Abstract
Purpose
We compared the effects of a eucaloric moderate-fat diet (18% protein, 36% fat, 46% carbohydrate), a eucaloric low-fat high-carbohydrate diet (18% protein, 18% fat, and 64% carbohydrate), and a low-calorie (33% reduced) low-fat high-carbohydrate diet on biomarkers of systemic inflammation.
Methods
We randomly assigned 102 participants (age 21–76 y, BMI 19.2–35.5 kg/m2) to the three different diets for six weeks in a parallel design intervention trial. All foods were provided. Ninety-three participants completed all study procedures; 92 were included in the analyses. Endpoints included plasma C-reactive protein (CRP), interleukin-6 (IL-6), soluble tumor necrosis factor receptors I & II (sTNFRI&II), and adiponectin.
Results
In the unadjusted primary analyses, none of the endpoints were differentially affected by the dietary interventions despite the significantly greater reductions in body weight and fat mass in participants consuming the low-calorie low-fat diet compared to the eucaloric diets (p<0.001). When including weight change in the model in secondary analysis, adiponectin tended to be increased with weight loss (time × weight change interaction, p=0.051). Adjusted for weight change, adiponectin was reduced in the groups consuming the low-fat diets relative to the moderate-fat diet (p=0.008). No effect of the intervention diets or weight loss on CRP, IL-6 or sTNFR I & II was seen in these secondary analyses.
Conclusions
In relatively healthy adults, moderate weight loss had minimal effects on systemic inflammation, and raised plasma adiponectin only modestly. A lower dietary fat and higher carbohydrate content had little impact on measures of systemic inflammation, but reduced adiponectin concentrations compared to a moderate-fat diet. The latter may be of concern given the consistent and strong inverse association of plasma adiponectin with many chronic diseases.
Keywords: Systemic inflammation, dietary fat, dietary carbohydrate, caloric restriction, adiponectin
Introduction
Chronic systemic and adipose tissue inflammation are considered major etiologic factors in many chronic diseases, including type 2 diabetes, cardiovascular disease, chronic kidney disease, and many types of cancer. Specifically, circulating markers of inflammation such as C-reactive protein (CRP), interleukin-6 (IL-6), and the soluble tumor necrosis factor receptors I & II (sTNFRI&II) are consistently associated with the risk of type 2 diabetes, cardiovascular disease, and cancer [1–6]. The adipocyte-derived hormone, adiponectin, has anti-inflammatory and insulin-sensitizing effects [7–9]. Plasma adiponectin concentration is inversely associated with adiposity [9]. This is due to the fact that obesity is associated with low-grade chronic adipose tissue inflammation, which in turn suppresses adipocyte adiponectin secretion [9]. Thus, reduced levels of plasma adiponectin may be considered a biomarker for adipose tissue inflammation. Plasma adiponectin concentration is inversely associated with the risk of type 2 diabetes, coronary heart disease, and hypertension [10–12], as well as a variety of cancers in observational studies [13]. In a mouse model of obesity, moderately increasing the circulating adiponectin concentration by overexpression completely prevented the development of diabetes as the mice became obese [14].
To this date, the major causes and contributing factors of chronic inflammation have remained largely elusive. Because an association between obesity and adipose tissue inflammation with systemic inflammation and hypoadiponectinemia is well established [15], it seems plausible to hypothesize that caloric excess and/or increased fat mass play major roles in the etiology of chronic inflammatory processes. Similarly, because chronic adipose tissue inflammation was first described in high-fat diet-fed mouse models of obesity [16–18], and because fatty acids trigger inflammatory activation in macrophages in cell culture [19], it is commonly assumed that dietary fat may be a major contributor to chronic adipose tissue and systemic inflammation. Consistent evidence shows that substantial weight loss following bariatric surgery or intensive lifestyle modifications reduces the plasma concentrations of biomarkers of inflammation and increases plasma adiponectin [20–25]. However, data on the effect of a more modest medium-term caloric deficit on measures of systemic inflammation and adiponectin concentrations are less consistent and therefore largely inconclusive. Similarly, the effect of macronutrient composition per se on measures of systemic inflammation and adiponectin concentrations in fasting plasma has remained largely unclear. This is because diets differing in their macronutrient composition commonly have differential impacts on body weight, making it difficult to assess whether any differential impact of such diets on biomarkers of chronic disease risk is due to differences in macronutrient composition or differences in weight loss. For example, Belza et al. [20] reported no impact of a low-calorie low-fat weight loss diet on adiponectin concentrations, while Kasim-Karakas and colleagues show a reduction in plasma adiponectin on a eucaloric low-fat diet, while consumption of that same diet ad libitum led to weight loss and an increase in adiponectin [21]. While this could be interpreted to suggest that weight loss increases and low fat intake decreases plasma adiponectin, the study by Kasim-Karakas et al. suffered from the fact that diets were administered sequentially without a control group. It has therefore remained unclear whether the effects of their dietary intervention may be due to hidden time effects. A recent review therefore concluded that data on the effect of macronutrient composition on plasma adiponectin concentrations are largely inconclusive [24]. The data for measures of low-grade systemic inflammation such as CRP are similarly inconclusive, again largely due to the difficulty in disentangling the relative effects of changes in macronutrient composition and energy balance on the concentrations of the plasma biomarkers.
We conducted a secondary analysis based on samples available from the Fatty Acids Marker Evaluation (FAME) Study, a study designed to evaluate the potential of using blood fatty acids as biomarkers of dietary fat intake. Relatively healthy normal weight to moderately obese men and women were randomly assigned to consume controlled diets that differed with regard to their fat content (18% vs. 36% of total energy intake, with reciprocal differences in carbohydrate content) and their energy content (eucaloric vs. 33% energy reduced) [26]. The objective of the work presented here was to assess the effects of these diets on biomarkers of systemic inflammation, which included the key insulin-sensitizing and anti-inflammatory adipokine, adiponectin.
Materials and Methods
Study participants
Participants were men (21–79 years old) and pre-menopausal women (21–44 years old). Exclusion criteria were any medical condition that required dietary modification (e.g. diabetes, renal disease, and hypercholesterolemia), regular consumption of the main meal outside the home more than twice per week, unsatisfactory completion of food records prior to randomization, unintended weight loss of more than 10% of usual body weight over the past six months, alcohol intake more than 3 standard drinks per day or more than 30 grams of alcohol per day, use of a nutritional supplement containing fatty acids, changes in thyroid medication over the past 6 months or recent diagnosis of thyroid dysfunction, possibility of becoming pregnant during the duration of the study, not having regular menstrual cycles, and body mass index (BMI) ≥ 35.
Study design
The FAME Study was a randomized, controlled, dietary intervention trial conducted at the Fred Hutchinson Cancer Research Center (FHCRC) and the University of Washington between September 1997 and April 2000. Participants were recruited from the Seattle area by targeted mailings, flyers and advertisements. Potential participants were screened and attended group and individual meetings at FHCRC. Those interested in and eligible for the study attended a baseline clinic visit (visit A) after an overnight fast of at least 12 hours. The baseline visit included a blood draw, assessment of body height and weight, and a dual-energy X-ray absorptiometry (DEXA) scan to assess body fat mass. Participants were then randomly assigned to consume one of 3 controlled diets for 6 weeks. Participants returned for clinic visits every two weeks after the commencement of the study diets. At the last clinic visit (visit D; the end of the six-week dietary period), procedures were performed as described above for the baseline visit. Study protocols were approved by the Institutional Review Boards of both institutions and all participants provided written, informed consent.
Study diets
Participants completed two 4-day food records before the baseline clinic visit to estimate their habitual energy intake. We used these data to estimate each participant’s energy needs during the feeding study. We designed 3 diets: a eucaloric moderate-fat diet, a eucaloric low-fat diet, and that same low-fat diet with a 33% caloric deficit (“low-calorie low-fat diet”). The eucaloric moderate-fat diet provided 36% of energy in the form of fat and 46% of energy in the form of carbohydrate. The two low-fat diets provided 18% of energy in the form of fat, and 64% of energy in the form of carbohydrate. Protein content was standardized across diets at 18% of total energy intake. At 2000 kcal per day, the low-fat diet contained 61g added sugars and 28g dietary fiber including 8g soluble fiber; at 2000 kcal per day, the moderate-fat diet contained 38g added sugars and 20g dietary fiber including 6g soluble fiber. We developed a 5-day cycle menu each for the moderate- and low-fat diets using the Diet Planner software, which used the USDA Standard Reference Database. A day’s dietary intake consisted of three meals and a snack. All study foods were prepared in advance at the Nutrition Research Kitchen at the University of Washington Clinical Research Center. Within each diet group, each day was matched for macronutrients (protein, carbohydrate, and fat), fatty acid composition, cholesterol, and dietary fiber. We supplied the diets at 13 different calorie levels, from 1200 kcal to 3600 kcal per day, in 200 kcal increments. In addition to weight measurements at the clinic visits, participants were also weighed in the kitchen when they picked up food twice a week. Energy intake was adjusted every 2 weeks to maintain baseline weight of participants in the 2 eucaloric arms. Any unconsumed food was returned to the Nutrition Research Kitchen where staff weighed and recorded the amount of remaining food. Actual intakes were calculated with Diet Planner software and summarized in Table 1.
Table 1.
Composition of the study dietsa
| Eucaloric moderate-fat diet (n=30) |
Eucaloric low-fat diet (n=31) |
Low-calorie low-fat diet (n=31) |
|
|---|---|---|---|
| Energy (kcal/d) | 2,623 ± 396 | 2,662 ± 365 | 1,881 ± 341 |
| Protein (% of total energy) | 17.5 ± 0.3 | 17.9 ± 0.2 | 17.7 ± 0.4 |
| Carbohydrate (% of total energy) | 46.5 ± 0.6 | 63.6 ± 0.6 | 63.9 ± 0.8 |
| Fat (% of total energy) | 36.0 ± 0.4 | 18.5 ± 0.6 | 18.4 ± 0.6 |
| Saturated fatty acids (% of total fat) | 33.1 ± 1.1 | 34.1 ± 0.3 | 34.0 ± 0.6 |
| Monounsaturated fatty acids (% of total fat) | 37.3 ± 0.8 | 37.1 ± 0.5 | 37.2 ± 0.7 |
| Polyunsaturated fatty acids (% of total fat) | 17.9 ± 0.6 | 18.4 ± 0.6 | 18.4 ± 0.8 |
| Fiber (g/d) | 25.4 ± 3.7 | 31.1 ± 4.6 | 22.7 ± 3.9 |
| Fiber (g/1,000 kcal) | 9.7 ± 0.3 | 11.7 ± 0.5 | 12.1 ± 0.6 |
| Cholesterol (mg/d) | 376 ± 58 | 264 ± 37 | 183 ± 35 |
| Cholesterol (mg/1,000 kcal) | 143 ± 4 | 99 ± 3 | 97 ± 5 |
Data are means ± SD
Laboratory methods
EDTA plasma samples, stored at −80°C since collection, from baseline (visit A) and the last clinic visit (visit D) were used for lab measurements in the current study. Concentrations of IL-6 (Human IL-6 Quantikine HS, R&D Systems), leptin (Human leptin, Millipore), and total adiponectin (Total Adiponectin, Alpco) were assayed by ELISA. Intra-assay coefficients of variation (CV) were 3.2%, 3.9% and 1.6%, and inter-assay CVs were 7.6%, 16.1%, and 2.72% for IL-6, leptin, and adiponectin, respectively. Plasma concentrations of sTNFRI and sTNFRII were measured using the MILLIPLEX MAP Human Soluble Cytokine Receptor Panel (Millipore). Intra-assay CVs were 2.8% and 3.1%, and inter-assay CVs were 6.2% and 2.0% for sTNFRI and sTNFRII, respectively. Plasma CRP concentrations were measured using the High-Sensitive CRP reagent (Kamiya Biochemical Company) on a Roche Cobas Mira Plus chemistry analyzer. The limit of quantification for this assay in our lab was 0.15 mg/L. Intra- and inter-assay CVs were 1.8% and 3.2%, respectively. All samples were run in duplicate and samples from the same individual were run in the same batch.
Statistical analyses
All statistical analyses were conducted with SPSS for Macintosh (version 20, IBM Corporation, Armonk, NY). Normal distribution of variables, or of residuals from repeated measures analysis of variance (RM-ANOVA) or multiple linear regression analyses, was confirmed by checking histograms and normal plots, and by conducting Shapiro-Wilk tests. Variables that were not consistent with a normal distribution (age, leptin, CRP, and IL-6) were log-transformed prior to statistical analyses. Baseline characteristics of the three diet groups were compared using one-way ANOVA. Whenever a measurement for an analyte fell below the limit of quantification for that assay, which was the case for plasma fasting leptin concentrations for several men, we used 50% of the lower limit of quantification as the best estimate of that value at that time point. In the primary analyses, changes in body weight, body fat mass, and plasma fasting concentrations of leptin, CRP, IL-6, total adiponectin, sTNFRI and sTNFRII were compared by RM-ANOVA, with the two time points (baseline and last clinic visits) as the two levels of the within-subjects factor (time) and diet group (eucaloric moderate-fat vs. eucaloric low-fat vs. low-calorie low-fat) as a between-subjects factor. In secondary analyses, we adjusted for the change in weight as well as the change in fat mass between baseline and last visits. For those outcome variables for which the RM-ANOVA indicated differential changes in the three diet groups (i.e., a “time × diet group” interaction) or changes dependent on weight change (i.e., a “time × weight change” interaction), we conducted post hoc independent samples t-tests and multiple linear regression analyses to assess the strength and direction of the observed interactions. For the independent samples t-test, we compared the changes between baseline and the last visit in the variable of interest between the three diets, with p-values adjusted for multiple testing using Bonferroni correction. In the regression analyses, the change in the outcome variable between baseline and the last visit was the dependent variable, and low-fat diet (yes/no) and weight change, were included as independent variables. These analyses allowed us to ascertain whether any time × diet group interaction could be explained by randomization to the low-fat as opposed to the moderate-fat diet, and by changes in body weight (which included changes in body weight in the low-calorie diet arm as well as random variations in body weight that occurred in the eucaloric diet arms). We also repeated all RM-ANOVA with additional adjustment for baseline fat mass (% of total) and gender to examine whether the response to the diets may have differed between men and women or may have depended on adiposity, and whether adiposity itself predicted changes in the endpoints over time. The level of significance was set to p<0.05 for all analyses.
Results
We enrolled 102 subjects into this randomized controlled feeding study. Of these, nine (three from each diet group) failed to complete the entire study for the following reasons: two left the Seattle area unexpectedly, three discontinued the study for personal reasons, and four dropped out because they did not like the study diet. Ninety-three participants, 45 women and 48 men, completed the entire dietary study and all clinic visits. One male participant had high baseline CRP concentration (>10 mg/L) indicative of an active infection or inflammatory process more severe than the low-grade inflammation of interest here, and was therefore excluded from the analysis. Of the remaining 92 participants, age ranged from 21 to 76 years, and their body mass index (BMI) from 19.2 to 35.5 kg/m2. At baseline, subjects randomized to the three diet arms did not differ with regard to age, gender distribution, weight or any of the biomarkers measured in this study (Table 2). Baseline fat mass tended to be different between the three diet groups in women (p=0.088), but not in men.
Table 2.
Baseline characteristics of participants who completed the study and were included in the analysesa
| All (n=92) | Eucaloric moderate-fat diet (n=30) |
Eucaloric low-fat diet (n=31) |
Low-calorie low-fat diet (n=31) |
One way ANOVA p-value |
|
|---|---|---|---|---|---|
| Age (years) | 36.4 (21.3 76.9) | 36.1 (21.4–70.8) | 36.2 (22.0–69.7) | 36.6 (21.3–76.9) | 0.911 |
| Women / Men | 45 / 47 | 15 / 15 | 15 / 16 | 15 / 16 | 0.990 |
| Weight (kg) | 77.4 ± 12.9 | 75.2 ± 12.7 | 77.4 ± 14.6 | 79.4 ± 11.2 | 0.462 |
| Body mass index (kg/m2) | 26.0 ± 3.4 | 25.3 ± 3.0 | 25.7 ± 3.8 | 26.9 ± 3.3 | 0.148 |
| Fat mass (%) | |||||
| - Women (n=45: 15 / 15 / 15) | 34.7 ± 7.1 | 34.5 ± 6.6 | 31.9 ± 7.1 | 37.6 ± 6.8 | 0.088 |
| - Men (n=47: 15 / 16 / 16) | 24.1 ± 8.0 | 21.6 ± 7.7 | 24.9 ± 8.0 | 25.3 ± 8.2 | 0.223 |
| Leptin (ng/mL) | |||||
| - Women (n=45: 15 / 15 / 15) | 11.4 (1.4–35.2) | 10.1 (1.5–25.7) | 9.9 (2.4–25.7) | 13.6 (1.4–35.2) | 0.531 |
| - Men (n=47: 15 / 16 / 16) | 2.5 (0.2–21.4) | 1.4 (0.4–9.5) | 2.7 (0.2–21.4) | 3.7 (0.2–10.2) | 0.243 |
| C-reactive protein (mg/L) | 0.69 (0.08–5.82) | 0.63 (0.08–4.46) | 0.64 (0.10–5.56) | 0.98 (0.18–5.82) | 0.257 |
| Intereukin-6 (pg/mL) | 1.05 (0.29–9.39) | 1.02 (0.29–9.39) | 0.78 (0.31–5.57) | 1.15 (0.45–4.65) | 0.143 |
| Total adiponectin (ng/mL) | 6,111 ± 2,394 | 5,913 ± 2,232 | 6,337 ± 2,675 | 6,077 ± 2,307 | 0.788 |
| Soluble TNF receptor I (pg/mL) | 1,110 ± 278 | 1,117 ± 266 | 1,129 ± 298 | 1,084 ± 277 | 0.812 |
| Soluble TNF receptor II (pg/mL) | 5,117 ± 1,172 | 5,153 ± 1,296 | 5,237 ± 1,337 | 4,962 ± 845 | 0.644 |
All data are means ± SD or median (range).
Abbreviations: ANOVA: analysis of variance, TNF tumor necrosis factor.
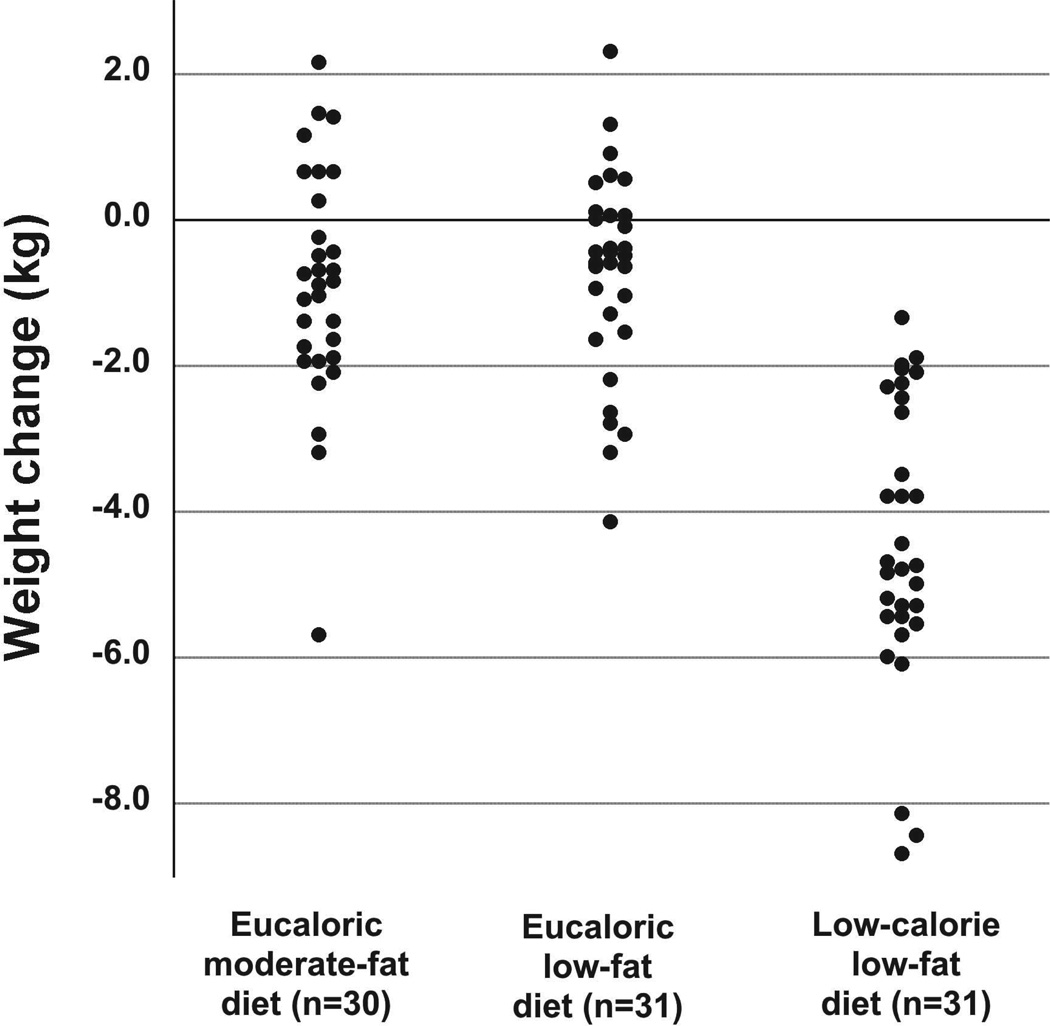
The study was designed to compare eucaloric moderate-fat and low-fat diets with each other, and with a low-calorie low-fat diet. RM-ANOVA showed differences in weight change overall (p<0.001, Table 3). Post hoc independent samples t-tests showed no statistically significant difference in weight change between the eucaloric moderate-fat and the eucaloric low-fat diet (p=0.671). As expected, the weight change was greater in the low-calorie low-fat diet (−4.4 ± 1.9 kg) than in the eucaloric low-fat diet (−0.7 ± 1.4 kg, p<0.001) or the eucaloric moderate-fat diet (−0.90 ± 1.60 kg, p<0.001). While the weight change in the two eucaloric groups was modest, it was subject to substantial inter-individual variation in all three groups (Figure 1). Changes in body fat mass paralleled those in body weight, with statistically significant differences between both eucaloric diets and the low-calorie low-fat diet (p<0.001 for both comparisons), but no statistically significant difference between the two eucaloric diets (p=0.157). Similar to body weight, we observed substantial inter-individual variation in the changes in fat mass in all three diet arms (data not shown). Interestingly, the diet group affiliation explained only 38% of the variation in weight change, and only 22% of the variation in fat mass change throughout the study. We therefore decided to conduct secondary analyses for all endpoints that included additional adjustment for the change in body weight or fat mass to determine whether a change in adiposity predicted changes in endpoints over time, and whether a differential effect of the moderate- vs. low-fat diet may become apparent after adjustment for changes in adiposity.
Table 3.
Effect of the study diets on fasting plasma leptin, adiponectin, and biomarkers of low-grade chronic systemic inflammationa
| RM-ANOVA p-values | ||||||
|---|---|---|---|---|---|---|
| n | Visit A (Baseline) |
Visit D (6 weeks) |
Time | Time × diet group |
||
| Body weight (kg) | Eucaloric moderate-fat diet | 30 | 75.2 ± 12.7 | 74.3 ± 12.6 | ||
| Eucaloric low-fat diet | 31 | 77.4 ± 14.6 | 76.7 ± 14.1 | <0.001 | <0.001 | |
| Low-calorie low-fat diet | 31 | 79.4 ± 11.2 | 74.9 ± 10.4 | |||
| Body fat mass (kg) | Eucaloric moderate-fat diet | 30 | 20.8 ± 8.8 | 19.4 ± 9.0 | ||
| Eucaloric low-fat diet | 31 | 22.2 ± 8.8 | 21.2 ± 8.9 | <0.001 | <0.001 | |
| Low-calorie low-fat diet | 31 | 24.8 ± 8.5 | 21.5 ± 8.4 | |||
| Leptin (ng/mL) | Eucaloric moderate-fat diet | 30 | 4.7 (0.4–25.7) | 4.2 (0.2–29.1) | ||
| Eucaloric low-fat diet | 31 | 4.7 (0.2–25.7) | 4.6 (0.2–23.7) | <0.001 | <0.001 | |
| Low-calorie low-fat diet | 31 | 7.3 (0.2–35.2) | 3.7 (0.2–23.4) | |||
| C-reactive protein (mg/L) | Eucaloric moderate-fat diet | 30 | 0.63 (0.08–4.46) | 0.66 (0.09–4.38) | ||
| Eucaloric low-fat diet | 31 | 0.64 (0.10–5.56) | 0.55 (0.12–5.89) | 0.025 | 0.175 | |
| Low-calorie low-fat diet | 31 | 0.98 (0.18–5.82) | 0.68 (0.08–7.14) | |||
| Interleukin-6 (pg/mL) | Eucaloric moderate-fat diet | 30 | 1.02 (0.29–9.39) | 1.02 (0.20–10.79) | ||
| Eucaloric low-fat diet | 31 | 0.78 (0.31–5.57) | 0.77 (0.39–6.32) | 0.354 | 0.861 | |
| Low-calorie low-fat diet | 31 | 1.15 (0.45–4.65) | 1.01 (0.30–5.82) | |||
| Total adiponectin (ng/mL) | Eucaloric moderate-fat diet | 30 | 5,913 ± 2,232 | 5,557 ± 2,329 | ||
| Eucaloric low-fat diet | 31 | 6,337 ± 2,675 | 5,448 ± 2,388 | <0.001 | 0.085 | |
| Low-calorie low-fat diet | 31 | 6,077 ± 2,307 | 5,336 ± 1,764 | |||
| Soluble TNF receptor I (pg/mL) | Eucaloric moderate-fat diet | 30 | 1,117 ± 266 | 1,130 ± 267 | ||
| Eucaloric low-fat diet | 31 | 1,129 ± 298 | 1,102 ± 299 | 0.588 | 0.597 | |
| Low-calorie low-fat diet | 31 | 1,084 ± 277 | 1,073 ± 264 | |||
| Soluble TNF receptor II (pg/mL) | Eucaloric moderate-fat diet | 30 | 5,153 ± 1,296 | 5,112 ± 1,202 | ||
| Eucaloric low-fat diet | 31 | 5,237 ± 1,337 | 5,163 ± 1,354 | 0.747 | 0.666 | |
| Low-calorie low-fat diet | 31 | 4,962 ± 845 | 5,017 ± 934 | |||
Data are means ± SD or median (range).
Abbreviations: RM-ANOVA: repeated measures analysis of variance, TNF: tumor necrosis factor.
Figure 1.
Change in body weight in the three diet arms over the 6-week dietary period
As expected in a study population losing weight and fat mass (Table 3), plasma fasting leptin concentrations decreased substantially over time, with statistically significant differences between the diet groups (time × diet group interaction, p<0.001, Table 3). As with body weight and fat mass, the change in plasma leptin was not statistically different between the two eucaloric diet groups in post hoc t-tests (p=0.431), while plasma leptin was reduced to a statistically significantly greater degree in the low-calorie low-fat group as compared to the eucaloric diet groups (p<0.001). When adjusting the overall RM-ANOVA for the change in either body weight or fat mass, the time × diet group interaction became non-significant (p=0.675 (Online Resource 1) and p=0.113 (not shown), respectively), while the interaction between time and the change in body weight (p<0.001) or the change in fat mass (p<0.001) was highly significant.
While CRP was reduced overall (p=0.025 in overall RM-ANOVA time effect, Table 3), the changes between the three diet groups were not significantly different (p=0.175 for the time × diet group interaction). In secondary analyses, we observed a trend towards a time × weight change interaction (p=0.060) for the changes in fasting plasma CRP concentrations (Online Resource 1). However, there was no trend towards a time × fat mass change interaction (p=0.109), and no trend was apparent for a consistent reduction in fasting CRP in the low-calorie group (Online Resource 2a), or with weight change (Online Resource 2b) or change in fat mass (Online Resource 2c). Further, multiple linear regression analyses did not reveal any statistically significant associations between the change in plasma CRP (computed as the difference between the log-transformed CRP data at baseline and follow-up) and the change in body weight (β=0.023, 95% CI: −0.009, 0.055, p=0.165), in a model adjusted for type of diet (low-fat diet yes/no). Consistent with the primary analysis (RM-ANOVA), type of diet (low-fat diet yes/no) was also not associated with the change in plasma CRP concentrations (β=−0.109, 95% CI: −0.272, 0.054, p=0.187) in this regression model, after adjustment for weight change. The other markers of chronic systemic inflammation, IL-6, sTNFRI and sTNFRII, did not change during this intervention study; and the type of diet consumed had no statistically significant effect on any of these endpoints (Table 3). In secondary analyses, we also did not observe an effect of change in weight or fat mass on any of these endpoints (Online Resource 1).
For fasting total adiponectin concentrations, RM-ANOVA showed a trend for a time × diet group interaction (p=0.085, Table 3). Adjustment for weight change in secondary analyses strengthened the time × diet group interaction (p=0.021, Online Resource 1), and indicated a trend towards a time × weight change interaction (p=0.051, Online Resource 1). Because the time × diet group interaction became significant only after adjusting for weight change, we conducted post hoc multiple linear regression analyses that included as covariates weight change and whether the diet was low-fat (yes/no). These analyses revealed that randomization to the low-fat diet compared to the moderate-fat diet led to a reduction in fasting total adiponectin concentrations by 597 ng/mL (9.4%, p=0.008), while weight loss of 1 kg led to an increase in fasting total adiponectin concentrations by 82 ng/mL (p=0.065, Table 4).
Table 4.
Multiple linear regression on the change in fasting plasma total adiponectin concentrations (ng/mL) between visits A and D
| Unstandardized coefficient β |
Standard error se(β) |
t | p | |
|---|---|---|---|---|
| Constant | −430 | 175 | ||
| Low-fat diet (0=no, 1=yes) | −597 | 221 | −2.705 | 0.008 |
| Body weight change (kg) | −82 | 44 | −1.872 | 0.065 |
Inclusion of baseline fat mass (in % of total body mass) and gender into the RM-ANOVA in addition to the change in body weight did not alter any of the findings.
Discussion
In this randomized controlled intervention trial, we found no differential impact of eucaloric moderate-fat, eucaloric low-fat, and low-calorie low-fat diets on measures of systemic inflammation. However, we found the anticipated modest impact of weight loss on plasma adiponectin concentrations. Further, plasma adiponectin concentrations were reduced by a low-fat compared to a moderate-fat diet when adjusting for weight change. The magnitude of that effect of ~10% of baseline adiponectin concentrations was sizeable and almost certainly clinically meaningful. On the other hand, it needs to be considered that the low-fat diet tested here was very low in fat, at 18% of total energy intake. Free-living individuals rarely consume such a low percentage of their energy as fat. It could also be argued that calorie intake was controlled, not ad libitum, and that consumption of a very low-fat diet ad libitum may lower energy intake, which in turn could alleviate any reductions in plasma adiponectin triggered by a lower dietary fat intake. However, it is important to note that adiponectin concentrations were reduced substantially even in the low-fat diet arm in which energy intake was reduced by 33% over 6 weeks. It seems unlikely that any diet would induce such a substantial caloric deficit over 6 weeks when consumed to satiety (ad libitum).
For CRP, although the RM-ANOVA in secondary analyses which included adjustment for weight change suggested a trend for reduced CRP concentrations with weight loss (p=0.060), no trend was apparent in linear regression analyses or in studying the changes in CRP in the three diet groups, or with weight loss (Online Resource 2a–c). Thus, it seems justified to conclude that the modest weight loss in this 6-week dietary intervention did not appreciably affect fasting plasma CRP concentrations, which is consistent with the literature. Belza et al. observed that a medium-term (8 week) very low calorie diet (~800 kcal/d) based on a low-fat formula drink led to substantial weight loss of ~13%, but had no significant impact on fasting plasma CRP concentrations [20]. In that study, significant reductions in CRP were seen only after a 4-week weight maintenance period and a second very low calorie diet-induced weight loss period of 4 weeks. These data suggest that changes in plasma CRP with weight loss either take more time than the initial 8 weeks, require the establishment of a new steady state body weight as the authors hypothesize, or that the weight loss needs to be more substantial than the 13% observed in the initial weight loss period. Other studies in this area suggest that the duration of the intervention period may be an important factor. For example, participants in two different studies reduced their plasma CRP concentrations by 22% and 25%, respectively, on weight loss that averaged only 6–8% on ad libitum diets over 6 and 8 months, respectively [21, 22]. Taken together, these data suggest that longer-term dietary interventions that lead to weight loss of at least 6–8% significantly reduce plasma CRP concentrations. Our relative lack of effect of the low-calorie diet or weight loss in general may have been due to the relatively short-term nature of our study (6 weeks) or due to the, on average, lower weight loss of only ~3% compared to the studies discussed above. Similarly, our lack of effect of calorie reduction or weight change on plasma IL-6 may have been due to the relatively short duration of our study, or the modest weight loss achieved. As for CRP, changes in IL-6 appear to require more substantial weight loss and/or longer-term caloric deficits [20]. The modest effect of weight change on plasma total adiponectin observed in this study is also consistent with the literature. A recent review found that consistently, significant increases in plasma adiponectin require substantial weight loss of ~7% [24]. While we observed a trend for a time × weight change interaction, confirming that body weight is an important factor in determining plasma adiponectin concentrations, the effect size was very modest, again likely related to the more modest average weight loss in this study compared to previous longer-term studies.
The effect of macronutrient composition, particularly higher-fat vs. low-fat diets, on measures of systemic inflammation and plasma adiponectin are much less consistent and conclusive. In the study by Nicklas et al. discussed above, reductions in body weight and plasma CRP concentrations were similar on four different weight loss-diets differing widely in their macronutrient composition [22]. This is in contrast to a 12-week study by Ruth and colleagues in which modest weight loss averaging ~6% over 12 weeks on either a low-fat or a high-fat diet led to reductions in plasma CRP only in the high-fat diet group [27]. Of note, the high-fat diet in that study also contained much more protein than the low-fat diet, and the differences observed in plasma CRP may have been due to the differences in the intake of protein or other associated, non-standardized dietary factors, rather than the differences in fat and carbohydrate content. In another study, a low-fat diet reduced plasma CRP concentrations after adjustment for changes in fat mass compared to a control group not instructed to lower fat intake [28]. However, effects were seen only in a subgroup — women suffering from metabolic syndrome — while no effects were seen in men or women without metabolic syndrome. Our study clearly shows that in the context of eucaloric intake, the dietary fat to carbohydrate ratio covering most common intakes for both macronutrient levels has little impact on plasma CRP concentrations per se. With regard to the impact of the dietary macronutrient composition on plasma adiponectin concentrations, the existing data have also been inconsistent. For example, AlSaleh and colleagues reported that 24 weeks of dietary treatment with two different high-fat diets (differing in fat composition) and one low-fat diet had no differential effect on plasma adiponectin concentrations [29]. In contrast, in settings of both weight loss and weight stability, adiponectin concentrations were higher after the consumption of a high-fat diet compared to a low-fat diet [27, 30]. These latter findings are consistent with our data showing a clear weight-change adjusted reduction in plasma adiponectin concentrations by ~10% in participants consuming the low-fat diet. Similarly, our data are largely consistent with those published by Kasim-Karakas and colleagues [21]. In their study, plasma adiponectin decreased by 14% when healthy postmenopausal women were switched from a eucaloric 35% fat diet to a eucaloric 15% fat diet, but bounced back to baseline when participants consumed the 15% fat diet ad libitum, which led to reduction in energy intake and body weight. A potential limitation of that study was the fact that the three diets were administered sequentially, without a concurrent control group; thus, any observed change may have been due to hidden time effects.
Low levels of circulating adiponectin are associated with a variety of diseases, such as type 2 diabetes and different types of cancer [12, 31]. Increased levels of adiponectin, on the other hand, provide metabolic benefits. For example, mice constitutively overexpressing adiponectin are protected against the negative metabolic effects of high-fat diets [32] and modestly increased circulating adiponectin levels completely prevented the development of diabetes in spite of massive obesity in mice [14]. Consistent with the mouse models, obese yet metabolically healthy individuals who maintain insulin sensitivity and glucose tolerance have favorable inflammation profiles including higher adiponectin and lower CRP concentrations [33, 34]. Therefore, it may be of concern that a diet low in fat but high in carbohydrate as tested in our study resulted in reduced circulating level of adiponectin.
The main strengths of our study were that it was a randomized controlled feeding trial in which all food was provided to participants and, by controlled feeding trial standards, its relatively large sample size. Ninety one percent of the 102 recruited participants completed the study diets, with 31 persons in each study arm. The study design was well suited to specifically compare moderate-fat vs. low-fat diets, as well as the impact of a moderate caloric restriction imposed on top of the low-fat diet. Although there were differences in carbohydrate quality between diets (i.e. the amount of fiber and added sugars were higher in the low-fat diet than those in the moderate-fat diet of the same calorie), these differences are viewed as a strength rather than a limitation because it mimics the carbohydrate type choices that free-living individuals are likely to make when consuming moderate vs. low fat diets in the real world. This study also had several limitations. Participants were on the study diets for 6 weeks only, which may not have been long enough to observe changes in certain markers, as discussed above. However, numerous previous studies have shown significant effects of dietary interventions lasting 2–6 weeks on the biomarkers measured here [35–44]. It is also possible that the observed decrease in plasma adiponectin on the low-fat diets may be transient, which would become apparent only in a longer-term study. The variation of biomarker concentrations at baseline among subjects was quite large; a cross-over design study may have been more sensitive in detecting changes within each individual after consuming the different diets. For the same reason, we may have had a better chance of detecting significant changes in the biomarkers had the subject population been more homogenous at baseline with regard to systemic inflammation or variables such as age or BMI. Though some of our subjects were moderately obese, they were relatively healthy individuals and concentrations of CRP and IL-6 were already low at baseline, potentially limiting further reduction. It is also a possible limitation that we measured total plasma adiponectin only, and not also the potentially more biologically relevant high molecular weight adiponectin concentration; however, the two are highly correlated [45, 46]. In addition, it is important to consider that because our subject population was relatively healthy (i.e., we excluded subjects with certain obesity-associated health conditions such as diabetes or renal disease), the results may not be generalizable to other populations, including those with more profound chronic systemic inflammation or metabolic syndrome.
In conclusion, we found that in relatively healthy men and women, and after adjusting for weight change experimentally and statistically, diets varying widely in fat and carbohydrate content did not differ in their impact on biomarkers of chronic systemic inflammation including CRP, IL-6, and sTNF RI&II. It is also worth emphasizing that the modest weight loss in the low-calorie group of about 6%, similar to what is commonly considered a realistic clinical goal, did not affect these measures of systemic inflammation. In contrast, plasma adiponectin concentrations were lowered substantially and to a clinically significant degree by a low-fat high-carbohydrate diet compared to a moderate-fat diet. As adiponectin is a key insulin-sensitizing and anti-inflammatory adipokine, its plasma concentrations may reflect the pro- to anti-inflammatory balance in adipose tissue. Our findings may motivate more research into the long-term consequences of the dietary macronutrient composition on inflammatory processes in adipose tissue and downstream factors including insulin sensitivity.
Supplementary Material
Acknowledgements
Research related to this paper was funded by U.S. National Institutes of Health / National Cancer Institute grant CA61712 and by Fred Hutchinson Cancer Research Center.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Aleksandrova K, Jenab M, Boeing H, Jansen E, Bueno-de-Mesquita HB, Rinaldi S, Riboli E, Overvad K, Dahm CC, Olsen A, Tjonneland A, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Palli D, Krogh V, Tumino R, Vineis P, Panico S, Kaaks R, Rohrmann S, Trichopoulou A, Lagiou P, Trichopoulos D, van Duijnhoven FJ, Leufkens AM, Peeters PH, Rodriguez L, Bonet C, Sanchez MJ, Dorronsoro M, Navarro C, Barricarte A, Palmqvist R, Hallmans G, Khaw KT, Wareham N, Allen NE, Spencer E, Romaguera D, Norat T, Pischon T. Circulating C-reactive protein concentrations and risks of colon and rectal cancer: a nested case-control study within the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172:407–418. doi: 10.1093/aje/kwq135. [DOI] [PubMed] [Google Scholar]
- 2.Heikkila K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44:937–945. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15:381–384. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. 2009;55:209–215. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- 5.Dossus L, Becker S, Rinaldi S, Lukanova A, Tjonneland A, Olsen A, Overvad K, Chabbert-Buffet N, Boutron-Ruault MC, Clavel-Chapelon F, Teucher B, Chang-Claude J, Pischon T, Boeing H, Trichopoulou A, Benetou V, Valanou E, Palli D, Sieri S, Tumino R, Sacerdote C, Galasso R, Redondo ML, Bonet CB, Molina-Montes E, Altzibar JM, Chirlaque MD, Ardanaz E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Onland-Moret NC, Lundin E, Idahl A, Khaw KT, Wareham N, Allen N, Romieu I, Fedirko V, Hainaut P, Romaguera D, Norat T, Riboli E, Kaaks R. Tumor necrosis factor (TNF)-alpha, soluble TNF receptors and endometrial cancer risk: the EPIC study. Int J Cancer. 2011;129:2032–2037. doi: 10.1002/ijc.25840. [DOI] [PubMed] [Google Scholar]
- 6.Shai I, Schulze MB, Manson JE, Rexrode KM, Stampfer MJ, Mantzoros C, Hu FB. A prospective study of soluble tumor necrosis factor-alpha receptor II (sTNF-RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes Care. 2005;28:1376–1382. doi: 10.2337/diacare.28.6.1376. [DOI] [PubMed] [Google Scholar]
- 7.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008;35:321–326. doi: 10.1016/S1673-8527(08)60047-8. [DOI] [PubMed] [Google Scholar]
- 9.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94:2137–2142. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Mather KJ, Funahashi T, Matsuzawa Y, Edelstein S, Bray GA, Kahn SE, Crandall J, Marcovina S, Goldstein B, Goldberg R, Diabetes Prevention P. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes. 2008;57:980–986. doi: 10.2337/db07-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 16.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 20.Belza A, Toubro S, Stender S, Astrup A. Effect of diet-induced energy deficit and body fat reduction on high-sensitive CRP and other inflammatory markers in obese subjects. Int J Obes (Lond) 2009;33:456–464. doi: 10.1038/ijo.2009.27. [DOI] [PubMed] [Google Scholar]
- 21.Kasim-Karakas SE, Tsodikov A, Singh U, Jialal I. Responses of inflammatory markers to a low-fat, high-carbohydrate diet: effects of energy intake. Am J Clin Nutr. 2006;83:774–779. doi: 10.1093/ajcn/83.4.774. [DOI] [PubMed] [Google Scholar]
- 22.Nicklas JM, Sacks FM, Smith SR, LeBoff MS, Rood JC, Bray GA, Ridker PM. Effect of dietary composition of weight loss diets on high-sensitivity c-reactive protein: the Randomized POUNDS LOST trial. Obesity (Silver Spring) 2013;21:681–689. doi: 10.1002/oby.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva FM, de Almeida JC, Feoli AM. Effect of diet on adiponectin levels in blood. Nutr Rev. 2011;69:599–612. doi: 10.1111/j.1753-4887.2011.00414.x. [DOI] [PubMed] [Google Scholar]
- 25.Herder C, Peltonen M, Svensson PA, Carstensen M, Jacobson P, Roden M, Sjostrom L, Carlsson L. Adiponectin and Bariatric Surgery: Associations With Diabetes and Cardiovascular Disease in the Swedish Obese Subjects Study. Diabetes Care. 2014 doi: 10.2337/dc13-1362. [DOI] [PubMed] [Google Scholar]
- 26.King IB, Lemaitre RN, Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83:227–236. doi: 10.1093/ajcn/83.2.227. [DOI] [PubMed] [Google Scholar]
- 27.Ruth MR, Port AM, Shah M, Bourland AC, Istfan NW, Nelson KP, Gokce N, Apovian CM. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism. 2013;62:1779–1787. doi: 10.1016/j.metabol.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camhi SM, Stefanick ML, Ridker PM, Young DR. Changes in C-reactive protein from low-fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism. 2010;59:54–61. doi: 10.1016/j.metabol.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AlSaleh A, O'Dell SD, Frost GS, Griffin BA, Lovegrove JA, Jebb SA, Sanders TA, Group RS. Single nucleotide polymorphisms at the ADIPOQ gene locus interact with age and dietary intake of fat to determine serum adiponectin in subjects at risk of the metabolic syndrome. The American journal of clinical nutrition. 2011;94:262–269. doi: 10.3945/ajcn.111.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung EH, Appel LJ, Miller ER, 3rd, Kao WH. The effects of macronutrient intake on total and high-molecular weight adiponectin: results from the OMNI-Heart trial. Obesity (Silver Spring) 2010;18:1632–1637. doi: 10.1038/oby.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol. 2010;176:1364–1376. doi: 10.2353/ajpath.2010.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 34.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372:1281–1283. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- 35.Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, Noar K, Song X, Lampe JW. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369–374. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King DE, Egan BM, Woolson RF, Mainous AG, 3rd, Al-Solaiman Y, Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–506. doi: 10.1001/archinte.167.5.502. [DOI] [PubMed] [Google Scholar]
- 37.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peppa M, Uribarri J, Cai W, Lu M, Vlassara H. Glycoxidation and inflammation in renal failure patients. Am J Kidney Dis. 2004;43:690–695. doi: 10.1053/j.ajkd.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 40.Sofi F, Whittaker A, Gori AM, Cesari F, Surrenti E, Abbate R, Gensini GF, Benedettelli S, Casini A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: a double-blinded randomised dietary intervention trial. Br J Nutr. 2014:1–8. doi: 10.1017/S000711451400018X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kralova Lesna I, Suchanek P, Brabcova E, Kovar J, Malinska H, Poledne R. Effect of different types of dietary fatty acids on subclinical inflammation in humans. Physiol Res. 2013;62:145–152. doi: 10.33549/physiolres.932439. [DOI] [PubMed] [Google Scholar]
- 42.Tovar J, Nilsson A, Johansson M, Ekesbo R, Aberg AM, Johansson U, Bjorck I. A diet based on multiple functional concepts improves cardiometabolic risk parameters in healthy subjects. Nutr Metab (Lond) 2012;9:29. doi: 10.1186/1743-7075-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franzini L, Ardigo D, Valtuena S, Pellegrini N, Del Rio D, Bianchi MA, Scazzina F, Piatti PM, Brighenti F, Zavaroni I. Food selection based on high total antioxidant capacity improves endothelial function in a low cardiovascular risk population. Nutr Metab Cardiovasc Dis. 2012;22:50–57. doi: 10.1016/j.numecd.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Navarro SL, Schwarz Y, Song X, Wang CY, Chen C, Trudo SP, Kristal AR, Kratz M, Eaton DL, Lampe JW. Cruciferous vegetables have variable effects on biomarkers of systemic inflammation in a randomized controlled trial in healthy young adults. J Nutr. 2014;144:1850–1857. doi: 10.3945/jn.114.197434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, Inukai T, Nakano Y. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–1960. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- 46.Almeda-Valdes P, Cuevas-Ramos D, Mehta R, Gomez-Perez FJ, Cruz-Bautista I, Arellano-Campos O, Navarrete-Lopez M, Aguilar-Salinas CA. Total and high molecular weight adiponectin have similar utility for the identification of insulin resistance. Cardiovasc Diabetol. 2010;9:26. doi: 10.1186/1475-2840-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.