Abstract
Purpose
We determined the likelihood that transurethral resection biopsy of the prostatic urethra adjacent to the verumontanum would detect prostatic involvement of urothelial carcinoma in patients with bladder carcinoma.
Materials and Methods
We compared precystectomy transurethral resection biopsy specimens of the prostatic urethra with those of the matched radical cystoprostatectomy in 272 patients with urothelial carcinoma of the bladder. All prostates were evaluated by whole mount step sections.
Results
Prostatic involvement by urothelial carcinoma was detected by transurethral resection biopsy or radical cystoprostatectomy in 101 patients (37.1%). Transurethral resection biopsy detected urothelial carcinoma in 72 cases with 71.3% sensitivity and 100% specificity. The overall accuracy of transurethral resection biopsy to detect urothelial carcinoma of the prostate was 89% (positive and negative predictive values 100% and 86%, respectively). Invasive prostatic urothelial carcinoma arising from the prostatic urethra was detected by transurethral resection biopsy in 21 of 26 patients (81%) while prostatic carcinoma in situ was detected in 39 of 52 (75%). Transurethral resection biopsy detected prostatic invasive urothelial carcinoma resulting from transmural invasion of a bladder tumor in 4 of 15 patients.
Conclusions
Prostatic involvement by urothelial carcinoma of the bladder was found in 37.1% of patients. Transurethral resection biopsy missed most tumors resulting from transmural invasion of the bladder primary lesion. Carcinoma in situ and invasive urothelial carcinoma arising from the prostatic urethra were detected in most cases. Transurethral resection biopsy of the prostatic urethra can complement staging and support clinical decision making with respect to neoadjuvant chemotherapy and planning for an orthotopic neobladder.
Keywords: prostate, urinary bladder, urothelium, carcinoma, biopsy
It was previously shown that involvement of the prostate is a common finding in patients with advanced urothelial carcinoma of the bladder. Synchronous neoplastic transformation and pagetoid spread of CIS from bladder neck to prostate appear to be the most relevant mechanisms for the development of prostatic urothelial carcinoma while direct transmural invasion of the prostate through the bladder wall accounts for fewer cases.1
The clinical significance and prognosis associated with prostatic involvement by urothelial carcinoma are clearly documented in several studies.2–7 Furthermore, the patterns and extent of prostatic involvement affect the outcome in patients with bladder cancer who have prostatic urothelial carcinoma.5,8,9
The reported incidence of prostatic urothelial carcinoma in patients with bladder cancer ranges widely from 16% to 48%.1,2,5,10 Whole mount step sectioning of the entire prostate is associated with much higher sensitivity for detecting prostatic urothelial carcinoma (range 32% to 48%2–5,11) than selective sections of the prostate with a detection rate of only 15.6% to 35% of prostatic urothelial carcinomas.1,6,7,12 A few studies suggest that the transurethral biopsy adjacent to the verumontanum detects most prostatic urothelial carcinomas since this portion of the prostatic urethra has the highest concentration of prostatic ducts.13–15
To our knowledge no study to date has described the precise patterns of prostatic involvement detected by transurethral biopsy and its correlation with whole mount step section. Our hypothesis was that precystectomy staging of the prostatic urethra with TUR biopsy is a sensitive method to detect prostatic urothelial carcinoma arising from the prostatic urethra and the prostatic ducts/acini. To determine the accuracy of transurethral biopsy for detecting prostatic UC and its concordance with whole mount specimens we examined prostatic tissue from 272 radical cystoprostatectomy specimens by whole mount sectioning of the entire prostate for the detection of prostatic urothelial carcinoma and correlated this with the final pathology results of the corresponding precystectomy transurethral biopsy.
MATERIALS AND METHODS
We identified 272 male patients who underwent prostatic urethral biopsy before radical cystoprostatectomy for bladder cancer from 1987 to 2013 from our institutional review board approved database (CAISIS, http://www.caisis.org/) and the pathology database at our private teaching hospital. Patients treated by other surgeons were included in the analysis. However, 204 of the 272 men were treated by a single surgeon (SPL) who performed a total of 278 male cystectomies at the teaching hospital during the study period. Pathological stage after cystectomy was pT0 in 21 cases, pTa/Tis in 62, pT1 in 45, pT2 in 50, pT3 in 63 and pT4 in 31. Transurethral biopsies were performed at the 5 and 7 o’clock positions adjacent to the verumontanum.
For prostatic urethral biopsy the entire specimen was submitted for processing and 3 levels of hematoxylin and eosin stained slides were prepared and examined. For pathological examination of the radical cystoprostatectomy specimen the prostate was separated from the bladder. Whole mount sections of the entire prostate were prepared and examined by at least 1 of 2 urological pathologists (SSS and TMW). The method of whole mount sectioning the prostate using a standard institutional protocol was described previously.16 Since 2005, we have applied a modified protocol by adding 2 sagittal whole mount sections of the prostate, which are cut along the prostatic urethra, and the remaining bilateral prostate tissue from apex to base is submitted (fig. 1). The specific types and patterns of prostatic involvement by UC that were evaluated included 1) prostatic urethral and ductal/acinar involvement by CIS, 2) lamina propria invasion of the prostatic urethra, 3) stromal invasion of the prostate, 4) periprostatic or seminal vesicle invasion and 5) direct penetrating invasion of the prostate through the bladder wall.
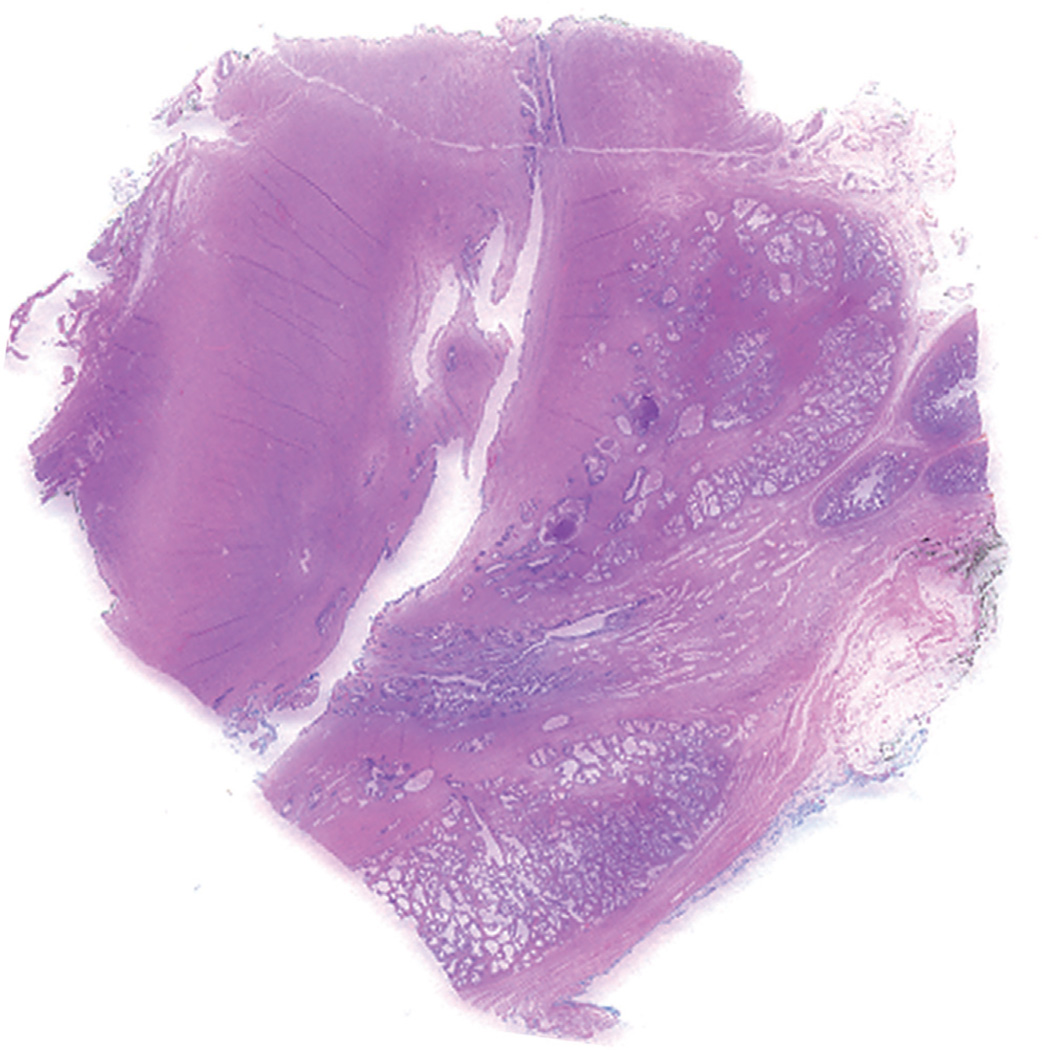
Figure 1.
Sagittal section of prostate enables evaluation of urethra in its entire longitude.
Categorical variables were summarized by the frequency and percent, and quantitative variables were summarized by the median and range. The Fisher exact or chi-square test was used to compare categorical variables among groups. Statistical analysis was done with SPSS® for Windows®, release 12.0. For positive and negative predictive values, and accuracy we defined positive TUR biopsy and negative whole mount as positive so that there were no false-positive results.
RESULTS
Patients with Bladder Cancer with vs without Prostatic Involvement
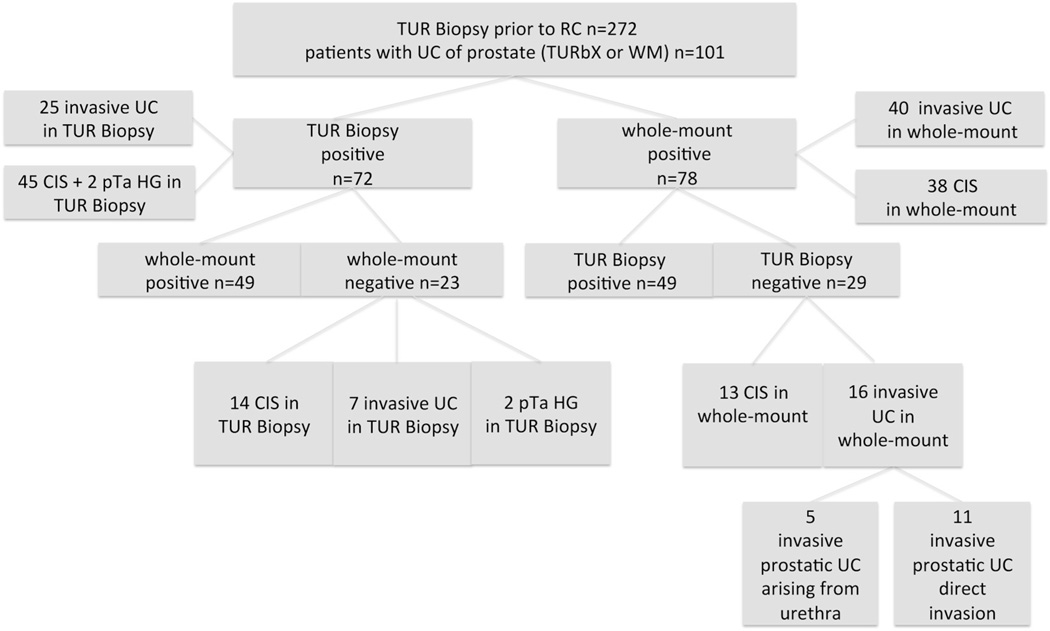
Prostatic urothelial carcinoma was identified in 101 of the 272 patients by TUR biopsy in 72 and/or whole mount section of the prostate in 78 for a combined incidence of 37.1% (fig. 2). CIS only at bladder tumor TUR was associated with a higher likelihood of prostatic involvement compared to tumors in the overall cohort (57% vs 37%, p = 0.043). Patients with prostatic UC involvement had a higher probability of pathological lymph node metastasis than those without prostatic UC (31.7% vs 20.5%, p = 0.042). Of the 48 patients with invasive prostatic UC on TUR biopsy or whole mount section 23 (47.9%) had positive lymph nodes (table 1). Those with invasive prostatic UC originating from direct penetration of the primary bladder tumor showed a significantly higher association with lymph node metastasis than patients with invasive prostatic UC arising from the prostatic urethra/duct (81% vs 33%, p = 0.002).
Figure 2.
Pathological findings in patients treated with TUR biopsy of prostatic urethra before radical cystectomy. In 49 patients UC was detected by TUR biopsy (TURBx) and by whole mount (WM). HG, high grade.
Table 1.
Pathological tumor stage stratified by prostate UC involvement
| No. Prostatic UC | |||
|---|---|---|---|
| No. Pts | Yes | No | |
| pT stage: | |||
| pT0 | 21 | 4 | 17 |
| pTa | 8 | 1 | 7 |
| pTis | 54 | 24 | 30 |
| pT1 | 45 | 15 | 30 |
| pT2 | 50 | 9 | 41 |
| pT3 | 63 | 17 | 46 |
| pT4 | 31 | 31 | 0 |
| Lymph node metastasis | 67 | 32 | 35 |
| Totals | 272 | 101 | 171 |
Prostatic UC
Pattern
Of the 101 patients with prostatic UC detected by TUR biopsy or whole mount sections 51 (50.5%) had CIS only, 48 (47.5%) had invasive urothelial carcinoma and 2 had papillary stage Ta, high grade disease (table 2). The latter 2 cases were detected only in the TUR biopsy. Of the 38 cases of prostatic CIS in the whole mount section 13 showed CIS in the prostatic urethra only and 25 involved the prostatic ducts and acini with (9) or without (16) involvement of the prostatic urethra. In 40 patients with invasive prostatic UC in the whole mount section the observed patterns were superficial lamina propria invasion in 9, prostatic stromal invasion in 18 and extracapsular and seminal vesicle invasion in 9 and 4, respectively (table 2). Figure 3 shows examples of prostatic CIS involving the urethra mucosa and ducts/acini as well as different patterns of invasive prostatic UC.
Table 2.
Prostatic UC incidence and patterns on prostate whole mount step section
| No. Prostatic UC | |
|---|---|
| CIS: | |
| Urethra only | 13 |
| Ductal/acini | 25 |
| Invasion: | |
| Lamina propria | 9 |
| Prostatic stromal | 18 |
| Periprostatic | 9 |
| Seminal vesicle | 4 |
| Total | 78 |
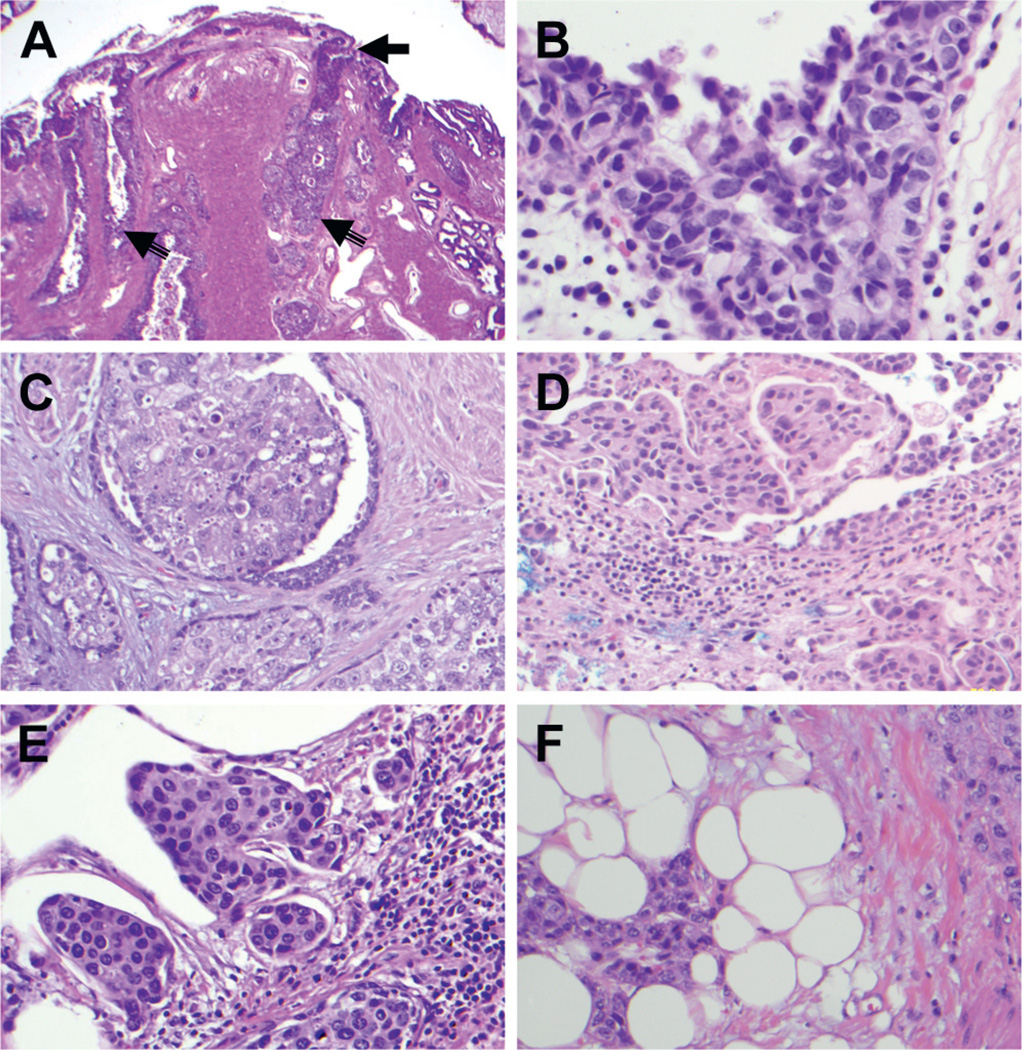
Figure 3.
Prostatic urothelial carcinoma patterns and levels. A, low power view shows prostatic verumontanum with urethral and ductal CIS (arrows). Reduced from ×20. B, prostatic urethral CIS with pagetoid spread. Reduced from ×400. C, prostatic ductal CIS spread with intact ductal epithelium. Reduced from ×400. D, superficial lamina propria invasion by prostatic urothelial carcinoma. Reduced from ×200. E, prostatic stromal invasion with nests and single tumor cells invading dense prostatic stromal tissue with inflammatory infiltrates. Reduced from ×200. F, periprostatic invasion by urothelial carcinoma with nests of tumor cells infiltrating adipose tissue. Reduced from ×200.
Detection by transurethral biopsy and whole mount section
Of the 29 cases of prostatic UC missed by TUR biopsy 11 were bladder tumors with transmural penetration into the prostate, 5 were invasive tumors arising from the prostatic duct/acini and 13 were urethral CIS. Of the 23 cases of prostatic UC detected by TUR biopsy but not identified on whole mount sections 14 were CIS, 7 were invasive UC and 2 were papillary stage Ta, high grade tumors (fig. 2).
The specific pattern of invasion detected by TUR biopsy did not always correlate with the type of invasion on the whole mount prostate sections. In 6 patients with CIS in the TUR biopsy the prostate whole mount sections revealed invasive UC and in 1 with invasive UC in the TUR biopsy the whole mount section showed only ductal CIS.
Transurethral biopsy detected prostatic UC in 72 cases with 71.3% sensitivity (95% CI 0.54–0.75) and 100% specificity (95% CI 0.97–1). The overall accuracy of TUR biopsy for detecting UC of the prostate was 89% with a positive predictive value of 100%. We counted TUR as positive and whole mount negative as positive. The negative predictive value was 86%.
Prostatic invasive UC via direct penetration of the bladder primary in 15 cases was detected in only 4 by TUR biopsy. Invasive prostatic UC arising from the prostatic urethra or ducts/acini was detected by TUR biopsy in 21 of 26 cases. This translated into an 81% detection rate with 81% sensitivity (95% CI 0.60–0.93) and 100% specificity (95% CI 0.97–1).
TUR biopsy was positive for prostatic CIS in 39 of 52 cases (75%), including 14 in which whole mount section revealed no CIS despite a positive biopsy. TUR biopsy had 75% sensitivity (95% CI 0.61–0.86) and 97% specificity (95% CI 0.93–0.99) to detect CIS. The negative predictive value of TUR biopsy to detect CIS and invasive UC arising from the urethra was 93% and 97%, respectively.
DISCUSSION
Identifying prostatic involvement by UC is essential for accurate clinical and pathological staging in patients with bladder cancer. It can help identify those with high risk disease (T4a) who may benefit from neoadjuvant cisplatin based chemotherapy and provide guidance for the urologist and the patient in choosing the optimal type of urinary diversion.
This study confirms the generally high incidence (37.0%) of prostatic UC in patients with urothelial bladder cancer who undergo radical cystoprostatectomy.1–3 Wood et al previously evaluated the accuracy of TUR biopsy in 25 cases treated with radical cystectomy and found that transurethral loop biopsy of the prostate adjacent to the verumontanum detected 9 of 10 cases of prostatic UC.14 Donat et al reported on 246 patients in whom precystectomy TUR biopsy of the prostatic urethra was performed.15 TUR biopsy detected 81% of those with prostatic UC. The investigators reported that positive biopsy was associated with an increased risk of second primary tumors of the urethra more often than negative biopsy (11% vs 2%).
Several studies suggest that the outcome in patients with prostatic UC depends on the type and level of prostatic invasion.5,8,9,17,18 Various terminologies have been used for prostatic stromal invasion in the recent literature. For the purpose of clarification we refer to the current 2010 AJCC (American Joint Committee on Cancer) staging classification, which categorizes stromal invasion of the prostate as pT2 for a primary urethral tumor. Primary bladder tumor staging classifies concomitant prostatic stromal invasion as pT4a. There is no differentiation between transmural penetration of the prostate from a bladder tumor or from stromal invasion arising from the prostatic urethra or ducts/acini. Thus, any stromal invasion of the prostate is staged as pT4a according to the AJCC classification in the presence of primary bladder cancer.
Whole mount or extensive sections of the prostate at and around the prostatic urethra can best determine the true incidence and extent of prostatic involvement. In our series 29 patients with prostatic UC identified by whole mount sections of the prostate had a negative TUR biopsy. Of these 29 cases 16 showed invasive UC in the prostatic stroma and, therefore, they were up staged to pT4a. These results suggest that extensive sections of prostate or whole mount step section are needed to identify any invasive prostatic UC regardless of transurethral biopsy results. Our study suggests that it is likely that many bladder cancers will be under staged if an inadequate number of prostatic blocks is examined. It is also important that the entire bladder neck be submitted to determine whether there is transmural bladder invasion into the prostate.
With respect to invasion type we distinguished between tumors that arose from the urethra and tumors that directly penetrated through the bladder wall into the prostate. Because the latter do not necessarily reach the level of the urethra, they would be expected to have a low probability of detection on transurethral biopsy. In tumors with direct transmural penetration into the prostate invasive UC and focal CIS account for most discordant findings of transurethral biopsy and prostate examination by whole mount section. This was reflected in our data, in which only 4 of 15 tumors with contiguous penetration were detected on precystectomy transurethral biopsy. For invasive tumors that arise from the prostate, including lamina propria invasion, the overall detection rate of TUR biopsy was 81%.
In general TUR biopsy of the prostatic urethra should be performed in all patients with bladder CIS. In our series 57% of patients with bladder CIS only at bladder tumor TUR were found to have prostatic UC in the biopsy or the whole mount sections of the prostate, a rate consistent with those of previous reports.12 This indicates that histological evaluation via TUR biopsy is imperative, particularly in patients with any CIS associated with the bladder primary tumor, as is now recommended by EAU (European Association of Urology) guidelines. To further illustrate this Huguet et al reported on 62 patients at high risk who had recurrence after bacillus Calmette-Guérin and were treated with radical cystectomy.19 In that series prostatic UC was the most important risk factor for muscle invasive cancer.
Moreover, it has been our practice to recommend prostatic urethra biopsy before radical cystoprostatectomy even in the presence of normal appearing mucosa. Furthermore, identifying prostatic UC before cystectomy can help counsel patients on the most suitable choice of urinary diversion. If present, it identifies the need for intraoperative frozen section of the apical urethral margin in patients planning to undergo orthotopic neobladder reconstruction.
The high proportion of patients with UC in the prostatic urethra also has implications for the surgeon considering PSC. While PSC may improve the recovery of sexual function and continence,20 as few as 10% of all patients might be candidates for PSC.21 We do not perform this procedure in light of the data that we report and we are concerned about the risk of spilling cancer or leaving residual UC in the prostate.
At our institution prostatic stromal involvement (cT4a), among other risk factors, is an indication for neoadjuvant chemotherapy. As a referral center we prefer to restage the case, obtain tissue to confirm the pathological diagnosis and perform TUR of the prostatic urethra. In the post-neoadjuvant chemotherapy setting we do not repeat these restaging procedures if the clinical situation does not require it.
Vanishing Cases with Positive Biopsy and Negative Whole Mount Section
Interestingly in our series of 101 prostatic UC cases 23 (23%) were identified by transurethral biopsy only since whole mount prostate sections revealed no UC. Of these patients 14 had CIS and 7 had invasive prostatic UC. These vanishing cases might be explained in part by the focal nature of CIS so that TUR may have removed the entire lesion. In addition to evaluating prostatic involvement by UC, TUR loop biopsy may have a therapeutic role in patients with focal noninvasive disease.
CONCLUSIONS
The current study shows that prostatic UC is a frequent finding in bladder cancer and invasive tumors arising from the prostatic urethra/ducts have a high likelihood of being detected by TUR biopsy. However, penetrating invasion of the prostate from bladder cancer is not detected by TUR biopsy in most cases. The identification of prostatic UC by urethral biopsy confers a significant risk of invasive urothelial carcinoma in the prostate. In patients planning cystectomy prostatic UC may be done to facilitate counseling for chemotherapy and urinary diversion type. Thorough histological examination of the prostate appears to be necessary for accurate staging in patients with bladder cancer regardless of biopsy findings.
Acknowledgments
Study received institutional review board approval.
Abbreviations and Acronyms
- CIS
carcinoma in situ
- PSC
prostate sparing cystoprostatectomy
- TUR
transurethral resection
- UC
urothelial cancer
Footnotes
Financial interest and/or other relationship with Digipath, Medscape, Molecular Health and DNA/Seq.
REFERENCES
- 1.Esrig D, Freeman JA, Elmajian DA, et al. Transitional cell carcinoma involving the prostate with a proposed staging classification for stromal invasion. J Urol. 1996;156:1071. [PubMed] [Google Scholar]
- 2.Wood DP, Jr, Montie JE, Pontes JE, et al. Transitional cell carcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. J Urol. 1989;141:346. doi: 10.1016/s0022-5347(17)40762-2. [DOI] [PubMed] [Google Scholar]
- 3.Reese JH, Freiha FS, Gelb AB, et al. Transitional cell carcinoma of the prostate in patients undergoing radical cystoprostatectomy. J Urol. 1992;147:92. doi: 10.1016/s0022-5347(17)37142-2. [DOI] [PubMed] [Google Scholar]
- 4.Revelo MP, Cookson MS, Chang SS, et al. Incidence and location of prostate and urothelial carcinoma in prostates from cystoprostatectomies: implications for possible apical sparing surgery. J Urol. 2004;171:646. doi: 10.1097/01.ju.0000107380.40481.bc. [DOI] [PubMed] [Google Scholar]
- 5.Shen SS, Lerner SP, Muezzinoglu B, et al. Prostatic involvement by transitional cell carcinoma in patients with bladder cancer and its prognostic significance. Hum Pathol. 2006;37:726. doi: 10.1016/j.humpath.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Schellhammer PF, Bean MA, Whitmore WF., Jr Prostatic involvement by transitional cell carcinoma: pathogenesis, patterns and prognosis. J Urol. 1977;118:399. doi: 10.1016/s0022-5347(17)58039-8. [DOI] [PubMed] [Google Scholar]
- 7.Hardeman SW, Soloway MS. Urethral recurrence following radical cystectomy. J Urol. 1990;144:666. doi: 10.1016/s0022-5347(17)39549-6. [DOI] [PubMed] [Google Scholar]
- 8.Barocas DA, Patel SG, Chang SS, et al. Outcomes of patients undergoing radical cystoprostatectomy for bladder cancer with prostatic involvement on final pathology. BJU Int. 2009;104:1091. doi: 10.1111/j.1464-410X.2009.08558.x. [DOI] [PubMed] [Google Scholar]
- 9.Knoedler JJ, Boorjian SA, Tollefson MK, et al. Urothelial carcinoma involving the prostate: the association of revised tumor stage and coexistent bladder cancer with survival following radical cystectomy. BJU Int. 2013 doi: 10.1111/bju.12486. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Njinou Ngninkeu B, Lorge F, Moulin P, et al. Transitional cell carcinoma involving the prostate: a clinicopathological retrospective study of 76 cases. J Urol. 2003;169:149. doi: 10.1016/S0022-5347(05)64056-6. [DOI] [PubMed] [Google Scholar]
- 11.Pettus JA, Al-Ahmadie H, Barocas DA, et al. Risk assessment of prostatic pathology in patients undergoing radical cystoprostatectomy. Eur Urol. 2008;53:370. doi: 10.1016/j.eururo.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Nixon RG, Chang SS, Lafleur BJ, et al. Carcinoma in situ and tumor multifocality predict the risk of prostatic urethral involvement at radical cystectomy in men with transitional cell carcinoma of the bladder. J Urol. 2002;167:502. doi: 10.1016/S0022-5347(01)69073-6. [DOI] [PubMed] [Google Scholar]
- 13.Rikken CH, van Helsdingen PJ, Kazzaz BA. Are biopsies from the prostatic urethra useful in patients with superficial bladder carcinoma? Br J Urol. 1987;59:145. doi: 10.1111/j.1464-410x.1987.tb04806.x. [DOI] [PubMed] [Google Scholar]
- 14.Wood DP, Jr, Montie JE, Pontes JE, et al. Identification of transitional cell carcinoma of the prostate in bladder cancer patients: a prospective study. J Urol. 1989;142:83. doi: 10.1016/s0022-5347(17)38667-6. [DOI] [PubMed] [Google Scholar]
- 15.Donat SM, Wei DC, McGuire MS, et al. The efficacy of transurethral biopsy for predicting the long-term clinical impact of prostatic invasive bladder cancer. J Urol. 2001;165:1580. [PubMed] [Google Scholar]
- 16.Wheeler TM. Anatomic considerations in carcinoma of the prostate. Urol Clin North Am. 1989;16:623. [PubMed] [Google Scholar]
- 17.Pagano F, Bassi P, Ferrante GL, et al. Is stage pT4a (D1) reliable in assessing transitional cell carcinoma involvement of the prostate in patients with a concurrent bladder cancer? A necessary distinction for contiguous or noncontiguous involvement. J Urol. 1996;155:244. [PubMed] [Google Scholar]
- 18.Ayyathurai R, Gomez P, Luongo T, et al. Prostatic involvement by urothelial carcinoma of the bladder: clinicopathological features and outcome after radical cystectomy. BJU Int. 2007;100:1021. doi: 10.1111/j.1464-410X.2007.07171.x. [DOI] [PubMed] [Google Scholar]
- 19.Huguet J, Crego M, Sabate S, et al. Cystectomy in patients with high risk superficial bladder tumors who fail intravesical BCG therapy: precystectomy prostate involvement as a prognostic factor. Eur Urol. 2005;48:53. doi: 10.1016/j.eururo.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Mertens LS, Meijer RP, de Vries RR, et al. Prostate sparing cystectomy for bladder cancer: 20 years single center experience. J Urol. 2014;191:1250. doi: 10.1016/j.juro.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Heidenreich A, Porres D, Pfister D. Treatment of bladder cancer. Value of radical prostatesparing cystectomy. Urologe A. 2012;51:813. doi: 10.1007/s00120-011-2786-1. [DOI] [PubMed] [Google Scholar]