Abstract
Background and Objectives
Previous research has demonstrated that depressed individuals have difficulty both disengaging from negative information and maintaining positive information in working memory (WM). The present study was conducted to examine whether the tendency for depressed individuals to maintain negative content in WM and to experience difficulties maintaining positive content in WM is due to negative mood (in)congruency effects during a depressive episode, or whether these tendencies are evident outside of a depressive episode.
Methods
Individuals who had recovered from a depressive episode and never disordered controls performed emotion 0-back and 2-back tasks designed to assess biases in updating emotional content in working memory.
Results
Similar to currently depressed individuals in previous studies, recovered depressed participants disengaged from happy stimuli more quickly and from sad stimuli more slowly than did their never-depressed counterparts.
Limitations
Despite the extension of a depression-specific finding to recovered depressed individuals, the present study does not test whether the identified emotion updating biases predict long-term relapse or recovery.
Conclusion
The obtained results suggest that a decreased ability to disengage from negative content and to maintain positive content in WM represents a trait-like cognitive style that impairs adaptive emotion regulation and may contribute to the recurrent nature of depression.
Keywords: Depression, recovered, emotion, updating, working memory
1.1 Introduction
Investigators have documented biases in the processing of emotional information that may impair emotion regulation and maintain episodes of Major Depressive Disorder (MDD). Recently, using an eye-tracking task, Duque and Vázquez (2015) found that individuals diagnosed with MDD fixated longer on sad faces and spent less time looking at happy faces. Similarly, Levens and Gotlib (2010) found that depressed individuals have difficulty both disengaging from negative information and maintaining positive information in working memory (WM). Theorists have postulated that the preferential processing of negative information impairs the ability of depressed individuals to effectively regulate their affect (Gotlib & Joormann, 2010; Bistricky, Atchley, Ingram, & O’Hare, 2014; MacCoon & Newman, 2006). Less is known, however, about the persistence of emotion processing biases beyond the depressive episode. The goal of the present study is to address this gap by examining biases in emotion updating that have been found to characterize currently depressed persons in a sample of individuals who have recovered from a depressive episode.
WM is at the intersection of attention, executive functioning, short-term memory, and long-term memory (Dudai, 2002), each of which has been implicated in emotion processing biases in depression. WM principally reflects the focus of individuals’ attention and cognitive resources, representing what people are aware of and thinking about at any given moment (Baddeley, 1986; Miyake & Shaw, 1999). Importantly, WM has been implicated in the regulation of emotion; indeed, maintaining emotional content in WM has been found to affect both positive and negative mood (Isen, 1984; Russel, 2003). Therefore, it is critical to elucidate how emotion processing may interact with specific executive functions in WM as a function of a depressive state.
Updating is an executive process that involves modifying existing representations in WM to accommodate new information (Morris and Jones, 1990). Specifically, updating monitors and codes incoming information for relevance to the task, and appropriately reviews items held in WM by replacing old, no-longer-relevant information with newer, more relevant information (Morris & Jones, 1990). For example, when an individual begins a new task, the contents of WM are updated and representations or information relevant to the new task become active, while representations/information central to the previous task become less active and fall out of WM. Importantly, when a representation enters WM, it interacts with the existing content in WM, forming associations and possibly modifying the existing content in relation to the new content (Dudai, 2002). Given that updating continually codes new and existing WM content for task relevance, it is an important executive process that might be related to more complex executive functions like goal pursuit, planning, and adaptively responding to environmental demands (Hervey et al., 2004).
In addition to updating, set-shifting and inhibitory control have also been examined in the context of depression (e.g., Grant, Thase, & Sweeney, 2001; Harvey et al., 2004; Merriam et al., 1999). Importantly, confirmatory factor analyses indicate that set-shifting, inhibition, and updating are clearly separable (Miyake et al., 2000), suggesting that each contributes differentially to the execution of complex tasks such as goal pursuit and emotion regulation. In this context, Harvey et al. (2004) documented concurrent deficits in set-shifting, inhibition, and updating in depressed individuals, yet found that only deficits in updating were correlated with the number of hospitalizations and the longitudinal course of the depressive illness.
Because updating involves modifying existing information to accommodate new input, the influence of new information on existing content may be particularly salient when the new content is emotional, or valenced. For example, when updating processes admit sad content to WM, this content interacts with, forms associations with, and modifies the existing content already in WM. Neutral content in WM that becomes associated with sad content may become negatively valenced, and/or the intensity of sad content already in WM may become heightened by the additional sad content. Similarly, when sad content enters WM, it may interact with positive content already in WM to dampen the salience or arousal of the positive content. A tendency to more readily admit sad (or happy) content to WM that occurs consistently would represent a stable bias that could facilitate the formation and maintenance of negative (or positive) moods that underlie individual differences in emotion regulation.
Levens and Gotlib, (2010), Yoon, LeMoult, and Joormann (2014), and Joormann and Gotlib (2008) examined updating of emotional content in the context of depression. Levens and Gotlib (2010) investigated emotion updating in depression by modifying the commonly used n-back task to include emotional content. In their study, diagnosed depressed and never-disordered controls performed an emotion 2-back task in which they were presented with a series of happy, sad, and neutral faces and were asked to indicate whether the current face had the same emotional expression as that presented two faces earlier (which required that participants match set) or a different emotional expression as that presented two faces earlier (which required that participants integrate new content or break a previously matched set). Participants also performed a 0-back task with the same emotional stimuli to serve as a control for perceptual processing. Levens and Gotlib found that depressed and nondepressed participants exhibited biases in updating emotional content that reflected the tendency to keep negative and positive information active in WM. Compared with controls, depressed participants were both slower to disengage from sad stimuli and faster to disengage from happy facial expressions. In contrast, nondepressed controls took longer to disengage from happy than from neutral or sad stimuli.
Critically, Levens and Gotlib’s (2010) findings implicate executive processing biases in WM as an important factor in the maintenance and, possibly, the recurrence of depression. Attenuated maintenance of positive information in WM would result in less elaboration and dedication of fewer cognitive resources to the processing of positive stimuli, leading to weaker representations in long-term memory, difficulty replacing negative stimuli with positive material, and difficulty using WM resources adaptively to regulate mood. It is not clear, however, whether these maladaptive biases in emotion updating are mood-congruent aspects of a depressive episode, or alternatively, reflect stable individual differences and/or scar effects that are present outside of a depressive episode and increase the likelihood that an individual will experience a recurrence of depression.
The present study was designed to examine whether these biases in emotion updating operate outside the depressive episode. Recovered depressed and never-depressed participants performed the emotion n-back task; they were presented with happy, neutral and sad faces for which they were required to indicate whether each face in a series of faces has the same or a different emotional expression as the expression on the face that was presented two trials before, matching the two expressions into a conceptual set on ‘same’ trials, and breaking or determining the absence of a conceptual set on ‘different’ trials. Although MDD is often characterized by recurrent depressive episodes (Boland & Keller, 2009), there are fewer studies examining emotion processing in persons who have recovered from a depressive episode. Previous studies have demonstrated that remitted depressed individuals have significant impairments in executive function and attention (Paelecke-Habermann et al., 2005; Weiland-Fiedler et al., 2004). Researchers have also found that attentional biases for negative stimuli persist beyond depression (Ehring et al., 2008; Joormann & Gotlib, 2007). In addition, investigators have documented that recovered depressed patients focus less attention on positive faces (Sears et al., 2011) and dampen positive emotion (Werner-Seidler et al., 2013). Based on this research and on the recurrent nature of depression, we predicted that the updating biases identified by Levens and Gotlib (2010) reflect stable emotion-cognitive biases that persist beyond the depressive episode rather than depression related mood (in)congruence effects. Specifically we predicted that, as we found in currently depressed individuals, recovered depressed individuals would exhibit longer reaction times to disengage from negative content and shorter reaction times to disengage from positive content in WM.
1.2 Method
1.2.1 Participants
Forty-seven individuals – 23 currently recovered from MDD (19 females1) and 24 never-disordered controls (13 females) – participated in this study. Participants were solicited from two outpatient psychiatry clinics in a university teaching hospital and through advertisements posted in numerous locations within the community (e.g., internet bulletin boards, university kiosks, supermarkets). Participants’ responses to a telephone interview provided initial selection information. Individuals were excluded if they were not fluent in English, were not between 18 and 60 years of age, and reported severe head trauma or learning disabilities, psychotic symptoms, bipolar disorder, or alcohol or substance abuse within the past six months. Potentially eligible persons were invited to come to the laboratory for a more extensive diagnostic interview. Participants who met the study inclusion criteria (see below) were then scheduled for a second session during which they completed the emotion n-back task.
Trained interviewers administered the Structured Clinical Interview for the DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2001) to individuals during their first session in the study. All interviewers had extensive training in the use of the SCID, and diagnostic reliability was continually assessed by randomly selecting and re-rating recorded interviews. Across multiple studies our team of interviewers has achieved excellent inter-rater reliability for recovery from a major depressive episode (k = .91) and for classifying participants as non-psychiatric controls (k = .92; Joormann & Gotlib, 2007; Levens & Gotlib, 2010). Participants were included in the recovered depressed group if they were not currently depressed but met the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV; American Psychiatric Association, 1994) criteria for a previous major depressive episode. In addition to the SCID, depressive symptoms were assessed by the Hamilton Depression Inventory (HDI; Kobak & Reynolds, 2000), a 17-item, self-report measure of the severity of depressive symptoms; the total HDI score was analyzed in this study. Participants were included in the never-depressed group if they had no current or past Axis I disorder.
1.2.2 Stimuli
A total of 138 digital grayscale images of faces from the NimStim Face Set (Tottenham et al., 2009) were used as stimuli. The set of 138 faces comprised 46 sad faces, 46 happy faces, and 46 neutral or calm faces from 23 different actors (12 female, 11 male). Half of the facial expressions of each emotion featured an open mouth, and the other half featured a closed mouth. Each emotional expression of each actor was presented approximately four times during the experiment. Each of the blocks of trials (see below) contained either only male or only female emotional faces. Face gender was counterbalanced across blocks: in the 0-back task segment, participants either viewed two blocks of female faces and one block of male faces, or vice versa; in the 2-back task segment, all participants viewed three blocks of female faces and three blocks of male faces.
1.2.3. Task Design
The experiment was divided into a 0-back task segment and a 2-back task segment. Participants performed the 0-back task first, followed immediately by the 2-back task. The experimental procedure was similar for each segment, and instructions were given to participants both orally and in writing. In both the 0-back and 2-back tasks, participants viewed emotional faces presented one at a time for 2 seconds, with an inter-trial-interval of 2.5 seconds. Response and response latency was recorded for each trial.
1.2.3.1 0-Back Task
The 0-back task, modeled after that used by Harvey et al. (2005) and Ladouceur et al. (2005), consisted of 129 trials separated into 3 blocks of 43 trials and an additional 8 practice trials that were not scored. Participants were presented with an “expression label” (happy, sad or neutral) and a sample face displaying that expression. The target emotional expression differed across blocks: one block was sad, one block was happy, and one block was neutral. Following the presentation of the target label and expression for each block, the trials began. Participants pressed a key labeled ‘Same’ if the facial expression was the same as that of the target expression, or a key labeled “Diff” if the facial expression was different than the target face (Figure 1a). The presentation order of the three blocks of 0-back trials was random.
Figure I.
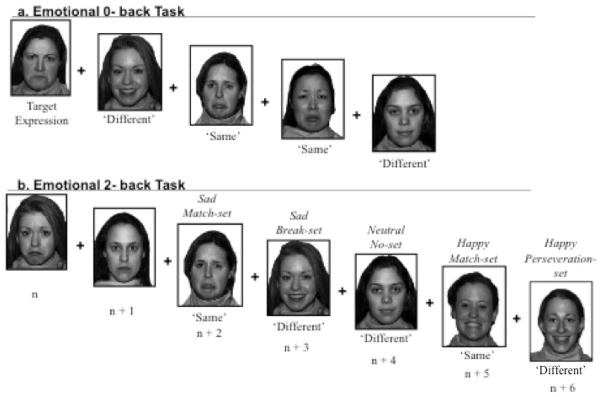
(A.) Sample target expression and Emotion 0-back task trials with correct responses. (B.) Sample of each Emotion 2-back trial type with correct response.
1.2.3.2 2-Back Task
The 2-back task consisted of 330 trials separated into 6 blocks of 55 trials, as well as an additional 10 practice trials that were not scored. Participants were asked to indicate whether the emotional expression of the currently presented face was the same as, or different than, the facial expression presented two faces earlier. Participants pressed a key labeled ‘Same’ if the facial expression was the same as the expression presented two faces before, or a key labeled “Diff” if the facial expression was different than the expression presented two faces earlier. For each block of trials, for the first two faces presented, participants were told to view the faces without pressing a key; from the third face on, participants were told to respond with the keys “Same” or “Diff” to each face presented, resulting in 53 usable trials per block.
1.2.3.3 Trial Types
On each trial, participants must perceptually process the presented facial expression, add that stimulus to their maintained set of stimuli, discard the facial expression presented three trials earlier, compare the current facial expression to the one presented two trials earlier, and then respond. What differ across trials are the valence of incoming and outgoing stimuli and the cognitive processes required, resulting in four trial types: “match-set” trials, “break-set” trials, “perseveration-set” trials, and “no-set” trials. In “match-set” or ’Same’ response trials, the current facial expression is the same as that presented two trials earlier, requiring participants to conceptually link the two expressions (see Figure 1b).
Trials requiring a ’Different’ response on the other hand involve a different set of cognitive processes. There are three types of ‘Different’ response trials: “break-set” trials, “perseveration-set” trials and “no-set” trials. A break-set trial immediately follows a match-set trial. Therefore, to respond to break-set trials, participants must break a set that they endorsed in the preceding trial. Thus, break-set trials assess participants’ ability to disconnect two paired valenced stimuli and disengage from the first face to remove it from WM. Perseveration-set trials are similar to break-set trials in that they must follow a match-set trial. In perseveration set trials, however, the current expression is the same valence as that in the preceding match trial, creating a lure. To respond correctly (‘Different’) the participant must not perseverate (respond ‘Same’) on the preceding match-set trial. Finally, no-set trials do not follow a match-set trial. On no-set trials participants integrate a valenced stimulus into WM and assess its relatedness to existing stimuli being held in WM to determine that no set exists and respond accordingly. For trial type examples and additional details of how trials are categorized, see Levens and Gotlib (2010).
1.2.4. Statistical Analysis
RTs and responses were recorded for each trial, and a mean RT and accuracy rate was calculated for correct trials for each trial type in the 0-back and 2-back tasks. In the present study, based on our joint goals of measuring the duration that a representation is in WM receiving resources and of following up on Levens and Gotlib’s (2010) findings in a sample of participants who have recovered from depression, our primary dependent variable is RT. In addition to RT and accuracy rates, response rates (i.e., the number of no-response trials) were recorded and calculated separately for the 0-back and 2-back segments.
To replicate Levens and Gotlib’s (2010) findings and to avoid spurious findings due to group differences in overall reaction time, all trial type RTs were converted to z-scores, as suggested by Faust et al. (1999). Trial type RTs were converted to z-scores in the following manner. For each participant, we calculated an overall mean (μ) and an overall standard deviation (σ) was calculated from the participant’s trial type reaction time means. Next, for each trial type, we subtracted the participant’s overall RT mean from the trial type RT mean (Χ), which was then divided by participants’ overall standard deviation ((μ−Χ)/σ). To separate the time required to perceive and categorize emotional faces from the time required to update and link content in WM, separate z-score transformations were conducted on the 0-back and 2-back RTs.
Next, a three-way (Group [Recovered, Never-depressed] repeated over Emotion [happy, neutral, sad] repeated over Response [‘Same,’ ‘Diff’]) ANOVA was conducted on 0-back RT z-scores and accuracy rates. Finally, to examine the more complex updating and linking processes required in the emotion 2-back task, separate two-way (Group [Recovered, Never-depressed] repeated over Emotion [happy, neutral, sad]) ANOVAs were conducted on match-set, break-set, perseveration-set, and no-set condition z-scores and accuracy rates.
1.3 Results
1.3.1 Participant Characteristics
Recovered and never-depressed participants did not differ significantly in age (recovered: M = 41; SD = 11; never-depressed: M = 37; SD = 12, t(45)=1.33) or education (recovered: M = 16 years; SD = 2.4; never-depressed: M =16 years; SD = 2.5, t(45)=.68, both ps > .05), nor did the HDI scores of recovered participants (M=0.5; SD=0.5) differ from those of never-depressed participants (M = 1.5; SD = 1.6, p >.05). Finally, 5 of the 23 recovered depressed participants were diagnosed with social phobia and 2 with obsessive compulsive disorder.
The findings are presented in two sections. In the first section we present the results of the 0-back and 2-back accuracy analyses. In the second section we present the results of the 0-back and 2-back match-set, break-set, perseveration-set, and no-set trial RT analyses.
Participants failed to respond to an average of three 0-back trials (SD = 2.6, range: 0–9); response rate did not differ by group, t(45) = 0.60, p > .1. All 0-back RT, z-score, and accuracy means and standard deviations are presented in Table I. In the 2-back task, participants failed to respond to an average of 11 2-back trials (SD = 11, range: 0–582); again, response rate did not differ by group, t(45) = 1.11, p > .1. All 2-back RT, z-score, and accuracy means and standard deviations are presented in Table II.
Table I.
0-back Trial Mean Reaction Times, Z-scores and Accuracy Rates for Recovered and Never-depressed Participants
| Never-depressed | Recovered | |||||
|---|---|---|---|---|---|---|
| RT | z-Score | Acc | RT | z-Score | Acc | |
| Happy facial expression | ||||||
| Same | 709 (93) | −0.60(0.75) | 98%(3%) | 715 (120) | −0.96(0.58) | 99%(2%) |
| Different | 711 (88) | −0.59(0.68) | 97%(9%) | 812 (122) | 0.03(0.65) | 98%(11%) |
| Neutral facial expression | ||||||
| Same | 805 (100) | 0.44(0.81) | 94%(7%) | 859 (169) | 0.43(0.73) | 96%(6%) |
| Different | 763 (117) | −0.07(0.76) | 94%(7%) | 895 (145) | 0.89(0.61) | 98%(4%) |
| Sad facial expression | ||||||
| Same | 783 (107) | 0.16(0.79) | 92%(9%) | 874 (162) | 0.48(0.65) | 92%(5%) |
| Different | 850 (166) | 0.66(0.94) | 92%(10%) | 735 (110) | −0.78(0.44) | 98%(3%) |
Note: Standard deviations are shown in parenthesis; RT = reaction time; Acc = Accuracy
Table II.
2-back Trial Mean Reaction Times, z-Score and Accuracy Rates for Recovered and Never-depressed Participants
| Never-depressed | Recovered | |||||
|---|---|---|---|---|---|---|
| RT | z-Score | Acc | RT | z-Score | Acc | |
| Match-set | ||||||
| Happy | 951 (160) | −1.42 (0.57) | 89%(11%) | 1064 (216) | −1.51 (0.51) | 92%(7%) |
| Neutral | 1134 (171) | −0.15 (0.91) | 90%(9%) | 1238 (214) | −0.30 (0.85) | 91%(9%) |
| Sad | 1140 (202) | −0.15 (0.65) | 76%(13%) | 1284 (204) | −0.05 (0.71) | 79%(12%) |
| Break-set | ||||||
| Happy | 1209 (196) | 0.28 (0.57) | 93%(9%) | 1231 (249) | −0.44 (0.53) | 93%(6%) |
| Neutral | 1178 (207) | −0.26 (0.55) | 94%(6%) | 1227 (274) | −0.35 (0.61) | 96%(5%) |
| Sad | 1122 (190) | −0.31 (0.54) | 93%(9%) | 1237 (232) | −0.10 (0.49) | 92%(10%) |
| Perseveration-set | ||||||
| Happy | 1285 (290) | 0.66 (0.94) | 90%(11%) | 1376 (265) | 0.66 (0.94) | 93%(10%) |
| Neutral | 1300 (299) | 64 (1.59) | 84%(14%) | 1390 (291) | 0.81 (0.89) | 87%(14%) |
| Sad | 1274 (244) | 0.68 (0.78) | 88%(10%) | 1393 (238) | 0.86 (0.68) | 92%(9%) |
| No-set | ||||||
| Happy | 1124 (214) | −0.29 (0.58) | 91%(8%) | 1268 (211) | 0.02 (0.62) | 93%(8%) |
| Neutral | 1177 (234) | 0.11 (0.85) | 88%(12%) | 1388 (237) | 0.81 (0.45) | 88%(9%) |
| Sad | 1212 (229) | 0.28 (0.51) | 87%(11%) | 1334 (223) | 0.40 (0.63) | 89%(8%) |
Note: Standard deviations are shown in parenthesis; RT = reaction time; Acc = Accuracy
1.3.2 0-back Accuracy Rates
The three-way ANOVA conducted on 0-back accuracy rates yielded a significant main effect of Emotion, F(2,90) = 6.92, p < .01, η2 = .13; no other effects were significant. Follow-up tests indicated that participants were more accurate in identifying happy expressions than both sad, t(46) = 3.71, p < .01, and neutral, t(46) = 1.96, p < .05, expressions.
1.3.3 2-back Accuracy Rates
Match-set
The two-way ANOVA conducted on match-set trial accuracy rates also yielded a significant effect only for Emotion, F(2,90) = 43.13, p < .001, η2 = .49. Accuracy rates for sad match-set trials were significantly lower than were rates for both neutral, t(46) = 6.58, p < .001, and happy, t(46) = 8.31, p < .001, match-set trials, which did not differ significantly from each other, t(46) = 0.52, p > .1.
Break-set
The ANOVA conducted on break-set trials also yielded only a main effect of Emotion, F(2,90) = 3.1, p < .05, η2 = .07, which was due to significantly lower accuracy rates for sad, t(46) = 2.17, p < .05, and happy, t(46) = 1.96, p < .05, than for neutral break-set trials.
Perseveration-set
The ANOVA conducted on perseveration-set trial accuracy rates also yielded only a main effect of Emotion, F(2,90) = 9.77, p < .001, η2 = .18, which reflected significantly lower accuracy rates for neutral than for sad, t(46) = 3.45, p < .01, and happy t(46) = 3.67, p < .01, perseveration-set trials.
No-set
Finally, the ANOVA conducted on no-set trial accuracy rates yielded only a main effect of Emotion, F(2,90) = 7.96, p < .001 η2 = .15, which was due to significantly lower accuracy rates for neutral, t(46) = 4.15, p < .01, and sad, t(46) = 2.74, p < .01, than for happy no-set trials.
In sum, recovered and never-depressed participants did not differ in their accuracy or response rates on the 0-back and 2-back tasks.
1.3.4 0-back Reaction Time Analysis
The three-way ANOVA conducted on z-scored RTs yielded a significant main effect of Emotion, F(2,88) = 36.48, p < .001, η2 = .45, which was qualified by a significant two-way interaction of Emotion and Group, F(2,88) = 10.35, p < .001, η2 = .19, Emotion and Response, F(2,88) = 7.52, p < .001, η2 = .15, and a significant three-way interaction of Group, Emotion, and Response, F(2,88) = 25.22, p < .001, η2 = .36. Follow-up tests indicated that the three-way interaction was due to significantly longer neutral ‘Different’ RTs for recovered than for never-depressed participants, t(45) = 4.73, p < .001, and significantly shorter sad ‘Different’ RTs for recovered than for never-depressed participants, t(45) = 6.70, p < .001; the two groups of participants did not differ on ‘Same’ response trial RTs or happy ‘Different’ RTs.
1.3.5 2-back Reaction Time Analysis
Match-set
The two-way ANOVA conducted on these RT z-scores yielded a significant main effect of Emotion, F(2,90) = 71.09, p < .001, η2 = .61, which reflected significantly faster happy match-set than neutral, t(46) = 8.97, p < .001, or sad, t(46) = 12.81, p < .001, match-set RTs.
Break-set
The two-way ANOVA conducted on z-scores yielded a main effect of Group, F(1,45) = 5.15, p < .05, η2 = .10, which was qualified by an interaction of Group and Emotion, F(2,90) = 8.42, p < .001, η2 = .16. One-way ANOVAs conducted within each group indicated that z-scores for both recovered, F(2,46) = 2.42, p < .1, η2 = .0.1, and never-depressed, F(2,44) = 7.98, p < .001, η2 = .26, participants were significantly different across emotions. Whereas recovered depressed participants broke sets of (or disengaged from) sad faces significantly more slowly than they did for happy faces, t(22) = 2.12, p < .05, never-depressed participants showed the opposite pattern, disengaging from happy faces significantly more slowly than they did from neutral, t(23) = 3.21, p < .01, and sad, t(23) = 3.79, p < .001, faces. Compared with never-depressed controls, recovered participants were also faster to disengage from happy faces, t(45) = 4.50, p < .001.
Perseveration-set
Mean RTs and standard deviations for the perseveration-set trials are presented in Table 2. The two-way ANOVA conducted on these z-scores yielded no significant main effects or interactions.
No-set
Mean RTs and standard deviations for the no-set trials are presented in Table 2. The two-way ANOVA conducted on no-set z-scores yielded significant main effects of Group, F(1,45) = 9.17, p < .01, η2 = .17, and Emotion, F(2,90) = 15.41, p < .001, η2 = .26, which were qualified by a significant interaction of Group and Emotion, F(2,90) = 3.23, p < .05, η2 = .07. One-way ANOVAs conducted within each group indicated that z-scores for both recovered, F(2,44) = 15.22, p < .001, η2 = .41, and never-depressed, F(2,46) = 5.39, p < .01, η2 = .19, participants differed significantly across emotions. Paired t-tests revealed that both recovered and never-depressed participants (respectively) integrated happy content faster than they did both sad, t(22) = 3.01, p < .01, and t(22) = 4.05, p < .001, and neutral, t(22) = 5.66, p < .001 and t(22) = 2.13, p < .05, content. The two groups differed, however, in their integration of neutral content: recovered depressed individuals integrated neutral content significantly more slowly than did never-depressed individuals, t(45) = 3.4, p < .01.
1.4 Discussion
The present study was designed to test whether emotion updating biases documented in currently depressed individuals by Levens and Gotlib (2010) are evident beyond the depressive episode. In their previous study, Levens and Gotlib found that, compared to never depressed controls, depressed participants were both slower to disengage from sad stimuli and faster to disengage from happy facial expressions. In contrast, never depressed controls took longer to disengage from happy than from neutral or sad stimuli. In the present study we predicted that both longer reaction times to disengage from negative content and faster reaction times to disengage from happy content would persist beyond the depressive episode. Consistent with our hypotheses and with Levens and Gotlib’s findings in currently depressed individuals, recovered depressed participants removed happy content from WM more quickly and sad stimuli from WM more slowly than did their never depressed counterparts. Therefore, similar to currently depressed individuals, recovered depressed persons are less able to maintain positive content and to disengage from sad content in WM than are never-disordered controls. This pattern of findings indicates that the inability to effectively regulate mood by maintaining positive content and expelling negative content from WM persists following recovery from depression.
Interestingly, the present results also illustrate that the longer time to disengage from sad content and the faster time to disengage from happy content documented in currently depressed individuals is not due simply to mood (in)congruency effects. Nor do the findings appear to be due to residual depression symptoms3. Instead, the tendency to maintain sad content and disengage from happy content appears to represent stable individual differences or scar effects that persist beyond the depressive episode. The findings from this study are critical because they indicate that 1) emotion-executive control interactions operate outside of a depressive state and may influence behavior and emotion regulation strategy; and 2) the tendency to maintain sad content and disengage from happy content may represent a stable risk factor that increases the likelihood of experiencing additional depressive episodes.
Results from the 0-back task indicated that while recovered depressed and never depressed participants did not differ in accuracy, recovered depressed participants took longer than did never depressed persons to respond to a facial expression that was not neutral, and were faster to respond to a facial expression that was not sad. Although these results do not replicate the null 0-back RT effects reported by Levens and Gotlib (2010), they are consistent with the literature on depression-associated biases in the processing of neutral and sad emotional expressions (Gollan, Pane, McCloskey & Coccaro, 2008; Karparova, Jersting & Suslow, 2005) suggesting that depression-associated biases in the processing of neutral and sad facial expressions also persist outside of depressive episodes.
The results of this study begin to elucidate the nature of the emotion-processing biases in WM that may be related to the recurrence of MDD. Researchers have shown that the recurrence of MDD (i.e., experiencing a depressive episode after having exhibited full and/or partial remission from a previous depressive episode) is high in the general population (35% after 15 years), and even higher in those treated at specialized mental health centers (60% after 5 years and 85% after 15 years; Hardevald, Spijker, De Graaf, Nolen, & Beekman, 2010). Both of the emotion updating biases documented by Levens and Gotlib (2010) in depressed individuals (i.e., impaired maintenance of positive content and impaired disengagement from negative content) appear to persist following recovery of depression, suggesting that these maladaptive biases underlie the recurrent nature of depression. Currently there are a number of theories regarding the specific cognitive and behavioral factors that may underlie the high rates of recurrence of depression.
One major theory is the stress kindling hypothesis, which posits that whereas the first episode of depression is likely to be due to a significant life stressor, subsequent episodes of depression can develop more autonomously (Monroe & Harkness, 2005; Post, 1992). Monroe and Harkness (2005) carefully elaborated on the stress-kindling formulation and proposed two possible routes to recurrence of depression: stress kindling/sensitization and stress autonomy. In the stress kindling/sensitization route, the first episode of depression would leave an emotion-cognitive scar or vulnerability that after the depression episode was over could ‘ignite’ more easily in response to stress (and not necessarily in proportion to the stressor). In contrast, the stress autonomy route suggests that depression becomes independent of stress episodes, regardless of their intensity. The present findings suggest a possible emotion-executive control scar that may underlie the stress kindling/sensitization pathway to recurrent depressive episodes. Recovered depressed participants not only take longer to disengage from negative content in WM, but they also disengage from happy content more quickly. In the context of a stressor, the pattern of updating biases identified in the present study suggests that the negative stressor related content receives more processing resources and greater opportunity for elaboration in WM than positive content. This would provide a route by which increased sensitivity to smaller stressors could cause relapse, even outside of a depressive episode; negative content receives more resources and elaboration, heightening the negative affect and creating a greater cumulative negative effect than the magnitude of the small stressor would indicate.
Despite the methodological strengths of the present study and the extension of a depression-specific finding to recovered depressed individuals, we should note that we were not able to test whether the identified emotion updating biases predict long-term relapse or recovery. It will be important in future research to examine whether individual differences in the tendency to maintain sad content and disengage from happy content represent a stable risk factor for developing and maintaining a negative mood that predict relapse or recovery. Longitudinal research should also be conducted to investigate the predictive validity of the present findings with respect to stress reactivity, and to examine whether interventions targeted at maintaining positive affect reduce stress reactivity and depression relapse rates (as proposed by Waugh & Koster, 2014). As a related point, future longitudinal research should also investigate whether the emotion updating biases identified in the present study exist prior to the onset of a depressive episode. The tendency to maintain negative content and disengage from positive content could be a stable individual difference, and perhaps a risk factor, for the onset of a first episode of depression. It is also possible, however, that the emotion updating biases are not present prior to depression and, instead, develop like stress sensitivity does following a stressful event. Finally, future research should investigate the operation of biases in emotion updating in related disorders such as Post Traumatic Stress Disorder and phobias to assess how updating biases may be related to specific trauma- or phobia-related content.
In sum, findings from this study suggest that currently depressed and recovered depressed individuals are characterized by similar emotional and cognitive deficits. These results suggest that following recovery from a depressive episode, individuals might be taught strategies to deliberately savor positive affect in order to actively counteract biases in emotion updating and thereby decrease the likelihood of relapse. To reduce recurrence of depression, it is essential to examine the specific emotional and cognitive biases that persist outside the depressive episode. In this context, the results of the present study contribute to our understanding of depression and biases in emotion-executive control processes and suggest interventions or coping strategies as well as directions for future research that might increase our ability to effectively treat depression and prevent the recurrence of depressive episodes.
Highlights.
Recovered depressed exhibit similar emotion biases as currently depressed individuals
Emotion biases may be trait-like cognitive vulnerabilities that underlie recurrent depression
Recovered depressed individuals disengage from negative content slower than controls
Recovered depressed individuals disengage from happy content quicker than controls
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH59259 awarded to Ian H. Gotlib. The authors thank Sarah Victor for their help running participants.
Footnotes
Because females are overrepresented in the recovered-depressed group, we conducted a post-hoc analyses in which we included gender as a covariate in all reaction-time and accuracy analyses. The four-way Gender [Male, Female] by Group [Recovered, Never-depressed] repeated over Emotion [happy, neutral, sad] repeated over Response [‘Same,’ ‘Diff’] ANOVAs conducted on 0-back RT z-scores and accuracy rates yielded no significant main effects or interactions with gender, all ps>.1. In addition, results of the separate three-way Gender [Male, Female] by Group [Recovered, Never-depressed] repeated over Emotion [happy, neutral, sad] ANOVAs conducted on match-set, break-set, perseveration-set, and no-set condition z-scores and accuracy rates also yielded no significant main effects or interactions with gender, all ps>.1
One participant had a total of 58 no-responses during the 2-back task. While performing the first block of 55 trials in the 2-back task the participant accidentally had her fingers placed on the wrong buttons on the keyboard. Consequently, no responses were recorded for this participant for that block of trials. On all other blocks of trials the participant’s response rate was quite high (approximately 99%) so we included the participant in analyses.
To address the possibility that the biases in emotion updating exhibited by recovered depressed individuals were due to residual effects of the depressive episode, one-way ANOVAs were conducted for each trial type with HDI scores entered as a continuous independent variable. If residual effects were related to current depressive symptomatology, then the emotion updating biases should vary as a function of HDI scores. The analyses yielded no main effects or interactions involving HDI, all ps>.1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Baddeley AD. Working Memory. Oxford University Press; New York, New York: 1986. [Google Scholar]
- Boland RJ, Keller MB. Course and outcome of depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. New York: Guilford; 2009. pp. 23–43. [Google Scholar]
- Bistricky SL, Atchley RA, Ingram R, O’Hare A. Biased processing of sad faces: an ERP marker candidate for depression susceptibility. Cognition and Emotion. 2014;28(3):470–92. doi: 10.1080/02699931.2013.837815. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. Activity in fusiform face area modulated as a function of working memory load. Brain Research. Cognitive Brain Research. 2001;10(3):355–64. doi: 10.1016/s0926-6410(00)00056-2. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Memory from A to Z: keywords, concepts, and beyond. Oxford University Press Inc; New York: 2002. [Google Scholar]
- Duque A, Vázquez C. Double attention bias for positive and negative emotional faces in clinical depression: evidence from an eye-tracking study. Journal of Behavior Therapy and Experimental Psychiatry 2015. 2015 Mar;46:107–14. doi: 10.1016/j.jbtep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Ehring T, Fischer S, Schnülle J, Bösterling A, Tuschen-Caffier B. Characteristics of emotion regulation in recovered depressed versus never depressed individuals. Personal Individ Differ. 2008;44:1574–84. [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychology Bulletin. 1999;125(6):777–99. doi: 10.1037/0033-2909.125.6.777. Review. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Gollan JK, Pane HT, McCloskey MS, Coccaro EF. Identifying differences in biased affective information processing in major depression. Psychiatry Res. 2008;159(1–2):18–24. doi: 10.1016/j.psychres.2007.06.011. Epub 2008 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: Evidence of modest impairment. Biological Psychiatry. 2001;50(1):35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardevald F, Spijker J, De Graaf R, Nolen WA, Beekman ATF. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatrica Scandinavica. 2010;122:184–191. doi: 10.1111/j.1600-0447.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, et al. Executive functions and updating of the contents of working memory in unipolar depression. Journal of Psychiatric Research. 2004;38(6):567–576. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, LeBastard G, Lehericy S, Alliaire JF, Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Isen AM. Toward understanding the role of affect in cognition. In: Wyer RS, Srull TS, editors. Handbook of social cognition. Hillsdale, N. J: Erlbaum; 1984. pp. 179–236. [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 2007. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117(1):182–92. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Karparova SP, Kersting A, Suslow T. Disengagement of attention from facial emotion in unipolar depression. Psychiatry Clin Neurosci. 2005;59(6):723–9. doi: 10.1111/j.1440-1819.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Effect of negative emotional content on working memory and long-term memory. Emotion. 2003;3(4):378–93. doi: 10.1037/1528-3542.3.4.378. [DOI] [PubMed] [Google Scholar]
- Kessler Y, Meiran N. All updateable objects in working memory are updated whenever any of them is modified: Evidence from the memory updating paradigm. Journal of Experimental Psychology: Learning, Memory and Cognition. 2006;32:570–585. doi: 10.1037/0278-7393.32.3.570. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Reynolds WM. The Hamilton Depression Inventory. In: Maruish ME, editor. Handbook of psychological assessment in primary care settings. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2000. pp. 423–461. [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey BJ. Altered emotional processing in Pediatric Anxiety, Depression, and comorbid Anxiety-Depression. Journal of Abnormal Child Psychology. 2005;33(2):165–177. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- Levens SM, Gotlib IH. Updating positive and negative stimuli in working memory in depression. J Exp Psychol Gen 2010. 2010 Nov;139(4):654–64. doi: 10.1037/a0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoon DG, Newman JP. Content meets process: Using attributions and standards to inform cognitive vulnerability in psychopathy, antisocial personality disorder, and depression. Journal of Social and Clinical Psychology. 2006;25(7):802–824. [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshayan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. American Journal of Psychiatry. 1999;156(5):780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge University Press; New York, New York: 1999. [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review. 2005;112(2):417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Morris N, Jones DM. Memory updating in working memory: The role of the central executive. British Journal of Psychology 1990. 1990;81:111–121. [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89(1–3):125–35. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Paramenter BA, Shucard JL, Benedict RHB, Shucard DW. Working memory deficits in multiple sclerosis: Comparison between the n-back task and the Paced Auditory Serial Addition Test. Journal of the International Neuopsychological Society. 2006;12:677–687. doi: 10.1017/S1355617706060826. [DOI] [PubMed] [Google Scholar]
- Paramenter BA, Shucard JL, Shucard DW. Information processing deficits in multiple sclerosis: A matter of complexity. Journal of the International Neuopsychological Society. 2007;13:417–423. doi: 10.1017/S1355617707070580. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychol Rev. 2003;110(1):145–72. doi: 10.1037/0033-295x.110.1.145. Review. [DOI] [PubMed] [Google Scholar]
- Sears CR, Newman KR, Ference JD, Thomas CL. Attention to emotional images in previously depressed individuals: an eye-tracking study. Cognitive Therapy Research. 2011;35:517–528. [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson CA. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Koster EHW. A resilience framework for promoting stable remission from depression. Clinical Psychology Review. doi: 10.1016/j.cpr.2014.05.004. (in press) [DOI] [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Charney DS, Neumeister A. Evidence for continuing neuropsychological impairments in depression. J Affect Disord. 2004;82(2):253–8. doi: 10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Werner-Seidler A, Banks R, Barnaby DD, Moulds ML. An investigation of the relationship between positive affect regulation and depression. Behavior Research and Therapy. 2013;51:46–56. doi: 10.1016/j.brat.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Yoon KL, LeMoult J, Joormann J. Updating emotional content in working memory: a depression-specific deficit? Journal of Behavioral Therapy and Experimental Psychiatry. 2014;45(3):368–74. doi: 10.1016/j.jbtep.2014.03.004. [DOI] [PubMed] [Google Scholar]


