Abstract
Objective
Alcoholic hepatitis (AH) is often associated with advanced fibrosis, which negatively impacts survival. We aimed at identifying kinases deregulated in livers from patients with AH and advanced fibrosis in order to discover novel molecular targets.
Design
Extensive phosphoprotein analysis by reverse phase protein microarrays was performed in AH (n=12) and normal human livers (n=7). Ribosomal S6 kinase (p90RSK) hepatic expression was assessed by qPCR, Western blot and immunohistochemistry. Kaempferol was used as a selective pharmacological inhibitor of the p90RSK pathway to assess the regulation of experimentally-induced liver fibrosis and injury, using in vivo and in vitro approaches.
Results
Proteomic analysis identified p90RSK as one of the most deregulated kinases in AH. Hepatic p90RSK gene and protein expression was also upregulated in livers with chronic liver disease. Immunohistochemistry studies showed increased p90RSK staining in areas of active fibrogenesis in cirrhotic livers. Therapeutic administration of kaempferol to carbon tetrachloride-treated mice resulted in decreased hepatic collagen deposition, and expression of profibrogenic and proinflammatory genes, compared to vehicle administration. In addition, kaempferol reduced the extent of hepatocellular injury and degree of apoptosis. In primary hepatic stellate cells, kaempferol and small interfering RNA decreased activation of p90RSK, which in turn regulated key profibrogenic actions. In primary hepatocytes, kaempferol attenuated proapoptotic signalling.
Conclusions
p90RSK is upregulated in patients with chronic liver disease and mediates liver fibrogenesis in vivo and in vitro. These results suggest that the p90RSK pathway could be a new therapeutic approach for liver diseases characterised by advanced fibrosis.
INTRODUCTION
Alcoholic hepatitis (AH) is a severe clinical condition found in patients with chronic liver disease and heavy alcohol consumption.1 AH is characterised not only by steatosis but also by extensive hepatocellular damage and inflammation as well as advanced fibrosis.2 The mortality of AH has not substantially decreased in the last decades, and 3-month mortality remains 30–50%.3 We recently identified the histological parameters associated with an unfavourable outcome in these patients. Among them, patients with severe fibrosis have a higher short-term mortality.4 Fibrosis contributes to severe portal hypertension, which underlies many of the most severe complications in these patients (ie, variceal bleeding or hepatic encephalopathy). The standard therapy for this disease (ie, corticosteroids or pentoxiphylline) is not effective in many patients so there is an urgent need to develop new target-specific therapies. In the past few years, different groups have identified several potential molecular targets to treat patients with advanced alcoholic liver disease including AH.5–13 Most of the studies performed in human samples focused on transcriptome analysis, while proteomic studies are lacking. This is partially due to the small amount of tissue obtained for research purposes using a transjugular biopsy. In the current study, we performed, for the first time, a proteomic analysis in liver tissue from patients with AH. We focused on kinases because they are well-characterised proteins and regulate key intracellular signalling pathways.14 We used reverse phase protein microarrays (RPPM) as a molecular technology, which allows the detection of multiple analytes on individual samples using specific primary antibodies,15 to provide us with an array of selected kinases that appear differentially regulated in AH and may be responsible for driving its pathophysiological events. One of the most up-regulated kinases in patients with AH compared to normal livers was the 90 kDa ribosomal S6 kinase (p90RSK). p90RSK is a serine/threonine kinase member of the S6 ribosomal kinase (RSK) family, which is downstream to the extracellular signal-regulated kinase (ERK) signalling pathway.16 p90RSK is known to participate in numerous other signalling pathways and regulate multiple cellular processes including cell proliferation, apoptosis, transformation, cytokine production and collagen synthesis, as well as to modulate tissue repair after chronic liver injury.17–21
To investigate the role of p90RSK on liver fibrogenesis, we performed a translational study using different approaches. First, we analysed the expression of p90RSK in livers from patients with different types of liver disease. Second, we investigated the role of p90RSK in a well-defined model of repeated liver injury and fibrogenesis, two key features of AH. Finally, the molecular mechanisms and biological effects of p90RSK inhibition were investigated in cultured hepatocytes and hepatic stellate cells (HSCs).
METHODS
Patients
Patients admitted consecutively to the Liver Unit, Hospital Clínic of Barcelona, with clinical, analytical and histological features of AH from 2009 to 2010, were included in the kinase analysis (n=12). Patients with hepatocellular carcinoma or any other potential cause of liver disease were excluded. All patients had an episode of AH confirmed by biopsy. Liver biopsy was obtained through a transjugular approach using tru-cut needles that allowed us to obtain enough tissue for clinical purposes and to perform proteomic studies.22 None of the patients fulfilled hepatorenal syndrome criteria at admission and 2 out of 12 patients (17%) developed hepatorenal syndrome during hospitalisation. One patient out of 12 had urinary track infection and another showed upper respiratory infection at admission. A fragment of liver tissue was frozen immediately in liquid nitrogen until proteomic analysis. Inclusion criteria and clinical management of these patients have been extensively explained in previous studies.3,5,6 For gene expression studies, additional patients with AH (n=9) were included in two control groups. First, patients with HCV-induced liver disease (genotype 1; n=9) without previous antiviral therapy and with a F3–F4 stage of liver fibrosis as assessed by the Metavir score, were included. Second, patients with morbid obesity who underwent a laparoscopic liver biopsy during bariatric surgery were included as diagnosed with non-alcoholic steatohepatitis (NASH n=9), according to Kleiner’s criteria.23 In addition, for protein expression studies, patients with compensated cirrhosis due to HCV (n=3) or history of alcohol abuse (in all cases patients were abstinent for at least 6 months; n=3) were included. To quantify protein expression in liver tissue by Western blot, we used wedge biopsies, which provide enough protein for this technique. Finally, fragments of normal liver tissue were obtained from optimal cadaveric liver donors (n=3) or resection of liver metastases (n=4), as described in detail previously,3,5,6 and included for kinase and gene/protein studies. All liver specimens were analysed by an expert pathologist and a part of the biopsy was submerged in a RNA stabilisation solution (RNAlater; Life Technologies Corporation, Carlsbad, California, USA). The protocol was approved by the Ethics Committee of the Hospital Clínic and all patients gave informed consent.
Reverse phase protein microarrays
Protein expression profiling was performed using RPPM on the ZeptoMARK assay platform (Zeptosens, Bayer, Wuppertal, Germany). Samples of frozen liver tissue were ground using a ball mill (Mikro-Dismembrator U; Sartorius, Goettingen, Germany) and lysated by adding 10-fold excess (vol/wt) of CeLyA Lysis Buffer CLB1 (Zeptosens). Briefly, microarrays were generated with an array layout containing four replicates of each sample at 0.4 μg/μL of concentrated protein. Detection of protein and protein modifications was performed using a direct two-step immunoassay using specific primary antibodies. Sixty-three analytes comprising an array of phosphorylated and non-phosphorylated kinases were included (see online supplementary table S1). Alexa647-labelled antispecies secondary antibodies (Life Technologies Corporation) were used to generate a fluorescence signal. Images of the microarrays were taken using a ZeptoREADER microarray imager (Zeptosens) and further analysis was performed using a ZeptoVIEW Pro V.2.0 software package (Zeptosens), as described in detail by Pirnia et al.15
Animals
Hepatic fibrosis was induced in male BALB/c mice following administration of carbon tetrachloride (CCl4 Sigma-Aldrich, St Louis, Missouri, USA) injected intraperitoneally at a dose of 0.5 mL/kg, twice a week for 4 weeks, as described in detail previously.24 Control groups received corn oil as vehicle for the same period. Pharmacological inhibition of p90RSK was performed by intraperitoneal administration of kaempferol (Sigma-Aldrich), a selective inhibitor of the p90RSK pathway,17,18,25,26 at a dose of 50 mg/kg, or dimethyl sulfoxide (Sigma-Aldrich) as vehicle, twice a week during the last 2 weeks of the injury period, once liver fibrosis and hepatocellular damage were established (see online supplementary figure S1). Mice were sacrificed and collection of liver and blood samples was performed. Samples were processed and analysed using molecular and histological techniques described in online supplementary methods. All animal experiments were approved by the Ethics Committee of Animal Experimentation of the University of Barcelona.
Cell cultures
Previous described procedures were followed for isolation of primary human HSCs from healthy livers27 and primary mouse hepatocytes.28 On overnight stabilisation in a serum-free medium, human primary HSCs were treated with recombinant human platelet-derived growth factor (PDGF), recombinant human tumour necrosis factor α (TNF-α) or lipopolysaccharide (LPS; Sigma-Aldrich). Additionally, HSCs were pre-treated with kaempferol 30 to 60 min before adding the other agents. In parallel, HSCs were transfected with small interference RNA sequences specific for the RSK2 isoform of p90RSK using the reagents and transfection protocols provided by JetPRIME Transfection Reagent kit (Polyplus Transfection, Illkirch, France). After treatments, RNA and protein extracts were obtained for assessing gene and protein expression by qPCR and Western blot, as described in online supplementary methods. HSC proliferation was assessed using the Cell Proliferation Reagent WST-1 (Roche Applied Science, Penzberg, Germany). In cultured mouse hepatocytes, apoptosis was induced by coincubation of mouse recombinant TNF-α with actinomycin D (Sigma-Aldrich), following kaempferol or Rsk2 siRNA transfection. The degree of hepatocyte apoptosis was tested by measuring caspase cleavage products using the Caspase-Glo 3/7 assay (Promega, Fitchburg, Wisconsin, USA) and by assessing transaminase release to cell supernatant and WST-1 metabolism.
Statistical analysis
Results of quantitative variables are expressed as mean±SE unless otherwise specified. The differences between groups were analysed using non-parametric tests (Mann-Whitney U test) for continuous variables. Statistical analysis was performed using SPSS V.14.0 for Windows (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Characteristics of patients with AH included in the proteomic study
Twelve patients were included in the kinase analysis with clinical, analytical and histological features of AH. Overall, 90-day mortality was 16%. The mean age of patients was 50 years and they were predominantly male (65%). Patients with AH at admission had an age, serum bilirrubin, INR and serum creatinine (ABIC) score >6.71 and 72% of patients had a Maddrey’s discriminant function >32. Importantly, all 12 patients had a high-liver fibrosis stage as assessed by Ishak (>5) and Metavir (F3–F4) scores. The main epidemiological, clinical and analytical characteristics of patients are shown in table 1.
Table 1.
Clinical, analytical and hepatic haemodynamic characteristics of patients with AH included in the Kinase analysis (n=12)
| Variables | Mean±SE or % |
|---|---|
| Age (years) | 49±1.12 |
| Male (%) | 65 |
| Alcohol intake (g/day) | 107.25±4.86 |
| 90-day mortality (%) | 16 |
| Severe AH (by Maddrey DF) (%) | 72 |
| Analytical and haemodynamic parameters | |
| Glucose (mg/dL) | 111±5 |
| Creatinine (mg/dL) | 0.84±0.06 |
| AST (U/L) | 155±12 |
| ALT (U/L) | 61±5 |
| GGT (U/L) | 600±83 |
| Bilirubin (mg/dL) | 12.5±1.21 |
| Albumin (mg/dL) | 26.2±0.55 |
| Platelet count (×109) | 139±14 |
| Leucocyte count (×109/L) | 10.04±7.43 |
| Neutrophils count (%) | 74±8.32 |
| INR | 1.92±0.12 |
| HVPG (mm Hg) | 19.35±0.85 |
| Cirrhosis (%) | 60 |
| Scoring systems | |
| Maddrey’s DF | 59.24±5.97 |
| MELD score | 22±0.8 |
| ABIC score | 7.65±0.19 |
ABIC, age, serum bilirrubin, INR and serum creatinine score; AH, alcoholic hepatitis; ALT, alanine transaminase; AST, aspartate transaminase; GGT, γ-glutamyl transpeptidase; HVPG, hepatic venous pressure gradient; INR, international normalised ratio; MELD, model of end-stage liver disease score.
Kinase analysis identifies p90RSK as a deregulated kinase in AH
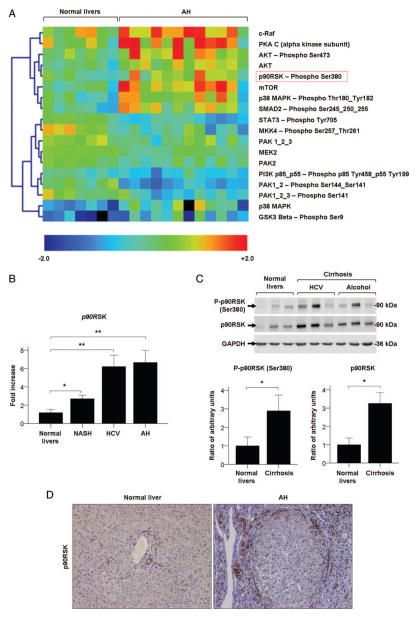
Among the 63 molecules that were analysed by RPPM, 18 appeared to be differentially regulated in livers from patients with AH compared to normal livers. Unsupervised clustering analysis revealed that patients with AH showed a proteomic profile consisting of increased levels of phosphorylated and total forms of protein kinase B (Akt) and p38, protein kinase A, subunit C (PKA C), mammalian target of rapamycin (mTOR) and phosphorylated forms of mothers against decapentaplegic homologue 2 (SMAD2), and glycogen synthase kinase 3 β (GSK3-β) compared with normal livers, but decreased phosphorylated signal transducer and regulator of transcription 3 (STAT3) as well as phosphatidylinositide 3-kinase (PI3K; figure 1A). In particular, p90RSK was the most significantly activated kinase found in patients with AH compared to normal livers, with an increased ratio point value of 1.60 (p<0.0001, table 2). p90RSK was selected for further analysis since it is an important downstream effector of the ERK pathway, a well known signalling pathway involved in liver injury.29 ERK1/2 appeared to be activated in AH as well, but without reaching statistical significance (p=0.068).
Figure 1.
(A) Kinase analysis of patients with alcoholic hepatitis (AH; n=12) compared to normal livers (n=7). Colour intensities represent the standardised ratio between the individual sample value and the average value of each analyte across all samples. Yellow to red coloured spots correspond to increased levels of the analyte in the indicated sample, whereas green to blue spots indicate decreased analyte levels. (B) p90RSK hepatic gene expression in patients with non-alcoholic steatohepatitis (NASH; n=9), HCV (n=9) and AH (n=9), compared to normal livers (n=7). (C) Western blot of phosphop90RSK (Ser380), total p90RSK and GAPDH on whole liver proteins from patients with compensated cirrhosis derived from HCV infection (n=3) or alcohol consumption (n=3), compared to normal livers (n=3). Protein levels are represented as mean ratio values quantified from protein bands of phospho-p90RSK (Ser380) or total p90RSK versus GAPDH compared to normal livers. (D) Immunohistochemistry of paraffin sections of liver biopsies from normal livers and from patients with AH, stained with the antiRSK2 antibody (×100 magnification). Significances are shown above bars: *p<0.05; **p<0.01.
Table 2.
Analytes differentially regulated in patients with AH compared to normal livers in the Kinase analysis
| Analyte name | Ratio | p Value |
|---|---|---|
| c-Raf | 1.552 | 0.028 |
| PKA C (α kinase subunit) | 2.261 | 0.010 |
| AKT—phospho Ser473 | 1.813 | 0.005 |
| AKT | 1.622 | 0.000 |
| p90RSK—phospho Ser380 | 1.599 | 0.000 |
| mTOR | 2.383 | 0.002 |
| p38 MAPK—phospho Thr180_Tyr182 | 1.846 | 0.004 |
| SMAD2—Phospho Ser245_250_255 | 1.917 | 0.005 |
| STAT3—Phospho Tyr705 | 0.576 | 0.000 |
| MKK4—Phospho Ser257_Thr261 | 0.539 | 0.003 |
| PAK 1_2_3 | 0.754 | 0.036 |
| MEK2 | 0.759 | 0.000 |
| PAK2 | 0.786 | 0.022 |
| PI3K p85_p55—Phospho p85 Tyr458_p55 Tyr199 | 0.758 | 0.010 |
| PAK1_2—Phospho Ser144_Ser141 | 0.456 | 0.000 |
| PAK1_2_3—Phospho Ser141 | 0.594 | 0.001 |
| p38 MAPK | 1.953 | 0.022 |
| GSK3 Beta—Phospho Ser9 | 1.950 | 0.013 |
Analyte levels are expressed as ratio values between the mean values of raw data quantified from patients with AH versus the mean values of raw data quantified from normal livers, in the kinase analysis.
AH, alcoholic hepatitis; PKA C, protein kinase A, subunit C; mTOR, mammalian target of rapamycin; SMAD2, decapentaplaegic homologue 2
p90RSK is overexpressed in patients with AH and other chronic liver diseases
We next assessed hepatic expression of p90RSK by qPCR in patients with AH and other types of chronic liver diseases including patients with HCV and NASH. The results revealed that p90RSK was up regulated in patients with chronic liver disease compared to normal livers (p<0.01), especially those patients with AH and HCV who had advanced fibrosis stage and, to a lesser extent, patients with NASH (figure 1B). Patients with compensated HCV and alcoholic cirrhosis showed increased hepatic protein levels of total p90RSK compared to normal livers (p<0.05). Moreover, protein levels of Serine 380 (Ser380)-phosphorylated p90RSK were slightly higher in patients with cirrhosis compared to normal livers (figure 1C). Next, we explored the tissue expression and distribution of p90RSK by immunohistochemistry using an antibody to detect total p90RSK. There was a marked staining of p90RSK in livers from patients with AH compared to normal livers. p90RSK immunostaining was mainly found in biliary ducts in both groups, but especially in areas of ductular reaction and myofibroblast expansion, such as in fibrotic capsule, in patients with AH (figure 1D). Similarly, phospho-ERK staining was increased in livers from patients with AH compared to normal livers and located in non-parenchymal cells (see online supplementary figure S2). Taken together, these results suggest that p90RSK is up regulated in patients with different types of liver disease, including AH. Expression and activation of p90RSK both seem to be related to the stage of liver fibrosis and not related to ALD exclusively, showing no differences between HCV-induced and alcohol-induced cirrhosis (figure 1B, C).
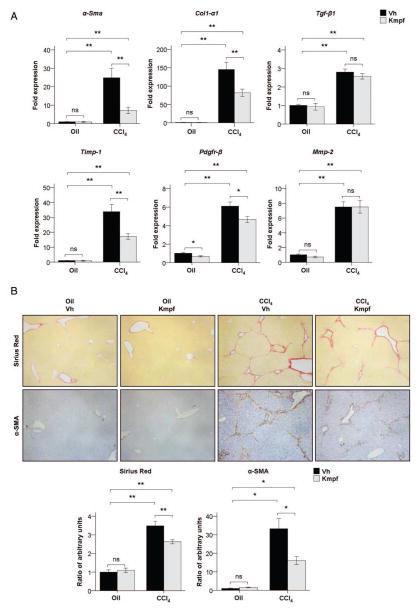
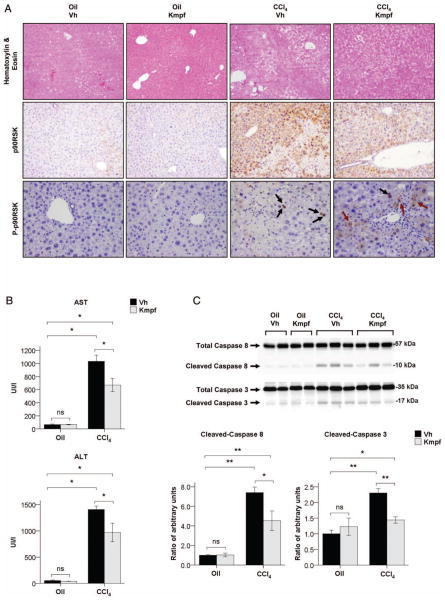
Kaempferol administration attenuates the p90RSK signalling pathway and parallels with reduced liver injury and fibrogenesis in CCl4-treated mice. We next assessed whether p90RSK inhibition attenuates fibrosis in vivo. Since there are no alcohol-based animal models that mimic the main prognostic histological findings of severe AH (mainly advanced fibrosis), we used a well-characterised experimental model of repeated liver injury that results in advanced liver fibrosis. In our study, we administered kaempferol, a selective inhibitor of the p90RSK pathway, to mice with ongoing liver injury after CCl4 exposure (therapeutic approach). Kaempferol-exposed mice showed a reduction in the degree of liver fibrosis and HSC expansion compared to vehicle-exposed mice after CCl4 treatment, as shown by decreased hepatic expression of profibrogenic genes such as Collagen type I, α 1 (Col1-α1) and tissue inhibitor of metalloproteinases 1 (Timp-1), as well as markers of HSC activation such as α-smooth muscle actin (α-Sma) and PDGF receptor β (Pdgfr-β; p<0.01, figure 2A). This finding was confirmed through morphometric quantification of Sirius Red and α-SMA immunostaining in liver paraffin sections, which revealed that kaempferol administration resulted in decreased deposition of extracellular matrix as well as decreased number of α-SMA-positive cells in CCl4-treated mice (p<0.01 both, figure 2B). Next, we evaluated whether p90RSK was induced by CCl4 treatment and whether this induction was attenuated after kaempferol administration. We performed H&E staining and immunohistochemistry studies to analyse p90RSK distribution within liver tissue. Blinded histological examination by an expert pathologist revealed reduced distorted liver architecture (cirrhosis-like) in CCl4-treated mice exposed to kaempferol compared to vehicle. In addition, p90RSK immunostaining analysis revealed that the total form of this kinase is increased in parenchymal cells surrounding areas of necrosis and high degree of hepatocellular damage, while its phosphorylated form was mainly found in the nuclei of damaged hepatocytes. Importantly, therapeutic administration of kaempferol to CCl4-treated mice resulted in decreased expression of total p90RSK in areas of necrosis, and changed the pattern of immunostaining of phospho-p90RSK, since it was expressed mainly in the cytoplasm of hepatocytes (figure 3A). These results suggest that p90RSK may regulate hepatocyte fate and that kaempferol could prevent p90RSK nuclear translocation in these cells. Moreover, we assessed the expression and activity of different kinases involved in liver fibrogenesis by Western blot. The results showed that kaempferol administration attenuated the CCl4-induced phosphorylation of p90RSK in both Ser380 and Ser363 key regulatory residues, as well as expression of total p90RSK. Similarly, kaempferol also decreased phosphorylation and expression of CCAAT/enhancer-binding protein β (C/EBP-β) transcription factor in CCl4-treated mice, which is a downstream mediator of p90RSK reported to mediate liver fibrogenesis in vivo.21 Additionally, we found that phosphorylation of ERK was also attenuated after kaempferol administration, whereas Akt signalling was induced. However, JNK and p38 signalling pathways were unmodified in CCl4-treated mice after kaempferol exposure (see online Supplementary figure S3A). Collectively, these results suggest that the inhibitory effects of kaempferol are not only restricted to p90RSK, but also to other kinases involved in liver fibrogenesis, such as ERK and C/EBP-β. We next explored whether the antifibrogenic effect of kaempferol was also associated with reduced inflammation and hepatocellular injury in CCl4-treated animals. Kaempferol-exposed mice had decreased hepatic gene expression of proinflammatory cytokines involved in the development of liver fibrogenesis, such as TNF-α, monocyte chemotactic protein 1 (Mcp-1), osteopontin (Opn) and Ccl20, compared to vehicle-exposed mice (p<0.05, see online supplementary figure S3B). Furthermore, mice exposed to kaempferol showed reduced levels of serum alanine transaminase (ALT) and serum aspartate transaminase (AST) compared to their vehicle-exposed counterparts (p<0.05, figure 3B), as well as blunted activation of caspases 8 and 3 after CCl4 administration (p<0.05, figure 3C). Taken together, these results suggest that kaempferol may exert hepatoprotective, anti-inflammatory and antifibrogenic effects in mice exposed to continuous hepatotoxins, by mediating the inhibition of the ERK-p90RSK signalling pathway.
Figure 2.
(A) α-Sma, Col1-α1, Tgf-β1, Timp-1, Pdgfr-β and Mmp-2 hepatic gene expression in corn oil or CCl4-treated mice after vehicle or kaempferol (Kmpf) exposure. (B) Representative Sirius Red and α smooth muscle actin (α-SMA) immunostaining in paraffin liver sections of corn oil or CCl4-treated mice after vehicle or kaempferol exposure (×100 magnification). Quantification of positive areas is presented in the graphs. Significances are shown above bars: *p<0.05; **p<0.01; ns=non-significant.
Figure 3.
(A) Representative H&E staining (×100 magnification), p90RSK immunostaining (×100×) and phosphop90RSK (Thr359/Ser363) immunostaining (×200) in paraffin liver sections of corn oil or CCl4-treated mice after vehicle or kaempferol (Kmpf) exposure. Black arrows show p90RSK nuclear translocation whereas dark red arrows show cytoplasmic presence of p90RSK in parenchymal cells. (B) Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels of corn oil or CCl4-treated mice after vehicle or kaempferol exposure. (C) Representative Western blot of total and cleaved caspase 8 and caspase 3 in whole liver proteins of corn oil or CCl4-treated mice after vehicle or kaempferol exposure. Caspase cleavage is represented as mean ratio values quantified from protein bands of cleaved caspase versus total caspase compared to oil+vehicle-treated group. Significances are shown above bars: *p<0.05; **p<0.01; ns=non-significant.
p90RSK mediates proliferation and activation of cultured primary human HSC
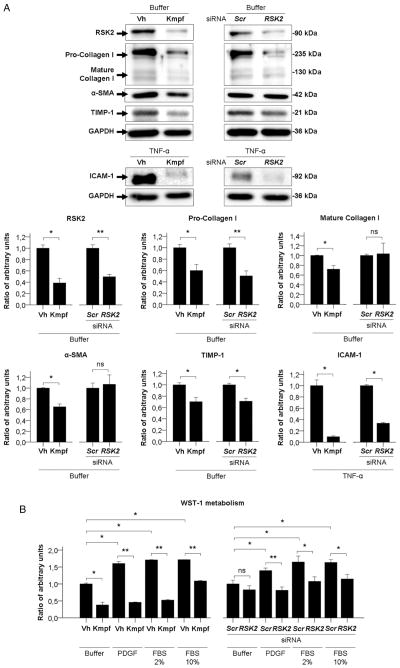
We next performed in vitro studies to confirm the mechanistic effects of kaempferol found in our animal model. We found that p90RSK was phosphorylated after a short-time exposure to key molecules known to regulate HSC activation, including TNF-α, PDGF and LPS (see online supplementary figure 4SA). Additional Western blot analysis revealed that time-course incubation with kaempferol blunted basal phosphorylation of p90RSK, ERK and C/EBP-β in cultured HSC (see online supplementary figure S4B), thus confirming our in vivo data showing that kaempferol inhibits ERK-p90RSK signalling pathway. Besides, we performed an interfering RNA assay to specifically inhibit p90RSK in these cells. RSK2 siRNA transfection in HSC resulted in blunted gene expression of the RSK2 isoform but not RSK1 and RSK3 isoforms (p<0.05, see online supplementary figure S4C). Further, RSK2 silencing decreased Pro-Collagen type I and TIMP-1 expression, as well as TNF-α-induced expression of ICAM-1 in culture-activated HSC (p<0.05, figure 4A). In addition to markers regulated by RSK2 silencing, kaempferol also decreased expression of Mature Collagen type I and α-SMA (p<0.05, figure 4A). Moreover, since the ERK signalling pathway is known to be involved in HSC activation and expansion, we next evaluated the role of p90RSK in HSC proliferation. We showed that kaempferol incubation and RSK2 silencing reduced the HSC proliferation after exposure to different mitogens, as assessed by WST-1 metabolism (p<0.05, figure 4B). Collectively, these results suggest that p90RSK is involved with in vitro activation and expansion of HSC, and that kaempferol may exert its antifibrogenic effect by targeting HSC.
Figure 4.
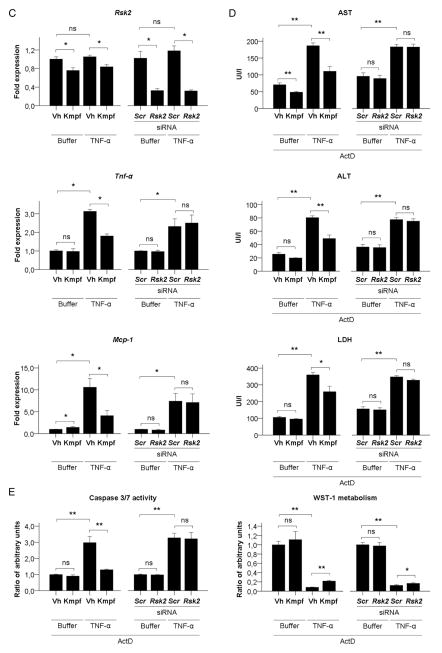
(A) Representative Western blot and quantification graphs of basal protein expression of RSK2, Collagen type I, α smooth muscle actin (α-SMA) and TIMP-1 and TNF-α-induced protein expression of ICAM-1, of primary human culture-activated hepatic stellate cells (HSC) incubated with vehicle (Vh) or kaempferol (Kmpf) or transfected with Scrambled control (Scr) or RSK2 siRNA. GAPDH has been used as an endogenous control for protein expression. Protein levels are represented as mean ratio values quantified from protein bands of each marker versus GAPDH compared to Vh-treated or Scr-treated HSC. (B) Platelet-derived growth factor (PDGF) or FBS-induced proliferation of primary human HSC incubated with vehicle (Vh) or kaempferol (Kmpf) or transfected with Scr or RSK2 siRNA. Proliferation rates are presented as mean ratio values quantified from absorbance data of WST-1 metabolism to formazan after 48 h of incubation compared to Vh-treated or Scr-treated, buffer-treated HSC. (C) Rsk2, Tnf-α and Mcp-1 gene expression of buffer-treated or TNF-α-treated primary mouse hepatocytes incubated with Vh or Kmpf or transfected with Scr or Rsk2 siRNA. (D) Aspartate transaminase (AST), alanine transaminase (ALT) and LDH release to cell culture supernatant from buffer-treated or TNF-α +actinomycin D (ActD)-treated primary mouse hepatocytes incubated with Vh or Kmpf or transfected with Scr or Rsk2 siRNA. (E) Caspase 3/7 activity and WST-1 metabolism of buffer-treated or TNF-α+ActD-treated primary mouse hepatocytes incubated with Vh or Kmpf or transfected with Scr or Rsk2 siRNA. Caspase activity is represented as mean ratio values quantified from luminescent data of caspase metabolites after 12 h of incubation compared to Vh-treated or Scr-treated, buffer-treated hepatocytes. Cell viability rates are presented as mean ratio values quantified from absorbance data of WST-1 metabolism to formazan after 24 h of incubation compared to Vh-treated or Scr-treated, buffer-treated hepatocytes. Significances are shown above bars: *p<0.05; **p<0.01; ns=non-significant.
Kaempferol protects cultured hepatocytes from apoptosis
Finally, we sought to assess whether the hepatoprotective effects of kaempferol seen in our animal model of chronic liver damage were caused by an inhibition of p90RSK signalling in hepatocytes. For this purpose, we evaluated hepatocellular damage in primary cultured mouse hepatocytes either incubated with kaempferol or transfected with an Rsk2 siRNA. As in cultured human HSC, Rsk2 silencing resulted in a specific depletion of both gene and protein expression of the Rsk2 isoform of p90RSK, but not of the other Rsk isoforms (p<0.05, see online supplementary figures S5A,B). TNF-α treatment induced an increased gene expression of proinflammatory cytokines Tnf-α and Mcp-1 in cultured hepatocytes (p<0.05, figure 4C) but did not induce transaminase release to the culture media (see online supplementary figure S5C). Interestingly, kaempferol, but not Rsk2 silencing, blunted TNF-α-induced proinflammatory effects in cultured hepatocytes (p<0.05, figure 4C). Next, we evaluated the effects of kaempferol and Rsk2 silencing on hepatocyte apoptosis after exposure to a combination of TNF-α and actinomycin D. Kaempferol treatment reduced hepatocyte damage, as shown by a reduction on AST, ALT and LDH release to the culture media, an inhibition of Caspase 3/7 activity, and an improvement of the 24 h-cell viability as assessed by WST-1 metabolism (p<0.05, figures 4D, E). In contrast, Rsk2 siRNA transfection showed no effect on promoting or blunting TNF-α-induced apoptosis (figures 4D, E). These results suggest that kaempferol may mediate hepatoprotective effects on hepatocytes through other mechanisms besides p90RSK.
DISCUSSION
The treatment of AH has not evolved in the last decades. The two available therapies (ie, prednisolone and pentoxiphylline) do not target specific molecular drivers of the disease. In order to identify key molecular determinants of AH, we performed the first proteomic analysis in biopsies from patients with well-characterised AH. We focused on kinases because they are highly druggable molecular targets. In this study, we identified a number of kinases that are up regulated and activated in AH by a RPPM approach. However, as in any broad screening analysis to conclude that a particular kinase is actually having a pathophysiological role, it is critical to validate each kinase in a confirmatory set of samples and to perform a functional validation in relevant cell or animal models. Several kinases identified are appealing as potential targets to explore for AH including mTOR, Akt or c-Raf. In this study we focused on p90RSK since it was one of the most significantly up regulated kinases identified in the kinase analysis and an important downstream effector of a well-described signalling pathway in liver disease.16 Upstream mediator ERK was also found activated in AH, yet the statistical analysis showed borderline significance, probably due to a limitation in the number of samples included in the study. On the contrary, upstream mediator MEK appeared to be downregulated in the kinase analysis. This fact may be regulated by negative feedback mechanisms of downstream mediators of the signalling pathway including p90RSK.16 p90RSK has been involved in the regulation of cell death and apoptosis, and has been implicated in diseases characterised by endothelial and parenchymal disruption, such as atherosclerosis and heart disease.30,31 In contrast with other organs, little is known on the role of p90RSK on chronic liver diseases. p90RSK is activated in HSC during liver fibrogenesis and phosphorylates C/EBP-β, a transcription factor that mediates collagen synthesis.21 Our study complements and expands these previous studies by showing that p90RSK is activated in human liver diseases characterised by severe fibrosis and portal hypertension. Also, we demonstrate that the described pathway not only regulates experimental liver fibrogenesis but also may be involved in hepatocellular injury.
Our translational approach consists of identifying potential molecular drivers of AH to perform functional studies in animal models. Severe AH is characterised by profound hepatocellular dysfunction, severe fibrosis and progenitor cell expansion.4,32 Unfortunately, there are no animal models that strongly mimic these histological features. Advanced fibrosis is an independent predictor of mortality in patients with AH and mediates many of the clinical complications.4 Moreover, our gene and protein experiments in patients revealed that p90RSK up regulation was not only restricted to alcohol-induced liver disease. Therefore, we used a well-characterised model of repeated hepatocellular injury and fibrosis based on chronic CCl4 administration. In order to perform a loss-of-function study, we chose a pharmacological approach using a selective inhibitor of the p90RSK pathway (kaempferol). Kaempferol is a flavonol with a molecular structure containing a planar heterocyclic ring system that is similar in size and shape to the adenine moiety of ATP, thus acting as a binding competitor for ATP to activate the catalytic subunit of p90RSK.26 We decided to use kaempferol instead of some other novel synthetic inhibitors25 because their specificity is not yet fully proven in animal models.33 In addition, mice lacking p90RSK have a very disturbed phenotype resembling the Coffin-Lowry syndrome (CLS), with serious developmental problems.33–35 In addition, there are studies showing that human fibroblasts from patients with CLS and fibroblasts from Rsk2-deficient mice displayed different mechanisms of p90RSK-dependent regulation of gene transcription, suggesting that these mice might not be the ideal model to study p90RSK function in vivo.33
The first finding of our study is that p90RSK is markedly deregulated in AH. Interestingly, overall phosphorylated p90RSK was found increased in patients with AH, confirming the results obtained in the kinase analysis. Moreover, we showed that p90RSK was transcriptionally regulated and increased in patients with advanced fibrosis at gene and protein level. Based on previous studies, we speculated that p90RSK could have pleiotropic effects in the livers of these patients. We studied fibrogenesis because advanced fibrosis is a strong predictor of poor outcome in these patients. In addition, we studied the potential role of p90RSK in inflammation and hepatocellular injury. We found that p90RSK is expressed in areas of myofibroblast expansion in livers from patients with AH, which are characterised for presenting a high stage of liver fibrosis. Our in vivo results clearly showed that therapeutic administration of kaempferol into CCl4-treated mice resulted in decreased HSC accumulation and collagen deposition, which paralleled with decreased ERK and p90RSK signalling. The in vitro results with cultured HSCs using a gene silencing approach strongly indicated that p90RSK mediates cell proliferation and collagen synthesis in response to well-known fibrogenic mediators, thus suggesting that this kinase is an appealing target to treat liver fibrosis. Interestingly, we observed that the antifibrogenic and anti-inflammatory properties of kaempferol were accompanied with reduced hepatocellular apoptosis, using in vivo and in vitro approaches. In CCl4-treated mice, this finding was paralleled with decreased expression of total p90RSK and retarded nuclear translocation of phosphop90RSK in hepatocytes, suggesting that kaempferol may modulate the expression and cellular localisation of this kinase. The difference in the pattern of tissue staining of p90RSK in human and animal samples suggests that the cell types up regulating p90RSK may be dependent on the type of injury and species. Nevertheless, we used a gene silencing approach to investigate the role of p90RSK in regulating apoptosis in cultured mouse hepatocytes, and found that inhibition of p90RSK is not sufficient to attenuate hepatocellular damage. In fact, it is well reported that p90RSK activity contributes to cell survival through different mechanisms, including induction of NFkB signalling and by regulating the expression of proapoptotic and antiapoptotic genes such as BAD and BCL-2.33 Importantly, the inhibitory effects of kaempferol are not only restricted to p90RSK, but also to other members involved in the fibrogenic pathway such as upstream regulator ERK and downstream mediator C/EBP-β in animal as well as cell models. These results are supported by other studies that report an inhibitory effect of kaempferol on other kinases.36–38 Owing to that, additional loss-of-function approaches in animal models should investigate the role of p90RSK signalling during experimental liver fibrogenesis and hepatocyte survival through kaempferol-independent mechanisms.
In this study, we identified the p90RSK pathway as a novel potential druggable molecular driver that may participate in the development and progression of liver fibrogenesis. As we discussed previously, there is an urgent need to develop targeted therapies for patients with advanced liver fibrosis, including patients with AH. In recent years, attempts made by the hepatology research community to identify molecular drivers of AH have been unsuccessful, probably because they were identified in animal models of moderate alcoholic liver disease (ie, steatosis and mild inflammation). Thus, our approach is based on molecular studies in liver samples from patients with well-characterised AH. Our kinase analysis included 63 analytes of phosphorylated and non-phosphorylated forms of a number of candidate kinases known to regulate liver injury and fibrogenesis.39 Additional in vitro and in vivo functional analysis will be required to address the role of each identified kinase in liver injury and disease. Moreover, studies using more sensitive proteomic techniques40 should be performed in the future to allow a more comprehensive analysis of the kinases that are potentially implicated in this disease.
In summary, the present study provides the first kinase analysis of patients with AH and has identified the p90RSK pathway as one of the most upregulated signalling pathways. In absence of a model of severe AH and fibrosis in rodents, we used alternative in vivo and in vitro approaches showing that the p90RSK pathway mediates liver fibrogenesis and hepatocellular injury in chronically damaged livers. Further preclinical studies should confirm our results.
Supplementary Material
Significance of this study.
What is already known about this subject?
Alcoholic hepatitis (AH) is a severe clinical condition that is associated with short-term mortality. Advanced liver fibrosis is independently associated with poor prognosis in these patients.
Kinases are enzymatic proteins involved in the transduction of signalling pathways that are involved in several pathological processes, and thus represent highly druggable targets for therapy.
Ribosomal S6 kinase (p90RSK) is a ribosomal protein involved in multiple cellular processes including protein synthesis, migration and transformation. It is a downstream mediator to extracellular signal-regulated kinase (ERK), which in turn is involved in development of liver fibrogenesis and hepatic stellate cell activation. Kaempferol is a plant-derived flavonol that has been used as an in vitro selective inhibitor of the p90RSK pathway.
What are the new findings?
Proteomic analysis in liver tissue has revealed a differentially regulated subset of kinases in patients with AH compared to normal livers. p90RSK appeared to be especially upregulated in livers from patients with AH and patients with chronic liver disease presenting a high stage of liver fibrosis. p90RSK has been found expressed in areas of active liver fibrogenesis.
Kaempferol inhibits the p90RSK pathway—including the upstream mediator ERK—in an experimental mouse model of liver fibrogenesis. This is paralleled with reduced collagen deposition and myofibroblastic cell expansion within the hepatic parenchyma and blunted expression of profibrogenic genes. Additionally, kaempferol administration also lowered the degree of hepatocellular damage.
Inhibition of p90RSK using either kaempferol or a gene silencing approach induced decreased expression of activation markers and proliferation rate in cultured hepatic stellate cells. Moreover, kaempferol confers resistance to apoptotic signalling in cultured hepatocytes.
How might it impact on clinical practice in the foreseeable future?
Liver fibrosis is one of the most important features associated with poor prognosis in different types of liver disease including AH. In this study, we present the first kinase screening performed in livers from patients with AH showing different kinases that may be involved in its pathogenesis. Importantly, we show that p90RSK is highly expressed during advanced liver fibrosis, and appears to be an important mediator of fibrosis development and progression. Although kaempferol is not a specific molecular inhibitor of p90RSK, it can be used as a wide-spectrum inhibitor for the fibrogenic pathway—comprising ERK, p90RSK and C/EBP-β—exerting in vivo as well as in vitro antifibrogenic and hepatoprotective effects. Thus, kaempferol-derived molecules may be suitable drugs to develop new treatments for patients with advanced liver fibrosis.
Acknowledgments
This work was performed in Centre Esther Koplowitz (CEK), Barcelona, Spain, and in the Medical and Biomolecular Research Building (MBRB) at UNC, Chapel Hill, NC. The authors would like to thank Bibiana Rius, Cristina López-Vicario, Dr Eva Morán-Salvador and Dr Esther Titos from the IDIBAPS for their help in providing for the experiments of cultured hepatocytes.
Funding This work was supported by grants from the Instituto de Salud Carlos III (FIS PI041538, FIS PI042380 and FIS PI080126 to Dr Bataller, Dr Caballería and Dr Ginès, respectively), by NIAAA grants 1U01AA021908, 1U01AA020821 and P60-AA011605 and by European Commission within its FP7 Cooperation Programme and the European Cosmetics Association (COLIPA) HeMiBio—HEALTH-F5-2010-266777. O Morales-Ibanez received a grant from la Generalitat de Catalunya for Research Stays Abroad, BE-DGR 2012. SA received a grant from IDIBAPS. DR-T received a grant from the Ministerio de Educación, Cultura y Deporte, FPU programme. Dr PS-B is funded by Instituto de Salud Carlos III, Miguel Servet (CP11/00071) and cofinanced by FEDER, Unión Europea, ‘Una manera de hacer Europa’. JA received a grant from Fundación Banco Bilbao Vizcaya Argentaria.
Footnotes
Contributors OM-I participated in the conception and design of this study, data analysis and interpretation, performance of the majority of the experiments and manuscript drafting. SA, DR-T, DB, CM, MC, LP, GO and MM performed additional experiments. TK and MFT performed the kinase analysis. JA performed the statistical analysis. RM performed the histological examination of human and animal samples. VA, PG and JC helped with the interpretation of the data and revision of the final version of the manuscript. PS-B and RB were involved in the conception, design and supervision of this paper, analysis and interpretation of the data and participated in drafting and critically revising the final version of the paper.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/gutjnl-2014-307979).
Competing interests None.
Patient consent Obtained.
Ethics approval Hospital Clínic of Barcelona, Barcelona, Catalonia, Spain.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroneterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 2.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–56. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 4.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–9. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colmenero J, Bataller R, Sancho-Bru P, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687–97. doi: 10.1053/j.gastro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 7.Affò S, Dominguez M, Lozano JJ, et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2013;62:452–60. doi: 10.1136/gutjnl-2011-301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales-Ibanez O, Domínguez M, Ki SH, et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 2013;58:1742–56. doi: 10.1002/hep.26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Affò S, Morales-Ibanez O, Rodrigo-Torres D, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782–92. doi: 10.1136/gutjnl-2013-306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrasek J, Bala S, Csak T, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–89. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan IS, Guy CD, Machado MV, et al. Alcohol activates the Hedgehog pathway and induces related procarcinogenic processes in the alcohol-preferring rat model of hepatocarcinogenesis. Alcohol Clin Exp Res. 2014;38:787–800. doi: 10.1111/acer.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes MA, McMullen MR, Roychowdhury S, et al. Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury, steatohepatitis, and steatosis. Hepatology. 2013;57:1980–91. doi: 10.1002/hep.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–66. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirnia F, Pawlak M, Thallinger GG, et al. Novel functional profiling approach combining reverse phase protein microarrays and human 3-D ex vivo tissue cultures: expression of apoptosis-related proteins in human colon cancer. Proteomics. 2009;9:3535–48. doi: 10.1002/pmic.200800159. [DOI] [PubMed] [Google Scholar]
- 16.Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 17.Peng C, Cho YY, Zhu F, et al. RSK2 mediates NF-κB activity through the phosphorylation of IκBα in the TNF-R1 pathway. FASEB J. 2010;24:3490–9. doi: 10.1096/fj.09-151290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho YY, Yao K, Kim HG, et al. Ribosomal S6 kinase 2 is a key regulator in tumor promoter–induced cell transformation. Cancer Res. 2007;67:8104–12. doi: 10.1158/0008-5472.CAN-06-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Moreau F, Hirota CL, et al. Proteinase-activated receptors induce interleukin-8 expression by intestinal epithelial cells through ERK/RSK90 activation and histone acetylation. FASEB J. 2010;24:1971–80. doi: 10.1096/fj.09-137646. [DOI] [PubMed] [Google Scholar]
- 20.Yang MF, Xie J, Gu XY, et al. Involvement of 90-kuD ribosomal S6 kinase in collagen type I expression in rat hepatic fibrosis. World J Gastroenterol. 2009;15:2109–15. doi: 10.3748/wjg.15.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck M, Chojkier M. A ribosomal S-6 kinase–mediated signal to C/EBP-b is critical for the development of liver fibrosis. PLoS ONE. 2007;2:e1372. doi: 10.1371/journal.pone.0001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bañares R, Alonso S, Catalina MV, et al. Randomized controlled trial of aspiration needle versus automated biopsy device for transjugular liver biopsy. J Vasc Interv Radiol. 2001;12:583–7. doi: 10.1016/s1051-0443(07)61479-1. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Moreno M, Chaves JF, Sancho-Bru P, et al. Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology. 2010;51:974–85. doi: 10.1002/hep.23421. [DOI] [PubMed] [Google Scholar]
- 25.Utepbergenov D, Derewenda U, Olekhnovich N, et al. Insights into the inhibition of the p90 ribosomal S6 kinase (RSK) by the flavonol glycoside SL0101 from the 1. 5 Å crystal structure of the N-terminal domain of RSK2 with bound inhibitor. Biochemistry. 2012;51:6499–510. doi: 10.1021/bi300620c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utepbergenov D, Derewenda ZS. The unusual mechanism of inhibition of the p90 ribosomal S6 kinase (RSK) by flavonol rhamnosides. Biochim Biophys Acta. 2013;1834:1285–91. doi: 10.1016/j.bbapap.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sancho-Bru P, Bataller R, Gasull X, et al. Genomic and functional characterization of stellate cells isolated from human cirrhotic livers. J Hepatol. 2005;43:272–82. doi: 10.1016/j.jhep.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Morán-Salvador E, Titos E, Rius B, et al. Cell-specific PPARγ deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. J Hepatol. 2013;59:1045–53. doi: 10.1016/j.jhep.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Bataller R, Paik YH, Lindquist JN, et al. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–40. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Le NT, Heo KS, Takei Y, et al. A crucial role for p90RSK-mediated reduction of ERK5 transcriptional activity in endothelial dysfunction and atherosclerosis. Circulation. 2013;127:486–99. doi: 10.1161/CIRCULATIONAHA.112.116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le NT, Takei Y, Shishido T, et al. p90RSK targets the ERK5-CHIP ubiquitin E3 ligase activity in diabetic hearts and promotes cardiac apoptosis and dysfunction. Circ Res. 2012;110:536–50. doi: 10.1161/CIRCRESAHA.111.254730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sancho-Bru P, Altamirano J, Rodrigo-Torres D, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–41. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 33.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–58. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 34.Trivier E, De Cesare D, Jacquot S, et al. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature. 1996;384:567–70. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- 35.Young ID. The Coffin-Lowry syndrome. J Med Genet. 1988;25:344–8. doi: 10.1136/jmg.25.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao J, Sun GB, Sun B, et al. Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology. 2012;292:53–62. doi: 10.1016/j.tox.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Chen HJ, Lin CM, Lee CY, et al. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep. 2013;30:925–32. doi: 10.3892/or.2013.2490. [DOI] [PubMed] [Google Scholar]
- 38.Park SE, Sapkota K, Kim S, et al. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br J Pharmacol. 2011;164:1008–25. doi: 10.1111/j.1476-5381.2011.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno M, Bataller R. Cytokines and renin-angiotensin system signaling in hepatic fibrosis. Clin Liver Dis. 2008;12:825–52. doi: 10.1016/j.cld.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Duncan JS, Whittle MC, Nakamura K, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–21. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.