving (neo)adjuvant trastuzumab consisted of antracycline (37%), antracycline and taxane (60%), taxane (2%), and no-antracycline, no-taxane-based therapy (0.9%) (supplemental online Table 2). Consequently, in only 3 of 230 (1%) patients, trastuzumab administration was not according to the labeled indication. We also monitored the incidence of cardiotoxicity; there were 36 patients who permanently stopped trastuzumab treatment because of presumed cardiotoxicity. Further data on cardiotoxicity will be shown elsewhere. No deaths caused by trastuzumab were seen.
Disease-Free and Overall Survival in Relation to Use of Trastuzumab
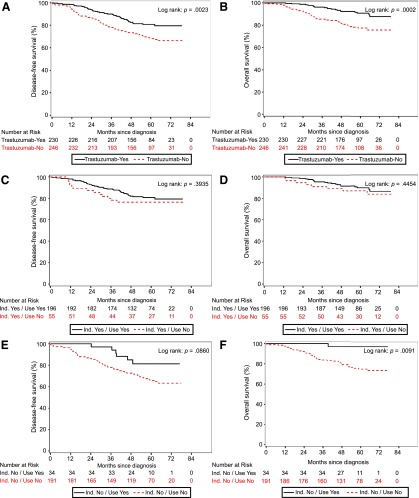
The median follow-up of the patients in this real-life study was 5.0 years (range 3.2–6.7). Five-year disease-free survival rate was 80.7% for 230 patients treated with trastuzumab versus 68.2% for 246 patients not treated with trastuzumab (p = .0023). The 5-year overall survival rates were 90.7% (trastuzumab) versus 77.4% (no trastuzumab), respectively (p = .0002). In the Cox proportional hazard model correcting for age, comorbidity, and tumor characteristics (size, nodal status, and grade), the administration of trastuzumab resulted in a hazard ratio for disease recurrence of 0.63 (95% confidence interval [CI], 0.37–1.06) (Tables 2, 3).
Table 2.
The HR for the effect on DFS and OS of treatment with trastuzumab (compared with without trastuzumab) corrected for one risk factor
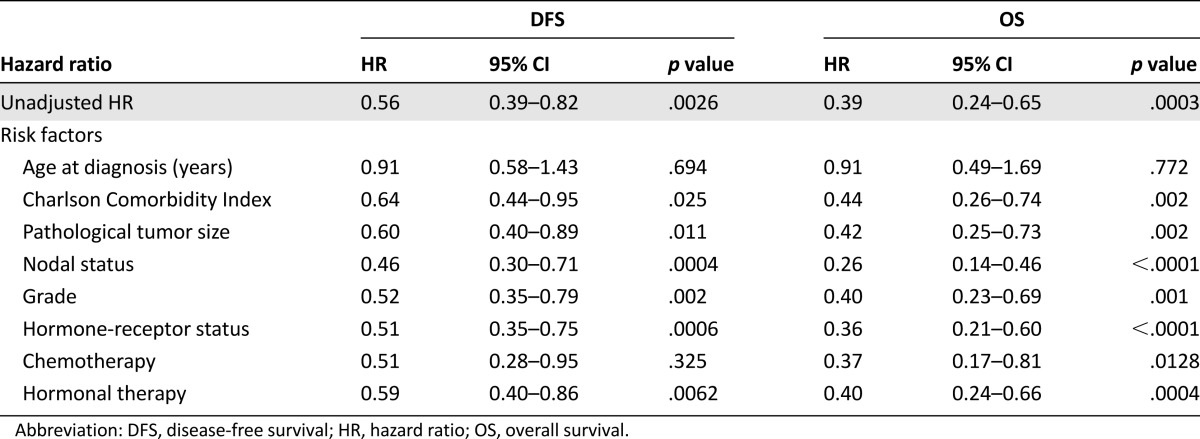
Table 3.
Unadjusted HR and hazard ratios adjusted for a combination of risk factors for the effect on DFS and OS of treatment with trastuzumab (compared with without trastuzumab)
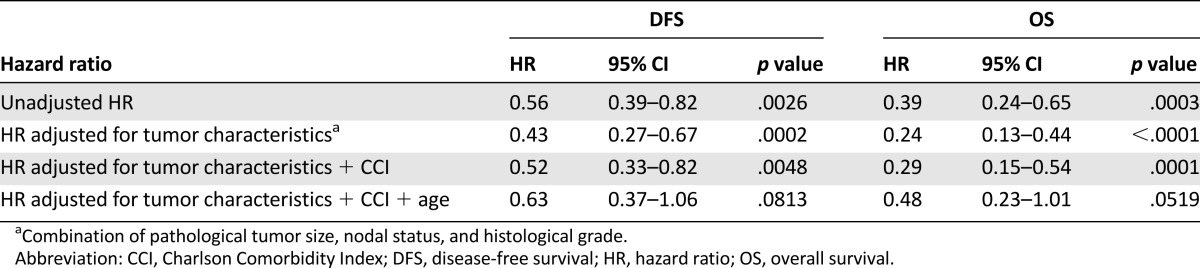
Additionally, we analyzed survival in patients with an indication for trastuzumab according to the 2005 guidelines, for both patients who did receive trastuzumab (n = 196) and those who did not receive trastuzumab for various reasons (mostly comorbidity) (n = 55). The 5-year disease-free survival was 80.6% in the patients treated with trastuzumab and 76.4% in those without trastuzumab (p = .3935). The 5-year overall survival rates were 89.7% (trastuzumab) versus 87.0% (no trastuzumab), respectively (p = .45). The Cox proportional hazards model resulted in a hazard ratio for disease recurrence of 0.56 (95% CI, 0.27–1.17).
In patients with no indication for trastuzumab according to the 2005 guidelines, the 5-year disease-free survival was 81.3% (n = 34) (trastuzumab) versus 65.8% (n = 191) (no trastuzumab), (p = .086). The 5-year overall survival rates were 97.1% and 74.7%, respectively (p = .009). In the multivariable analysis, the administration of trastuzumab resulted in a hazard ratio for disease recurrence of 0.75 (95% CI, 0.30–1.88) (Fig. 2).
Figure 2.
Survival of patients diagnosed with HER2-positive disease in the southeast Netherlands diagnosed in 2005–2007, receiving trastuzumab. Kaplan-Meier estimates of disease-free survival (A) and overall survival (B) of patients treated with 1 year trastuzumab versus no trastuzumab in real life irrespective of eligibility according to 2005 guidelines. Disease-free survival (C) and overall survival (D) of patients treated with or without trastuzumab while having an indication according to 2005 Dutch guidelines. Disease-free survival (E) and overall survival (F) of patients treated with or without trastuzumab with no indication according to 2005 Dutch guidelines. Disease-free survival included recurrence of breast cancer at any site, ipsilateral or contralateral breast cancer, second non-breast malignant disease, or death from any cause. Overall survival is defined as the interval from the date of first diagnosis to the date of death or date of last follow-up.
Abbreviation: Ind., Indication.
Factors That Influenced Disease-Free Survival/Real-Life Effectiveness of Trastuzumab
Trastuzumab treatment improved the disease-free survival; a hazard ratio for disease recurrence of 0.56 (95% CI, 0.39–0.82) was observed in the unadjusted analysis. The patients treated with trastuzumab generally had less favorable tumor characteristics (Table 1). After adjustment for these tumor characteristics in the multivariable analysis, the disease-free survival was even more favorable with a hazard ratio of 0.43 (95% CI, 0.27–0.67). The more favorable patient characteristic, age and comorbidity (Table 1), in the users contributes to this positive outcome. The adjusted hazard ratio corrected for tumor and patients characteristics appeared to be 0.63 (95% CI, 0.37–1.06) (supplemental online Table 2).
Discussion
In this study, we assessed to whom adjuvant trastuzumab was given in daily practice in the initial years since its reimbursement in September 2005 in The Netherlands and with what outcome. Of 2,684 patients diagnosed with stage I–III breast cancer, 17.7% had HER2-positive disease. The administration of trastuzumab was according to the label in 99% of patients. Approximately half of the patients with HER2-positive disease did not receive adjuvant trastuzumab: three-quarters because of the lack of an indication according to prevailing guidelines in 2005 and nearly one-fifth because of comorbidities or patient preference. Of the patients who received trastuzumab, 85% fulfilled the criteria of 2005 guidelines, and the remaining 15% would have had an indication according to the 2012 guidelines (supplemental online Table 1). Outcome was significantly improved by the use of trastuzumab, and the risk on recurrence was significantly reduced. Patients treated with trastuzumab were younger and had less comorbidity than patients not treated but instead had less favorable tumor characteristics.
Only a few studies have addressed the real-life implementation of adjuvant trastuzumab. de Munck et al. [13] reported that of all women with HER2-positive breast cancers receiving adjuvant chemotherapy, only 6% did not receive trastuzumab. The most common reasons to withhold trastuzumab were cardiovascular disease and patient refusal. In our cohort, 3% of patients did not start with trastuzumab. Whitfield et al. [15] reported for Australia and New Zealand that during 2006 and 2008, the percentage of breast cancers tested rose from 77% to 91%. In our cohort, only 4% of HER2 results were missing. Of their patients, 14% had HER2-positive tumors, which is comparable to our 17.7%. Remarkably, in the Australian study, the proportion of patients with HER2-positive tumors treated with trastuzumab rose to 74% in 2008, compared with 48% in our cohort. Currently, our national guidelines also have extended the indication for trastuzumab to smaller tumors. In a German study, 17.3% of patients had a HER2-positive tumor in 2007, and 61% of the patients with a HER2-positive breast tumor larger than 1 cm received trastuzumab [16]. In contrast to our real-life analyses, patients with node-negative breast cancer of >0.5 cm can be considered for adjuvant trastuzumab according to current guidelines. Furthermore, age is by itself not an exclusion criteria anymore. If a patient is above 70 years of age but yet in a clinical good condition, adjuvant treatment with trastuzumab may be an option depending on tumor characteristics. According to the national guidelines of 2005, patients above 70 (in some cases 60) years of age were generally considered not eligible for adjuvant chemotherapy and for that reason also not for adjuvant trastuzumab. However, as the average age of people all over the world is increasing and the elderly are becoming more vital, nowadays we discuss adjuvant treatment options with those above 70 years of age. Nonetheless, we notice within our country that most elderly patients look differently at calculated benefits and risks of additional treatment. As a result, the majority still does not choose to be treated with systemic therapy apart from endocrine therapy.
The age and tumor characteristics of the patients treated with trastuzumab in our cohort study largely resembled those of the prior reported randomized trials [2–6]. One difference is that in our daily practice, more patients with node-negative tumors received adjuvant systemic therapy. Although the benefit of trastuzumab is comparable in node-positive and node-negative disease, the absolute benefit results were smaller for the more favorable node-negative patient group, which accounts for 50% of our HER2-positive group.
Furthermore, 10%–15% of breast cancer patients have a cardiovascular disease. These patients have been largely excluded from treatment with trastuzumab, both in randomized trials and in daily clinical practice. In our analysis, we corrected for this factor by using the CCI, which resulted in a slight increase of the hazard ratio but still confirmed the favorable outcome for patients treated with trastuzumab. More important, what can be learned from a real-life analysis in contrast to results from randomized controlled trials is the size and the outcome of patients that are not candidates for the new treatment because of contraindications.
The disease-free survival results for patients treated with and without trastuzumab in real life are well comparable to those in the pivotal randomized controlled trials [14, 17, 20, 21] (supplemental online Table 3). It brings reassurance when eligibility and treatment results of clinical trials can be translated to the real-life setting. It confirms that implementation in real life has been successful. Additional information was found when comparing treatment with trastuzumab with or without indication. Hence, for reliable assessment of real-life effectiveness, we recommend assessing the outcome of both treated and untreated patients and whether their outcome is in line with clinical trial results or not. Thus it can be confirmed that implementation in real life is effectively done.
Additional improvement in treatment of adjuvant breast cancer patients has been achieved by the introduction of taxanes in the same period of time as trastuzumab. Taxanes established additional improvement in disease-free survival compared with trastuzumab and anthracyclines alone [5, 18]. In our multivariable analysis, we observed a trend for additional benefit of taxanes in patients with HER2-positive disease compared with those with HER2-negative disease (data not further shown).
A limitation of our study, related to its observational design, is that some missing data could not be retrieved. Also the number of patients in some subgroups became too small to draw firm conclusions.
To our knowledge, this is one of the few studies in which real-life treatment decisions on use of adjuvant trastuzumab were related to outcome. A study that did look at outcome was that of Seal et al. [19]; this was a population-based analysis that found that adjuvant trastuzumab use demonstrates excellent survival outcomes among women with HER2-positive early breast cancer. A randomized trial shows the maximum treatment effect that can be obtained by optimal patient selection. In real life, indications for use will generally get less strict with growing expertise. In the first years after the introduction of trastuzumab in The Netherlands, its use was in accordance with the eligibility criteria of the randomized trials. However, currently almost all patients with HER2-positive disease will be offered trastuzumab.
Conclusion
Adherence in real life to guidelines regarding indications for trastuzumab was excellent. The characteristics and the survival rates of the patients who received trastuzumab were comparable to those in the clinical trials. For informative decision making, real-life data may be of additional value, because real-life studies provide insight in specific patient groups that cannot be obtained from randomized trials.
Supplementary Material
Acknowledgments
We thank Wim A.J.G. Lemmens for his assistance with statistical analysis. This work was supported by Netherlands Organization for Health Research and Development Grant ZonMw 80-82500-98-9056 and Roche Netherlands.
Author Contributions
Conception/Design: Shanly C. Seferina, Manuela A. Joore, Adri C. Voogd, Vivianne C.G. Tjan-Heijnen
Provision of study material or patients: Shanly C. Seferina, Dorien J.A. Lobbezoo, M. Wouter Derckson, Franchette van den Berkmortel, Roel J.W. van Kampen, Agnès J. van de Wouw
Collection and/or assembly of data: Shanly C. Seferina, Dorien J.A. Lobbezoo, Roel J.W. van Kampen, Agnès J. van de Wouw
Data analysis and interpretation: Shanly C. Seferina, Maaike de Boer, Bart de Vries, Manuela A. Joore, Petronella G.M. Peer, Adri C. Voogd, Vivianne C.G. Tjan-Heijnen
Manuscript writing: Shanly C. Seferina, Dorien J.A. Lobbezoo, Maaike de Boer, M. Wouter Derckson, Franchette van den Berkmortel, Roel J.W. van Kampen, Agnès J. van de Wouw, Bart de Vries, Manuela A. Joore, Petronella G.M. Peer, Adri C. Voogd, Vivianne C.G. Tjan-Heijnen
Disclosures
Vivianne C.G. Tjan-Heijnen: Roche BV (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Richtlijn mammacarcinoom version 2.0 Consensus based 2012-02-13. Available at http://www.oncoline.nl. 2012. Accessed February 24, 2012.
- 2.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 6.Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 7.Vos EJ, Linn SC, Rodenhuis S. [Effects and costs of adjuvant chemotherapy for operable lymph node positive breast cancer with HER2/neu overexpression] Ned Tijdschr Geneeskd. 2006;150:776–780. [PubMed] [Google Scholar]
- 8.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27:5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: Summary of the Consensus Discussion. Breast Care (Basel) 2011;6:136–141. [DOI] [PMC free article] [PubMed]
- 11.Richtlijn “Behandeling van het Mammacarcinoom.” Utrecht, The Netherlands: Kwaliteitsinstituut voor de Gezondheidszorg CBO/Vereniging van Integrale Kankercentra en Nationaal Borstkanker Overleg Nederland (NABON), 2005.
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.de Munck L, Schaapveld M, Siesling S, et al. Implementation of trastuzumab in conjunction with adjuvant chemotherapy in the treatment of non-metastatic breast cancer in the Netherlands. Breast Cancer Res Treat. 2011;129:229–233. doi: 10.1007/s10549-011-1451-0. [DOI] [PubMed] [Google Scholar]
- 14.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitfield R, Kollias J, De Silva P, et al. Use of trastuzumab in Australia and New Zealand: Results from the National Breast Cancer Audit. ANZ J Surg. 2012;82:234–239. doi: 10.1111/j.1445-2197.2011.05998.x. [DOI] [PubMed] [Google Scholar]
- 16.Liebrich C, Unger G, Dlugosch B, et al. Adopting guidelines into clinical practice: Implementation of trastuzumab in the adjuvant treatment of breast cancer in Lower Saxony, Germany, in 2007. Breast Care (Basel) 2011;6:43–50. doi: 10.1159/000324048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 19.Seal MD, Speers CH, O’Reilly S, et al. Outcomes of women with early-stage breast cancer receiving adjuvant trastuzumab. Curr Oncol. 2012;19:197–201. doi: 10.3747/co.19.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 21.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.