Abstract
Objectives
To investigate the association between lymphovascular invasion (LVI) and clinical outcome in organ-confined, node-negative urothelial cancer of the bladder (UCB) in a post hoc analysis of a prospective clinical trial. To explore the effect of adjuvant chemotherapy with methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) on outcome in the subset of patients whose tumours exhibited LVI.
Patients and Methods
Surgical and tumour factors were extracted from the operative and pathology reports of 499 patients who had undergone radical cystectomy (RC) for pT1–T2 N0 UCB in the p53-MVAC trial (Southwest Oncology Group 4B951/NCT00005047). The presence or absence of LVI was determined by pathological examination of transurethral resection or RC specimens. Variables were examined in univariate and multivariate Cox proportional hazards models for associations with time to recurrence (TTR) and overall survival (OS).
Results
Among 499 patients with a median follow-up of 4.9 years, a subset of 102 (20%) had LVI-positive tumours. Of these, 34 patients had pT1 and 68 had pT2 disease. LVI was significantly associated with TTR with a hazard ratio (HR) of 1.78 [95% confidence interval (CI) 1.15–2.77; number of events (EV) 95; P = 0.01) and with OS with a HR of 2.02 (95% CI 1.31–3.11; EV 98; P = 0.001) after adjustment for pathological stage. Among 27 patients with LVI-positive tumours who were randomised to receive adjuvant chemotherapy, receiving MVAC was not significantly associated with TTR (HR 0.70, 95% CI 0.16–3.17; EV 7; P = 0.65) or with OS (HR 0.45, 95% CI 0.11–1.83; EV 9; P = 0.26).
Conclusions
Our post hoc analysis of the p53-MVAC trial revealed an association between LVI and shorter TTR and OS in patients with pT1–T2N0 disease. The analysis did not show a statistically significant benefit of adjuvant MVAC chemotherapy in patients with LVI, although a possible benefit was not excluded.
Keywords: adjuvant chemotherapy, lymphovascular invasion, SWOG, urothelial cancer
Introduction
In patients with muscle-invasive and high-risk non-muscle-invasive urothelial cancer of the bladder (UCB), radical cystectomy (RC) and pelvic lymphadenectomy (PLND) provide adequate loco-regional control and are the ‘gold standard’ of treatment [1,2]. Even in patients with organ-confined disease up to 40% subsequently experience a recurrence. Among other variables including stage and nodal involvement, the presence of lymphovascular invasion (LVI) has been defined as a risk factor for recurrence and survival after RC in retrospective multi-institutional analyses [3]. Our primary aim was to confirm the association between LVI and clinical outcome in a prospective study. We addressed this question by performing a post hoc analysis of the University of Southern California led p53-MVAC trial [Southwest Oncology Group (SWOG) 4B951/NCT00005047], which investigated the role of adjuvant chemotherapy in patients with p53-positive, organ–confined tumours (pT1–T2N0) [4]. Our secondary aim was to examine the putative benefit of adjuvant chemotherapy in the presence of LVI in patients with organ-confined, node-negative disease.
Patients and Methods
All patients provided written informed consent and the protocol was approved by the local Institutional Review Board at all participating institutions. The results of the primary endpoint of the clinical trial and the outcome of the entire cohort have been reported [4]. Methods for this analysis are briefly described.
Patients with pT1–T2N0 UCB who had undergone RC and bilateral PLND within the prior 9 weeks were eligible. Patients with pT0, pTa, or pTis disease after RC were included if cT1 or cT2 disease was present on the pre-RC transurethral resection (TUR) specimen submitted in toto for histological examination. Patients were required to have ≥15 lymph nodes identified on pathology or a normal postoperative abdominal/pelvic CT if fewer were identified. Patients with p53-positive immunohistochemistry (≥10% nuclear immunoreactivity) were randomised to adjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) or observation.
Assessment of LVI was a required pathological variable for study entry and was prospectively recorded by the participating institutions and pathology reports were centrally reviewed. Immunohistochemistry was not performed to confirm or identify LVI as a standard analysis. Tumours were considered to be LVI negative when LVI was determined to be absent by the pathologist or when there was no mention of LVI in the pathology report. Follow-up was every 3 months for the first year, biannually for the following 4 years, and annually thereafter. Patients who did not agree to randomisation were observed.
Statistical Analyses
Operative and pathology reports were reviewed to determine relevant surgical and tumour factors, including pathological stage and the presence or absence of LVI. Differences in sample means were assessed using the unpaired Student’s t-test. Differences among categorical variables were assessed using Pearson’s chi-squared test with Yates’ continuity correction or Fisher’s exact test. Variables were examined in univariate and multivariate Cox proportional hazards models for associations with time to recurrence (TTR) and overall survival (OS). TTR was the time from registration to the first observation of disease recurrence, censuring patients who died from unrelated causes. OS was the time from registration to time of death due to any cause. For the subset comparison of adjuvant chemotherapy vs observation in patients with LVI-positive tumours, TTR and OS were calculated from the date of randomisation. Reported P values were not adjusted for multiple testing.
Results
In all, 521 patients from 39 sites were enrolled between August 1997 and January 2006. This analysis is based on the 499 patients who were eligible for the trial. Pathology reports were available for all 499 patients (Table 1); 400 were male and 99 were female. The median (interquartile range, IQR) duration of follow-up for these patients was 4.9 (3.0–6.8) years. In all, 95 patients experienced a recurrence and 98 died. The 5-year TTR and OS probabilities (previously reported [4] but recalculated for this subset of patients) were 0.80 and 0.83, respectively.
Table 1.
The patients’ demographic and clinical characteristics.
| Variable | LVI-positive patients, n (%) | LVI-negative patients, n (%) | P |
|---|---|---|---|
| Patients | 102 | 397 | |
| Age at RC, years | |||
| >65 | 46 (45) | 167 (42) | 0.73 |
| <65 | 56 (55) | 230 (58) | |
| Gender | |||
| Female | 22 (22) | 77 (19) | 0.73 |
| Male | 80 (78) | 320 (81) | |
| Pathological stage | |||
| pT1 | 34 (33) | 223 (56) | <0.001 |
| pT2 | 68 (67) | 174 (44) | |
| Bladder CIS | |||
| Present | 66 (65) | 236 (59) | 0.41 |
| Absent | 36 (35) | 160 (40) | |
| Missing | 0 | 1 (<1) | |
| Urethrectomy | |||
| Yes | 3 (3) | 21 (5) | 0.60 |
| No | 80 (78) | 321 (81) | |
| Missing | 19 (19) | 55 (14) | |
| p53 status | |||
| Positive | 56 (55) | 216 (54) | 0.91 |
| Negative | 46 (45) | 181 (46) | |
| Lymph-node yield | |||
| ≥15 | 61 (60) | 226 (57) | 0.73 |
| <15 | 31 (30) | 122 (31) | |
| Missing | 10 (10) | 49 (12) | |
LVI as a Prognostic Marker in UCB
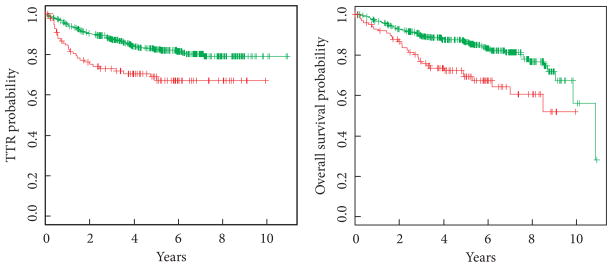
A subset of 102 (20%) patients exhibited LVI in the TUR or RC specimen; 80 were male and 22 were female. In all, 34 had pT1 and 68 had pT2 stage disease at the time of RC. Among the patients with LVI-positive tumours, 30 recurred and 32 died (Fig. 1). In comparison to the overall study population, the TTR and OS probabilities for patients with LVI were 0.69 and 0.69, respectively, with a median (IQR) follow-up of 4.3 (2.4–6.0) years. For patients without LVI, 5-year TTR and OS probabilities were 0.83 and 0.86, respectively.
Fig. 1.
TTR and OS stratified by LVI status. Red, LVI present; green, LVI absent. LVI-positive patients were about twice as likely to recur or die at any given time during the study. TTR: HR 2.06 (95% CI 1.34–3.18; EV 95; P = 0.001). OS: HR 2.17 (95% CI 1.42–3.31; EV 98; P < 0.001).
Among all 499 patients, LVI status (present vs absent) was significantly associated with TTR with a hazard ratio (HR) of 2.06 [95% CI 1.34–3.18; number of events (EV) 95; P = 0.001] in unadjusted Cox regression analysis (Fig. 2). It was similarly associated with OS with a HR of 2.17 (95% CI 1.42–3.31; EV 98; P < 0.001). Patients with LVI-positive tumours were about twice as likely to recur or die after RC. The association between LVI and clinical outcome persisted when adjusted for disease stage. Kaplan–Meier plots of TTR and OS stratified by LVI and pathological stage (pT1 vs pT2) are shown in Fig. 3. After adjusting the Cox regression models for disease stage, LVI was still significantly associated with TTR with a HR of 1.78 (95% CI 1.15–2.77; EV 95; P = 0.01) and with OS with a HR of 2.02 (95% CI 1.31–3.11; EV 98; P = 0.001).
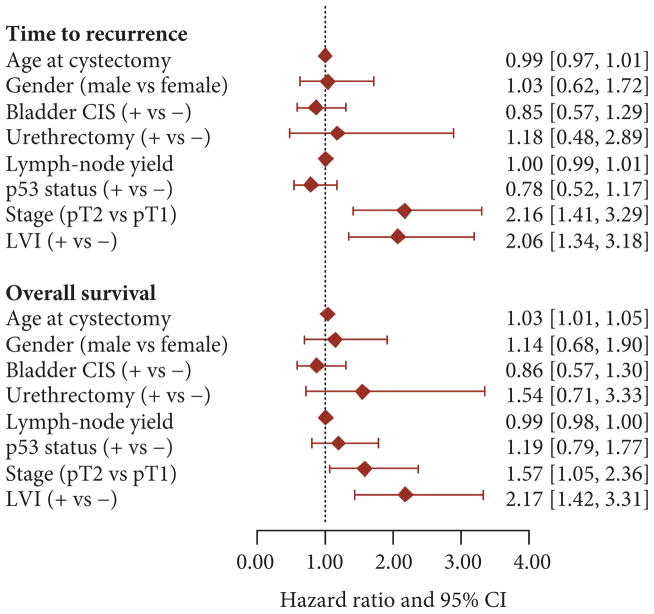
Fig. 2.
Forest plots of TTR and OS HRs with 95% CIs from unadjusted Cox proportional hazards regression analyses.
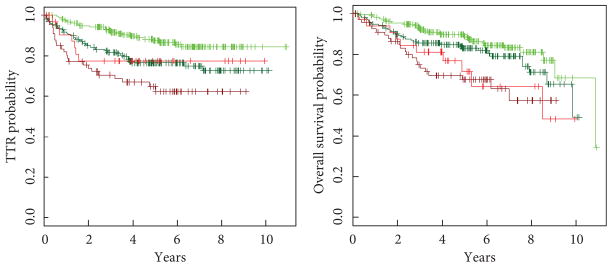
Fig. 3.
TTR and OS stratified by LVI status and pathologic stage. Light green, pT1 patients without LVI; dark green, pT2 patients without LVI; light red, pT1 patients with LVI; dark red, pT2 patients with LVI. TTR: for LVI, HR 1.8 (95% CI 1.1–2.8; EV 95; P = 0.01); for pathological stage, HR 2.0 (95% CI 1.3–3.0; EV 95; P = 0.002). OS: For LVI, HR 2.0 (95% CI 1.3–3.1; EV 98; P = 0.001); for pathological stage, HR 1.4 (95% CI 0.9–2.1; EV 98; P = 0.1).
For sake of comparison, the HRs for other demographic and pathological variables are also presented in Fig. 2. There was no univariate association between age at RC, gender, presence of associated carcinoma in situ (CIS), positive urethral margin, lymph-node count, or p53 status and TTR. Similarly, none of these factors (apart from age) was significantly associated with OS. As expected, pathological stage (pT2 vs pT1) was significantly associated with TTR with a HR of 2.16 (95% CI 1.41–3.29; EV 95; P < 0.001) and with OS with a HR of 1.57 (95% CI 1.05–2.36; EV 98; P = 0.03) in univariate analysis.
Effect of Adjuvant Chemotherapy on Patients with LVI-Positive Tumours
In the p53-MVAC trial, patients with altered p53 were offered the opportunity to be randomised to receive three cycles of MVAC chemotherapy or to be observed. In all, 58 patients were randomised to receive chemotherapy and 56 patients were randomised to observation. In addition, 158 patients with altered p53 declined randomisation and were also observed, as were 227 patients with normal p53.
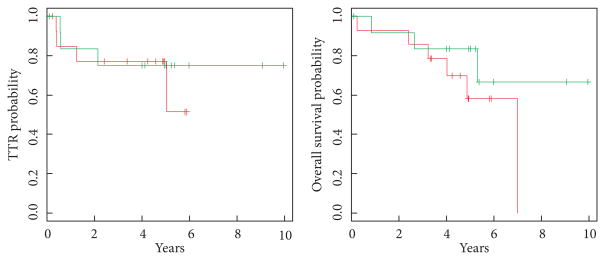
Of 102 patients with LVI, 27 with altered p53 agreed to randomisation; 13 (pT1, six; pT2, seven) were assigned to receive three cycles of MVAC chemotherapy while 14 (pT1, seven; pT2, seven) were assigned to observation after RC. Patients who had received chemotherapy after RC recurred in three of the 13 cases while only four of 14 patients who had been observed recurred (Fig. 4). This difference was not statistically significant. The 5-year TTR and OS probabilities for patients with LVI who received chemotherapy were 0.75 and 0.83, respectively. The 5-year TTR and OS probabilities for patients with LVI randomised to the observation arm were lower, and were 0.51 and 0.58, respectively. Receiving chemotherapy (yes vs no) was not significantly associated with TTR with a HR of 0.70 (95% CI 0.16–3.17; EV 7; P = 0.65) or with OS with a HR of 0.45 (95% CI 0.11–1.83; EV 9; P = 0.26) in univariate analysis.
Fig. 4.
TTR and OS of LVI-positive patients stratified by the administration of adjuvant chemotherapy. Green, patients receiving chemotherapy; red, patients who were observed. TTR: HR 0.70 (95% CI 0.16–3.17; EV 7; P = 0.65). OS: HR 0.45 (95% CI 0.11–1.83; EV 9; P = 0.26).
Among the 58 patients in the intervention arm (i.e. those with altered p53 who received MVAC chemotherapy), 13 had LVI and 45 did not. A subgroup analysis re-examining LVI status (present vs absent) as a prognostic marker among only these patients showed no statistically significant difference in TTR (HR 2.29; 95% CI 0.55–9.58; EV 8; P = 0.26) or OS (HR 1.3; 95% CI 0.34–4.93; EV 12; P = 0.70), although our ability to detect any association was probably limited by the small sample size in the comparison.
Discussion
Our secondary analysis of the p53-MVAC trial revealed an association of LVI with TTR and OS in patients with organ-confined, node-negative UCB. LVI-positive patients were about two-times more likely to recur or die after RC independent of pathological stage. The prevalence of LVI in the present study was 20%. This was somewhat lower than that reported in other series (33–54%) [5–7], which we attribute to the fact that this prospective clinical trial excluded patients with a tumour stage >pT2 and lymph-node metastasis.
The presence of LVI was assessed in TUR and RC specimens. This fact is important, as previous studies have shown that there is a considerable discrepancy in the detection of LVI between TUR and RC specimens, particularly for cT1 tumours [8]. As previously reported in other studies, we were able to confirm that LVI is not only present in early stage, invasive cancers but also that the incidence of LVI is much lower in pT1 tumours (13%) than in pT2 tumours (28%) [5].
We acknowledge the fact that there was no centralised re-review of the specimens for LVI and that the use of immunohistochemistry was not a standard requirement. However, the diagnosis of LVI remains primarily morphological (i.e. in the haematoxylin and eosin stain) and immunohistochemistry is reserved for select cases of indeterminate morphology [8]. While there may be some variance present in our study because of the involvement of multiple institutions, the effect of LVI was unequivocal on TTR and OS.
Our present results are in agreement with previous studies reporting that LVI is an independent risk factor for cancer recurrence and cancer-related survival in organ-confined, node-negative UCB. Lotan et al. [9] reported that the presence of LVI was associated with worse outcomes in node-negative disease. Across all stages, LVI was independently associated with cancer-specific survival and OS in 151 patients with pTa–T4N0 UCB. Another study by Tilki et al. [10] examined LVI in patients with pT1 disease and negative lymph nodes. They observed an association of LVI with recurrence and cancer-specific survival. However, the small number of recorded events (six) markedly limited the statistical power of their analysis. A similar observation was made by Branchereau et al. [11], who determined LVI to be a significant prognostic factor for OS when it was present in the first TUR specimen. While Lotan et al. included 10 patients with pT1 and 61 patients with pT2 UCB after RC, our present study included 34 and 68 for each stage, respectively. While there are other studies involving large cohorts of patients, those included individuals with regional metastatic disease at the time of RC. Thus, our present study reports on the largest prospective cohort of patients with pT1–T2N0 UCB and LVI to date.
Other parameters such as age, gender, presence of CIS, urethrectomy, lymph node count, and p53 positivity were not associated with TTR or OS in our present study. A difference in gender-specific outcome in other studies has been associated with a higher number of advanced stage tumours in women at the time of initial diagnosis [12]. Our present study was limited to patients with lower stage tumours, which may underlie the fact that we did not observe such an association. Similarly, urethral disease or the possible effect of lymph node count on outcome are typically associated with higher stage tumours. It must be stressed that the present trial did not include patients with positive lymph nodes at the time of RC.
Level I evidence supports that patients with muscle-invasive UCB benefit from cisplatin-based neoadjuvant chemotherapy (NAC) [13]. However, treating patients with non-muscle invasive cancer involves the potential risk of exposing patients to unnecessary side-effects or ineffective chemotherapy when RC alone might provide comparable loco-regional control. Only 40% of patients achieve a pT0 stage after NAC and patients with residual muscle-invasive or node-positive disease have poorer outcomes. In all, 80% of patients with organ-confined UCB may be cured by RC as evidenced by the findings from this trial [4]. Some authors advocate a risk-adapted strategy to identify patients who may benefit from NAC. Based on the presence of LVI and other risk factors including aberrant histology, clinical T3b-T4a disease, or hydronephrosis, patients can be classified as low or high risk [14]. As reported by Culp et al. [14], even in patients without these features the final pathology can still result in a higher risk category due to a higher stage, lymph-node metastasis, or the presence of LVI. The authors reported upstaging in 49% of low-risk patients. Yet those patients still exhibited better 5-year progression-free (84% vs 62%) and OS (65% vs 47%) compared with the pre-RC high-risk group.
In patients with organ-confined, node-negative UCB who present with LVI on final pathology, the role of adjuvant chemotherapy is unclear. Our secondary aim was therefore to explore the putative benefit of adjuvant chemotherapy. We did not observe a significant difference in TTR or OS for patients undergoing adjuvant MVAC chemotherapy when LVI was present. However, the magnitude and direction of the detected HR did not exclude a possible benefit. A larger study would be needed to ultimately answer this question. The original p53-MVAC study design planned for the randomisation of 190 patients with p53-positive tumours. However, at the time of the interim analysis, which included 110 patients, the data and safety monitoring board recommended study closure and only 39 of 58 patients had received three cycles of MVAC. The rationale for adjuvant chemotherapy in patients with organ-confined disease with LVI is based on the presumably higher risk of recurrence, but is not currently evidence based.
A prior, non-contemporary study reported in 1988 evaluated the effect of combined cisplatin, doxorubicin, and cyclophosphamide chemotherapy in patients with UCB in the adjuvant setting [15]. In all, 71 patients underwent adjuvant chemotherapy and 62 with high-risk features were included as controls. Patients with high-risk features such as resected nodal metastasis, extravesical extension (pT3), and direct invasion of pelvic viscera (pT4) benefitted from adjuvant chemotherapy, while no beneficial effect was noted for patients with LVI as the only risk factor.
Our present study has important limitations. It is a post hoc analysis of data from a prospective trial that was not specifically designed to examine the effect of LVI on outcome in patients receiving adjuvant chemotherapy. There was no central pathology re-review to assess for LVI, but the presence of LVI was recorded on the study forms by the participating institutions. Last, the cohort of patients with LVI who received adjuvant chemotherapy was small, limiting the power of this post hoc analysis. The original study did not report on surgical quality parameters with regards to surgeon volume or compliance with the surgical protocol. This is an inherent problem in multi-centre clinical trials.
In conclusion, this secondary analysis of the p53-MVAC trial showed that the presence of LVI was strongly associated with an increased risk for recurrence and death in patients with organ-confined, node-negative UCB. These patients were approximately twice as likely to recur or die. The rationale for adjuvant chemotherapy in the presence of LVI is currently based on the generally higher risk for recurrence but lacks definitive scientific evidence regarding its true benefit. Among the patients who did have LVI, we did not observe a statistically significant protective association between adjuvant chemotherapy administration and clinical outcome. Although our present analysis did not exclude a possible benefit of chemotherapy, further prospective studies are needed to define its role in this setting.
Acknowledgments
Funding
Supported by grants CA71921 (R.J.C.), CA70903 (R.J.C.), and CA14089 and in part by Public Health Service Cooperative Agreement grants CA180888, CA180819, CA180830, CA46368, CA180801, CA35192, CA180835, CA46282, CA67575, and CA180834 from the National Cancer Institute, Department of Health and Human Services. The University of Southern California and SWOG, a cancer research cooperative group, were responsible for coordinating this multi-institution study. F.-C.v.R. is a recipient of a grant from the German Research Foundation (DFG).
Abbreviations
- CIS
carcinoma in situ
- EV
number of events
- HR
hazard ratio
- IQR
interquartile range
- LVI
lymphovascular invasion
- MVAC
methotrexate, vinblastine, doxorubicin, and cisplatin
- NAC
neoadjuvant chemotherapy
- OS
overall survival
- PLND
pelvic lymphadenectomy
- RC
radical cystectomy
- SWOG
Southwest Oncology Group
- TTR
time to recurrence
- TUR
transurethral resection
- UCB
urothelial cancer of the bladder
Footnotes
Conflicts of Interest
F.C.v.R. reports grants from the DFG (German Research Foundation) during the conduct of the study. G.G. reports personal fees from Endo Pharmaceuticals Inc., outside the submitted work. All other authors report no conflicts of interest.
References
- 1.Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011;59:1009–18. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Kim M, Kwak C, Kim HH, Ku JH. Prognostic significance of lymphovascular invasion in radical cystectomy on patients with bladder cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e89259. doi: 10.1371/journal.pone.0089259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stadler WM, Lerner SP, Groshen S, et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol. 2011;29:3443–9. doi: 10.1200/JCO.2010.34.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shariat SF, Svatek RS, Tilki D, et al. International validation of the prognostic value of lymphovascular invasion in patients treated with radical cystectomy. BJU Int. 2010;105:1402–12. doi: 10.1111/j.1464-410X.2010.09217.x. [DOI] [PubMed] [Google Scholar]
- 6.Hara S, Miyake H, Fujisawa M, et al. Prognostic variables in patients who have undergone radical cystectomy for transitional cell carcinoma of the bladder. Jpn J Clin Oncol. 2001;31:399–402. doi: 10.1093/jjco/hye086. [DOI] [PubMed] [Google Scholar]
- 7.Leissner J, Koeppen C, Wolf HK. Prognostic significance of vascular and perineural invasion in urothelial bladder cancer treated with radical cystectomy. J Urol. 2003;169:955–60. doi: 10.1097/01.ju.0000043639.55877.17. [DOI] [PubMed] [Google Scholar]
- 8.Kunju LP, You L, Zhang Y, Daignault S, Montie JE, Lee CT. Lymphovascular invasion of urothelial cancer in matched transurethral bladder tumor resection and radical cystectomy specimens. J Urol. 2008;180:1928–32. doi: 10.1016/j.juro.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Lotan Y, Gupta A, Shariat SF, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol. 2005;23:6533–9. doi: 10.1200/JCO.2005.05.516. [DOI] [PubMed] [Google Scholar]
- 10.Tilki D, Shariat SF, Lotan Y, et al. Lymphovascular invasion is independently associated with bladder cancer recurrence and survival in patients with final stage T1 disease and negative lymph nodes after radical cystectomy. BJU Int. 2013;111:1215–21. doi: 10.1111/j.1464-410X.2012.11455.x. [DOI] [PubMed] [Google Scholar]
- 11.Branchereau J, Larue S, Vayleux B, Karam G, Bouchot O, Rigaud J. Prognostic value of the lymphovascular invasion in high-grade stage pT1 bladder cancer. Clin Genitourin Cancer. 2013;11:182–8. doi: 10.1016/j.clgc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Kluth LA, Rieken M, Xylinas E, et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: an international multi-institutional study of more than 8000 patients. Eur Urol. 2014;66:913–9. doi: 10.1016/j.eururo.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 14.Culp SH, Dickstein RJ, Grossman HB, et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol. 2014;191:40–7. doi: 10.1016/j.juro.2013.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logothetis CJ, Johnson DE, Chong C, et al. Adjuvant cyclophosphamide, doxorubicin, and cisplatin chemotherapy for bladder cancer: an update. J Clin Oncol. 1988;6:1590–6. doi: 10.1200/JCO.1988.6.10.1590. [DOI] [PubMed] [Google Scholar]