Abstract
Background
Active engagement in intellectually enriching activities (e.g., reading, hobbies) builds “reserve” against memory decline in elders and persons with multiple sclerosis (MS), but the neural basis for this protective influence of enrichment is unknown. Herein we investigate the neuroanatomical basis of reserve against memory decline in MS patients.
Methods
Relapse-onset MS patients (N=187) underwent 3.0T MRI of the brain to quantify T2 lesion volume (T2LV) and normalized volumes of total brain, total white, total grey (using SIENAX) and thalamus, caudate, putamen, pallidum, amygdala, and hippocampus (using FIRST). Patients completed a survey quantifying their engagement in early life intellectual enrichment (i.e., reading, hobbies). Verbal and visuospatial episodic memory was assessed with neuropsychological tasks in a representative subsample (N=97).
Results
Controlling for demographics and T2LV, intellectual enrichment was specifically linked to larger normalized hippocampal volume (rp=.213, p=.004), with no link to other brain volumes/structures. Moreover, greater intellectual enrichment moderated/attenuated the negative relationship between normalized total brain volume (i.e., overall cerebral atrophy) and normalized hippocampal volume (i.e., hippocampal atrophy; p=.001) whereby patients who engaged in more early life intellectual enrichment better maintained hippocampal volume in the face of worse overall cerebral atrophy. Finally, the link between greater intellectual enrichment and better memory was partially mediated through larger hippocampal volume.
Conclusions
These findings support larger hippocampal volume as one key component of the neuroanatomical basis of reserve against memory decline in MS. These findings are consistent with previous literature on experience-dependent neuroplasticity within the hippocampus.
Keywords: Multiple Sclerosis, Memory, Cognitive Disorders, Cognitive Reserve, Hippocampus
Introduction
Many persons with multiple sclerosis (MS) suffer memory impairment [1-2], which is characterized mostly by difficulty transferring verbal and visuospatial information into long-term storage (i.e., episodic memory deficits) due in part to hippocampal atrophy [3]. Importantly, however, many other MS patients maintain adequate memory despite comparable disease burden (i.e., cerebral atrophy) [4]. This disconnect between disease burden and memory is partially explained by the cognitive reserve hypothesis [5], which states that elders (for review [6]) and MS patients [7-9] with greater intellectual enrichment (e.g., cognitive leisure) are less likely to suffer memory impairment despite disease. It remains unclear how enriching life experiences protect memory. One possibility is that intellectual enrichment impacts the hippocampus, which is consistent with (a) the greater impact of experience/environment (less heritability) on the hippocampus relative to other brain regions [10] and (b) evidence for a link between intellectual enrichment and hippocampal volumes in healthy adults [11-13]. Herein we investigate whether early life engagement in mentally-stimulating activities (a) is specifically linked to larger hippocampal volume in MS patients, and (b) attenuates the negative impact of MS disease burden (estimated with overall cerebral atrophy) on hippocampal volume. We also examine (c) whether larger hippocampal volume mediates the positive relationship between cognitive leisure and memory.
Methods
Sample
Subjects were 187 relapse-onset MS patients [14] (109 women; age: 43.9±10.6 years; disease duration: 13.0±8.4 years; EDSS: 3.2±2.0; 135 RRMS, 52 SPMS) with no exacerbation or corticosteroid use within four weeks. Patients reported cognitive leisure from their early 20s, so all patients enrolled were at least 25 years old. We excluded pediatric-onset MS patients due to potential developmental differences. This study was approved by the Institutional Review Board of the San Raffaele Hospital in Milan. Written informed consent was obtained from all patients.
Cognitive Leisure
As described and validated previously [15], patients were surveyed to quantify participation in cognitive leisure activities during their early 20's (Table 1). Previous validation showed no difference in item endorsement between MS patients and healthy persons [15], indicating that early life cognitive leisure is unaffected by preclinical or early MS disease.
Table 1.
Cognitive Leisure Scale [7, 15]. Patients were asked to report the frequency of their participation in seven common cognitive leisure activities. Frequency was reported as: daily (5), several times per week (4), several times per month (3), several times per year (2), once per year or less (1).
| Cognitive Leisure Activities |
|---|
| Read books |
| Read magazines or newspapers |
| Produce art (e.g., painting, poetry, sculpture) |
| Produce non-artistic writing (e.g., newsletter, diary, essays) |
| Play a musical instrument |
| Play structured games (e.g., board games, cards, crossword puzzles) |
| Participate in hobbies (e.g., model building, gardening, web design) |
Global and Focal Cerebral Atrophy
A 3.0 Tesla Philips Intera scanner acquired a 3D T1-weighted fast field echo (FFE;TR=25ms, TE=4.6ms, FA=30°, FOV=230mm2, matrix= 256×256, slice thickness=1mm, 220 contiguous axial slices, in-plane resolution=0.89×0.89mm2). After T1-hypointense lesion refilling [16], cerebral atrophy was measured as normalized total brain volume (grey matter [GM]+white matter [WM]) obtained using SIENAX (version 2.6, part of FSL 4.1). Normalized volumes of deep grey matter structures (thalamus, caudate, putamen, pallidum, amygdala, hippocampus) were obtained using FIRST, then applying the same scaling factor calculated with SIENAX. To correct for misclassification of WM lesions, all pixels classified as GM, but laying neither in cortical GM nor in subcortical GM, were reassigned to WM, before volume calculation. The scaling factor within SIENAX is derived from the transformation that matches the extracted brain and skull to standard space brain and skull images (derived from the MNI152 standard image): values higher than one were obtained for heads with small ICV, values lower than one for ICVs larger than the MNI atlas. An advantage of this approach is that it does not require that CSF is robustly estimated, being difficult to distinguish between CSF and skull voxels in T1 images.
A dual-echo turbo spin echo was also acquired (TR/TE=3500/24-120ms; FA=150°; FOV=240mm2; matrix=256×256; echo train length [ETL]=5; 44 contiguous, 3-mm-thick axial slices), and T2 lesion volume (T2LV) was measured using a local thresholding segmentation technique (Jim 5.0, Xinapse System, www.xinapse.com). T2LV was log-transformed to promote normality.
Memory Subgroup
Memory was assessed in a subsample of 97 patients (71 RRMS, 26 SPMS), which did not differ from the 90 non-assessed patients in age, education, disease duration, disease subtype, EDSS, cognitive leisure, or any MRI parameter (all Ps>.1). There was a more equal proportion of women to men within this subsample (47 women). Verbal memory was assessed with the Selective Reminding Test (SRT), which requires patients to learn twelve unrelated words across six trials, and then retrieve these words on a 30-minute delayed free recall task. Long-term store (LTS) and consistent long-term retrieval (CLTR) scores indicate the amount of information entered into secondary/long-term memory during the learning trials (i.e., as words could not have been retrieved from primary/working memory). Delayed recall (DR) also requires retrieval from secondary/long-term memory. Norm-referenced [17] z-scores were calculated for LTS, CLTR, and DR, and a composite (mean) verbal memory z-score was calculated. (Correlations among these three scores ranged from .813 to .909, indicating contributions to a common construct: long-term verbal memory.) Mean verbal memory z-score was -0.82 ± 1.2 (21st percentile), with about 35% of patients performing at least 1.5 SDs below normal. Visuospatial memory was assessed with the Spatial Recall Test (SPART), which requires patients to learn and reproduce spatial locations of checkers within a grid across three trials, and then reproduce these locations after a 15-minute delay. Norm-referenced [17] z-scores were calculated for total learning (across the three initial trials) and delayed recall, and a composite (mean) visuospatial memory z-score was calculated. (Total learning and delayed recall were correlated .733, indicating contributions to a common construct: long-term visuospatial memory.) Mean visuospatial memory z-score was -0.61 ± 1.0 (27th percentile), with about 20% of patients performing at least 1.5 SDs below normal. An overall memory composite was also derived as the mean of verbal and visuospatial memory z-scores. Mean z-score was -0.72±0.94 (24th percentile), with about 20% of the sample performing at least 1.5 SDs below normal. Subsequent memory analyses are performed with the overall memory composite score first; and then performed again separately for verbal and visuospatial memory.
Statistical Analyses
We first performed partial correlations between cognitive leisure and all MRI estimates of global and focal atrophy, controlling for demographics (age, sex, education, disease duration, phenotype [RRMS, SPMS]). We performed these analyses a second time also controlling for T2LV (n = 186). We predict that cognitive leisure will be specifically related to normalized hippocampal volume, and not other brain regions (including other deep grey matter structures). We then performed a hierarchical regression predicting normalized hippocampal volume (i.e., hippocampal atrophy), with demographics (above), normalized total brain volume (i.e., overall cerebral atrophy) and cognitive leisure in block one, and the interaction term between normalized brain volume and cognitive leisure in block two. We predict an interaction between overall cerebral atrophy and cognitive leisure on hippocampal atrophy, such that MS patients with greater cognitive leisure will better maintain hippocampal volume (resist hippocampal atrophy) in the face of worse overall cerebral atrophy. Finally, we assessed intercorrelations among memory, cognitive leisure, and hippocampal volume to explore whether the relationship between cognitive leisure and memory may be at least partially explained by hippocampal volume.
Results
Correlations between cognitive leisure and global/regional atrophy
As shown (Table 2), greater cognitive leisure was specifically linked to larger normalized hippocampal volume (Figure 1a), with no relationship between cognitive leisure and any other MRI parameter (all Ps > .3), with the exception of a trend for the amygdala. That is, patients who engaged in more early life leisure activity suffered less hippocampal atrophy (better maintained hippocampal volume). [This pattern of results remained (a) when also controlling for T2LV in addition to demographic variables (Table 2) and (b) without controlling for T2LV or demographic variables (correlation between cognitive leisure and hippocampal volume =.220, p=.002).]
Table 2.
Partial correlations between cognitive leisure and normalized brain volumes, controlling for demographic variables (A), and also controlling for T2LV (B).
| Total Brain | Grey | White | Thalamus | Caudate | Putamen | Pallidum | Amygdala | Hippocampus | |
|---|---|---|---|---|---|---|---|---|---|
| A | |||||||||
| rp | .016 | .073 | -.041 | .048 | .012 | .051 | -.009 | .139 | .199 |
| p | .830 | .329 | .579 | .523 | .877 | .495 | .906 | .062 | .007 |
| B | |||||||||
| rp | .007 | .074 | -.049 | .041 | .005 | .048 | -.020 | .135 | .213 |
| p | .924 | .326 | .518 | .584 | .952 | .519 | .790 | .071 | .004 |
Figure 1.
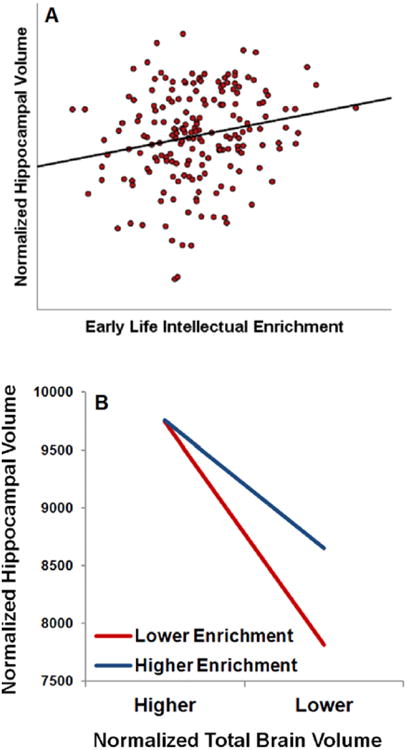
(A) Scatterplot depicting the partial correlation between early life intellectual enrichment (cognitive leisure activity) and current normalized hippocampal volume, controlling for demographic variables. (B) Plot of the regression equation depicting the interaction between intellectual enrichment and normalized total brain volume (overall cerebral atrophy) on normalized hippocampal volume. Lower and higher cognitive leisure and normalized total brain volume were plotted using values at the 16th and 84th percentile of our sample (i.e., ± 1 standard deviation). As shown, MS patients with greater early life intellectual enrichment better maintain hippocampal volume, even at higher levels of total cerebral atrophy. That is, greater cognitive leisure attenuates the negative relationship between overall cerebral atrophy and hippocampal atrophy.
Regression predicting hippocampal atrophy
The regression predicting normalized hippocampal volume was significant (F[8,178]=21.04, p<.001; multiple R2=.486). Controlling for demographic variables, lower normalized brain volume (worse overall cerebral atrophy) was associated with lower normalized hippocampal volume (worse hippocampal atrophy; rp=.579, t=9.49, p<.001), and greater early life cognitive leisure activity was associated with less hippocampal atrophy (greater normalized hippocampal volume; rp=.231, t=3.18, p=.002). Finally, there was an interaction between cognitive leisure and cerebral atrophy (block two) whereby greater early life cognitive leisure moderated/attenuated the negative association between overall cerebral atrophy and hippocampal volume (rp=-.223, t=3.05, p=.003). That is, as shown (figure 1b), MS patients who engaged in more early life cognitive leisure were better able to maintain hippocampal volume (combat hippocampal atrophy) even in the context of high overall cerebral atrophy (an estimate of overall disease-related brain change).
Memory, cognitive leisure, and hippocampal atrophy
Consistent with results for the full sample (N=187), cognitive leisure and normalized hippocampal volume were positively correlated (r=.230, p=.024) within the memory subsample (N=97). Memory function was positively correlated with both cognitive leisure (r=.414, p<.001) and hippocampal volume (r=.330, p=.001). Next, we were interested in whether hippocampal volume mediates the relationship between cognitive leisure and memory (using established mediation concepts [18]). When controlling for hippocampal volume, the correlation between cognitive leisure and memory was weakened (rp=.368, p<.001) but not eliminated: hippocampal volume accounted for about 20% of the relationship between cognitive leisure and memory. That is, the relationship between greater cognitive leisure and better memory is partially mediated through larger hippocampal volume.
Verbal memory was positively correlated with both cognitive leisure (r=.428, p<.001) and hippocampal volume (r=.243, p=.016). Controlling for hippocampal volume, the correlation between cognitive leisure and verbal memory was weakened (rp=.394, p<.001) but not eliminated, indicating partial mediation. Visuospatial memory was positively correlated with both cognitive leisure (r=.252, p=.013) and hippocampal volume (r=.315, p=.002). Controlling for hippocampal volume, the correlation between cognitive leisure and visuospatial memory was weakened and no longer significant (rp=.194, p=.058), suggesting greater partial mediation.
Discussion
Greater early life intellectual enrichment (cognitive leisure) was reliably and specifically associated with larger normalized hippocampal volume in MS patients (figure 1a). There was no relationship between cognitive leisure and other commonly-investigated global and regional brain volumes investigated (including other deep grey matter structures), nor was there a relationship with T2LV. These findings are consistent with the greater impact of life experience/environment (lower heritability) on hippocampal volume relative to other brain regions [10]. Moreover, cognitive leisure attenuated the negative impact of overall cerebral atrophy on hippocampal atrophy (figure 1b). That is, MS patients who engaged in more mentally-stimulating lifestyles earlier in life were better able to maintain hippocampal volume (resist hippocampal atrophy) despite overall cerebral atrophy.
Greater cognitive leisure and larger hippocampal volume were associated with better memory, and larger hippocampal volume partially mediated the relationship between greater cognitive leisure and better memory. As such, larger hippocampal volume may represent part of the neuroanatomical basis of cognitive reserve in MS. This is consistent with evidence linking educational attainment [11, 13] and lifetime mental activity [12] to hippocampal structure in healthy adults. In fact, Noble and colleagues [11] showed that the negative effects of aging on hippocampal volume are diminished among persons with higher educational attainment, which is consistent with our finding that the negative effects of MS disease progression (estimated with overall cerebral atrophy) on hippocampal volume are diminished in patients with greater intellectual enrichment. [Note that the contribution of cognitive leisure in our study was observed even when controlling for educational attainment.]
Unlike the aging literature [e.g., 11], however, further analysis within our MS sample did not reveal a correlation between education attainment and any volumetric measurements, including hippocampal volume (all Ps > .10). One explanation may be restricted variance in education (mean ± SD = 13.3 ± 3.4, range = 16) relative to intellectual enrichment (19.2 ± 5.1, range = 28). Note that education was only modestly correlated with early intellectual enrichment (r = .235, p = .001), indicating that these variables maintain a large degree of independence. Other factors such as socioeconomic status may contribute more to education than to leisure activity (see [8]).
There are other important neuroanatomical bases of memory function in MS patients, such as white matter integrity within the fornix [19-20]. Importantly, enrichment in the form of cognitive training has been linked to improvements of white matter integrity in humans [21], and new learning in humans induces white matter changes within the fornix [22-23]. Future research should investigate whether intellectual enrichment is associated with white matter architectural changes within the fornix and other tracts subserving memory function in MS patients. Interestingly, memory function among MS patients has also recently been positively linked to glutamate concentrations within the hippocampus (Muhlert et al., 2014) [24], highlighting another potential biomarker of memory-related hippocampal integrity, and a potential outcome measure in studies of intellectual enrichment and memory in MS patients.
The brain's default network consists largely of limbic structures [25], including the hippocampus, and has been implicated in memory [26]. Indeed, functional MRI (fMRI) studies have linked memory impairment in MS patients to reduced default network activation during a task [27] and reduced default network functional connectivity during rest [28]. We have previously shown that MS patients with greater intellectual enrichment exhibit greater activation within the brain's default network [29]. These links among intellectual enrichment, default network activity, and memory are consistent with our current finding that premorbid intellectual enrichment is positively and specifically linked to larger hippocampal volume in MS patients. Together, hippocampal volume (MRI), function (fMRI), and behavior (memory) are linked to intellectual enrichment in MS patients, thereby highlighting the hippocampus as one potential component of the neuroanatomical basis of cognitive reserve against memory decline in MS.
This study has limitations. The cross-sectional design and absence of data from healthy controls makes it difficult to know whether the positive relationship between intellectual enrichment and normalized hippocampal volume is due to (a) larger premorbid normalized hippocampi in persons with greater early intellectual enrichment, or (b) less disease-related hippocampal atrophy in those with greater early intellectual enrichment. This can be better answered by future longitudinal research. Also, a within-subjects, prospective design would likely provide a more accurate effect size estimate of the relationship between enrichment and changes in hippocampal volume, as the current cross-sectional design does not account for myriad sources of error due to individual differences in genetic, environmental, and pathological factors possibly related to hippocampal volume. A within-subjects design would control for many of these sources of error/variability, perhaps leading to larger correlations between early intellectual enrichment and hippocampal volume than observed in the current cross-sectional study. Finally, future experimental and/or clinical trial research is needed to draw a causal link between intellectual enrichment and both hippocampal volume and memory. This is true for the cognitive reserve literature across populations, which is based almost exclusively on observational research.
Acknowledgments
This project was funded in part by the National Institutes of Health (R00HD060765 to JFS).
Footnotes
Site of Data Collection: San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Via Olgettina, 60, Milan, Italy
Disclosure of Conflicts of Interest: None.
References
- 1.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 2.Thornton AE, Raz N. Memory impairment in multiple sclerosis: a quantitative review. Neuropsychology. 1997;11:357–366. doi: 10.1037//0894-4105.11.3.357. [DOI] [PubMed] [Google Scholar]
- 3.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 4.Filippi M, Rocca MA, Benedict RH, et al. The contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology. 2010;75:2121–2128. doi: 10.1212/WNL.0b013e318200d768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- 6.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumowski JF, Leavitt VM. Cognitive reserve in multiple sclerosis. Multiple Sclerosis Journal. 2013;19:1122–1127. doi: 10.1177/1352458513498834. [DOI] [PubMed] [Google Scholar]
- 8.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve in multiple sclerosis: what you've got and how you use it. Neurology. 2013;80:2186–2193. doi: 10.1212/WNL.0b013e318296e98b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumowski JF, Rocca M, Leavitt VM, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. 2014;82:1776–83. doi: 10.1212/WNL.0000000000000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- 11.Noble KG, Grieve SM, Korgaonkar MS, et al. Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience. 2012;6:307. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3:e2598. doi: 10.1371/journal.pone.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piras F, Cherubini A, Caltagirone C, Spalletta G. Education mediates microstructural changes in bilateral hippocampus. Human Brain Mapping. 2011;32:282–289. doi: 10.1002/hbm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumowski JF, Wylie GR, Gonnella A, et al. Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology. 2010;75:1428–1431. doi: 10.1212/WNL.0b013e3181f881a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CA. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. Journal of magnetic resonance imaging. 2010;32:223–228. doi: 10.1002/jmri.22214. [DOI] [PubMed] [Google Scholar]
- 17.Boringa JB, Lazeron RH, Reuling IE, et al. The brief repeatable battery of neuropsychological tests: normative values allow application in multiple sclerosis clinical practice. Multiple Sclerosis. 2001;7:263–267. doi: 10.1177/135245850100700409. [DOI] [PubMed] [Google Scholar]
- 18.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 19.Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239–249. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- 20.Kern KC, Ekstrom AD, Suthana NA, et al. Fornix damage limits verbal memory functional compensation in multiple sclerosis. NeuroImage. 2012;59:2932–2940. doi: 10.1016/j.neuroimage.2011.09.071. [DOI] [PubMed] [Google Scholar]
- 21.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y. Short-term learning induces white matter plasticity in the fornix. Journal of Neuroscience. 2013;33:12844–12850. doi: 10.1523/JNEUROSCI.4520-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Muhlert N, Atzori M, De Vita E, et al. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. Journal of neurology, neurosurgery, and psychiatry. 2014;85:833–839. doi: 10.1136/jnnp-2013-306662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H. A dual-subsystem model of the brain's default network: self-referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage. 2012;61:966–977. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Sumowski JF, Wylie GR, Leavitt VM, et al. Default network activity is a sensitive and specific biomarker of memory in multiple sclerosis. Multiple Sclerosis Journal. 2013;19:199–208. doi: 10.1177/1352458512448267. [DOI] [PubMed] [Google Scholar]
- 28.Leavitt VM, Paxton J, Sumowski JF. Default network connectivity is linked to memory status in multiple sclerosis. Journal of the International Neuropsychological Society. 2014 doi: 10.1017/S1355617714000800. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133:362–374. doi: 10.1093/brain/awp307. [DOI] [PMC free article] [PubMed] [Google Scholar]


