Abstract
Background
Psychiatric disorders such as schizophrenia are worsened by stress, and working memory deficits are often a central feature of illness. Working memory is mediated by the persistent firing of prefrontal cortical (PFC) pyramidal neurons. Stress impairs working memory via high levels of dopamine D1 receptor (D1R) activation of cAMP signaling, which reduces PFC neuronal firing. The current study examined whether D1R-cAMP signaling reduces neuronal firing and impairs working memory by increasing the open state of hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels, which are concentrated on dendritic spines where PFC pyramidal neurons interconnect.
Methods
A variety of methods were employed to test this hypothesis: dual immunoelectron microscopy localized D1R and HCN channels, in vitro recordings tested for D1R actions on HCN channel current (Ih), while recordings in monkeys performing a working memory task tested for D1R-HCN channel interactions in vivo. Finally, cognitive assessments following intra-PFC infusions of drugs examined D1R-HCN channel interactions on working memory performance.
Results
Immunoelectron microscopy confirmed D1R colocalization with HCN channels near excitatory-like synapses on dendritic spines in primate PFC. Mouse PFC slice recordings demonstrated that D1R stimulation increased Ih, while local HCN channel blockade in primate PFC protected task-related firing from D1R-mediated suppression. D1R stimulation in rat or monkey PFC impaired working memory performance, while HCN channel blockade in PFC prevented this impairment in rats exposed to either stress or D1R stimulation.
Conclusions
These findings suggest that D1R stimulation or stress weakens PFC function via opening of HCN channels at network synapses.
Keywords: Prefrontal cortex, working memory, stress, D1 dopamine receptor, cAMP, HCN channel
Introduction
Dysfunction of the prefrontal cortex (PFC) is central to cognitive deficits observed in many psychiatric disorders (1). The PFC uses “representational knowledge” to provide top-down guidance of behavior, thought and affect (2). The working memory functions of PFC are rapidly and markedly impaired by exposure to even mild, uncontrollable stress (3). Dopamine (DA) D1 receptor (D1R) signaling via cyclic adenosine monophosphate (cAMP) plays a critical role in PFC function, producing an inverted-U-shaped dose-response curve whereby moderate levels improve PFC function, while higher levels impair function, e.g. taking PFC “off-line” during stress (4, 5). While the PFC expands and differentiates greatly from rodents to primates, there are some effects that bridge across species, e.g. the D1R inverted-U dose-response curve with impairment at high levels of DA stimulation has been observed in mice (6), rats (4), monkeys (7) and humans (8, 9). The detrimental effects of high levels of D1R stimulation during stress are particularly important to understand, as D1Rs are altered in disorders such as schizophrenia (10, 11), and the symptoms of such disorders are often precipitated or exacerbated by stress (3, 12).
Much of the research on the molecular mechanisms of PFC function has focused on spatial working memory in animal models. In primates, spatial working memory is mediated by recurrent excitation among networks of pyramidal neurons in layer III and likely layer V that sustain firing over a delay period in dorsolateral PFC (DLPFC), considered to be the electrophysiological correlate of representational knowledge (2, 13, 14). Maintenance of persistent firing depends upon effective contacts between pyramidal neurons via NMDA receptor synapses at dendritic spines (2, 15). These networks can be spatially tuned by lateral inhibition from gamma-aminobutyric acidergic (GABAergic) interneurons (2, 16, 17), and are dynamically altered by neuromodulators via intracellular signaling at the spine, referred to as Dynamic Network Connectivity (18). Moderate levels of D1R stimulation enhance spatial tuning by scultping away “noise,” reducing neuronal firing for non-preferred directions, while higher levels of D1R stimulation erode all task-related firing (5).
Exposure to stress produces high levels of DA release in the rodent PFC, and D1R-mediated generation of cAMP reduces PFC network firing and impairs working memory (4, 5, 19). Similarly, direct application of D1R agonists onto DLPFC neurons reduces delay-related firing via increased cAMP in monkeys performing a working memory task (5), and high doses of D1R agonists impair spatial working memory in mice (6), rats (4) and monkeys (7). However, it is not understood how D1R stimulation reduces PFC neuronal firing, as D1Rs can have multiple actions in PFC, e.g. in layer V neurons of ferrets and rodents, D1R stimulation can reduce glutamate release from axon terminals (20) or alter opening of calcium channels (21). D1Rs may also reduce firing by increasing the open probability of hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels via cAMP. Previous research has shown that cAMP reduces PFC neuronal firing by increasing the open state of HCN channels on dendritic spines (22). In cerebral cortex, HCN1 and HCN2 subunits form heterotetramers that are particularly responsive to cAMP (23–25), and associate with cAMP-regulating proteins in spines of monkey layer III DLPFC (22, 26). In monkeys performing a working memory task, low doses of an HCN channel blocker, such as ZD7288, enhance task-related DLPFC neuronal firing, whereas treatments that increase the HCN channel current (Ih) reduce firing (22).
The current study investigated whether high levels of D1R stimulation, as occur during stress exposure, reduce PFC neuronal firing and impair working memory through cAMP interactions with HCN channels. Understanding the molecular basis of cognitive function requires molecular, cellular and behavioral approaches. Thus, we employed a cross-species approach, integrating monkey anatomy and in vivo physiology, mouse in vitro physiology, and rat and monkey behavior.
Methods and Materials
All procedures were approved by the Yale Institutional Animal Care and Use Committee.
Immunoelectron microscopy
Brains of two adult, male rhesus macaques (Macaca mulatta) were fixed via transcardial perfusion and blocked coronally. Sections of DLPFC were used for dual immunolabeling using peroxidase and/or gold immunoprobes for D1Rs and the HCN1 subunit of HCN channels. Tissue processing and all immuno-procedures, including antibody characterization, are described in detail in Paspalas et al. (26).
Monkey in vivo recordings
Subjects
Two adult, male rhesus macaques, C (13 years old) and P (7 years old), were housed individually under standard laboratory conditions. A highly palatable juice reward was used to minimize need for dietary regulation. Water was provided ad libitum, and animals were fed monkey chow (Purina Mills, Gray Summit, MO) and fruit immediately following testing. Care was taken to habituate them to all procedures.
Spatial working memory task, physiological recording and iontophoresis in monkey DLPFC
Monkeys were trained on the oculomotor delayed response (ODR) task, a test of spatial working memory as outlined in Figure 1A. Single-unit recordings were performed in the principal sulcus region of DLPFC (Fig. 1B), the brain region typically associated with performance of the ODR task (13). Many regularly spiking neurons (presumed pyramidal neurons) in DLPFC exhibit persistent firing that is maintained throughout the delay period (Delay cells). Spatial tuning is observed in a large subset of Delay cells, whereby they increase firing during the delay period following a cue in their preferred direction, but show smaller increases or even inhibition of firing for non-preferred directions (2) (Fig. 1C). The microcircuitry underlying spatially tuned delay-related firing is shown in Figure 1D.
Figure 1.
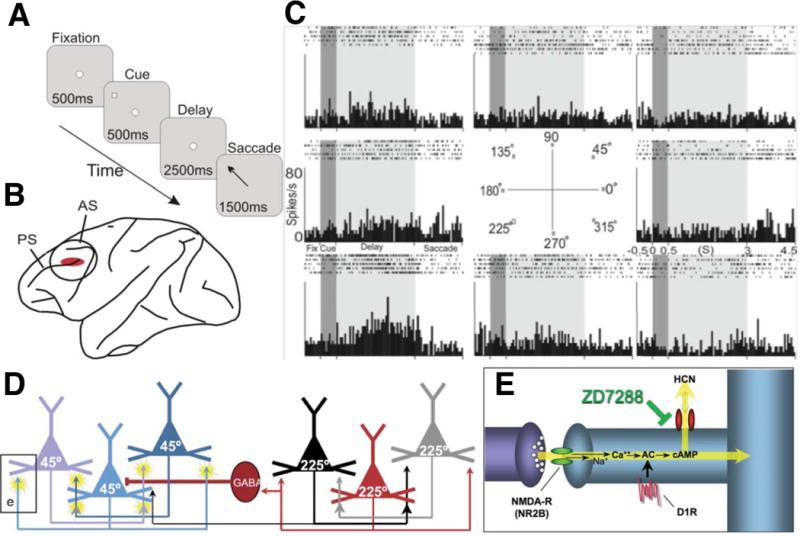
Single-unit recording from monkey dorsolateral prefrontal cortex (DLPFC) was combined with drug iontophoresis in monkeys performing the oculomotor delayed response (ODR) task.
A. The ODR task, a test of spatial working memory. The monkey was seated in front of a screen, and the trial began when he fixated on a central target on the screen for 0.5s (fixation period). Next, a cue appeared briefly (0.5s) in one of eight peripheral locations on the screen (cue period), followed by a 2.5s delay period, during which the monkey continued to maintain fixation. At the end of the delay period, the fixation target was extinguished, and the monkey was required to make a memory-guided saccade to the remembered location of the cue (response period); monkeys were rewarded with a drop of juice for each correct response. Each test session consisted of hundreds of trials across which the cued location randomly changed, thus requiring the monkey to update his working memory. The TEMPO Experiment Control System (Reflective Computing, St. Louis, MO) generated the task, while the ISCAN Eye Movement Monitoring System (ISCAN Inc., Woburn, MA) monitored eye position.
B. Single-unit recording was performed in DLPFC. AS: arcuate sulcus; PS: principal sulcus.
C. Firing patterns of a sample neuron from the current study. Under optimal conditions, neurons show delay-related firing for a preferred direction (e.g. 225°), but suppress firing for non-preferred directions (2). The dark gray background indicates the cue period, and the light gray background indicates the delay period.
D. Circuit basis for spatial working memory (2). Spatial working memory is maintained in DLPFC by recurrent excitation among networks of NMDA-receptor (NMDA-R) glutamatergic pyramidal neurons with shared stimulus inputs (e.g. 225°). Spatial tuning is enhanced by lateral inhibition of non-preferred inputs (e.g. 45°) from gamma-aminobutyric acidergic (GABAergic) interneurons (16).
E. Working model of molecular mechanisms that weaken PFC network connectivity. Cyclic adenosine monophosphate (cAMP) directly increases the open probability of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, while ZD7288 (ZD) blocks them. Dynamic Network Connectivity signaling proteins are often found in long, thin spines with narrow spine necks at NR2B NMDA-R synapses in layer III monkey DLPFC (2). AC: adenylyl cyclase.
Once Delay cell firing was stabilized, drugs were applied via iontophoresis in minute amounts that were insufficient to alter behavior (Supplementary Methods). Drugs were applied in the following sequence for at least 6 trials for each of the 8 cue locations: 1) Control, 2) ZD7288 (5–10nA; Tocris, Ellisville, MO), 3) ZD7288+SKF38393 (20–25nA; Tocris) 4) SKF38393 and 5) Recovery (Supplementary Table S1 lists the drug conditions applied to each neuron). Data were analyzed using ANOVA and circular regression using Stata (StataCorp LP, College Station, TX) (Supplementary Methods). A working model of molecular mechanisms that weaken PFC network connectivity at dendritic spines is illustrated in Figure 1E.
Mouse in vitro recordings
Whole-cell current-clamp recordings were obtained from layer V pyramidal neurons in mouse (P22–28) PFC slices; very young animals are needed to enhance slice viability. HCN channels are prominent along the dendrites of layer V pyramidal neurons in both monkeys and rodents (26, 27). In electrophysiological recordings, these cells exhibit a prominent “sag” current in response to hyperpolarizing voltage steps, corresponding to the slow activation kinetics of Ih (27, 28).
To measure Ih, a 400ms hyperpolarizing current (−300pA) was injected via the patch pipette. Ih was quantified as the ratio of the peak hyperpolarization to the steady state value during the pulse. SKF81297 (10μM; Tocris) was bath-applied, and the response in membrane potential was measured. Ih values were compared between Control and SKF81297 conditions using paired t-tests.
Rat behavioral studies
Subjects
Young adult male Sprague Dawley rats (Taconic, Germantown, NY) were housed individually under standard laboratory conditions. They were kept on a 12h light/dark cycle, and behavioral experiments were conducted during the light phase. Highly palatable rewards (chocolate chips) were used during the experiments to minimize need for dietary regulation. Water was provided ad libitum, and animals were fed 12–16g of autoclaved rat chow (Purina Mills, Gray Summit, MO) immediately following testing. They were weighed weekly, and weights were maintained at 400–450g. The rats were habituated to all procedures and tested by a single experimenter, who was blind to drug treatment conditions.
Spatial working memory task and drug infusions into rat prelimbic PFC
Rats were trained individually in a delayed alternation spatial working memory task in a T-shaped maze (Supplementary Methods).
The medial PFC, including prelimbic PFC, is required for delayed response tasks and behavioral flexibility in rats (29–35). Rats were implanted with chronic infusion cannulae directed above prelimbic PFC (AP +3.2mm; ML +/−0.75mm; DV −4.2mm) (36), or in a control region above the dorsal anterior cingulate cortex (ACd, AP +3.2mm; ML +/−0.75mm; DV −2.0mm), a region that is not needed for the delayed alternation task (37, 38) (Fig. 2). Drug was slowly infused at 0.25μL/min. Mock treatments adapted rats to all procedures (Supplementary Methods).
Figure 2.
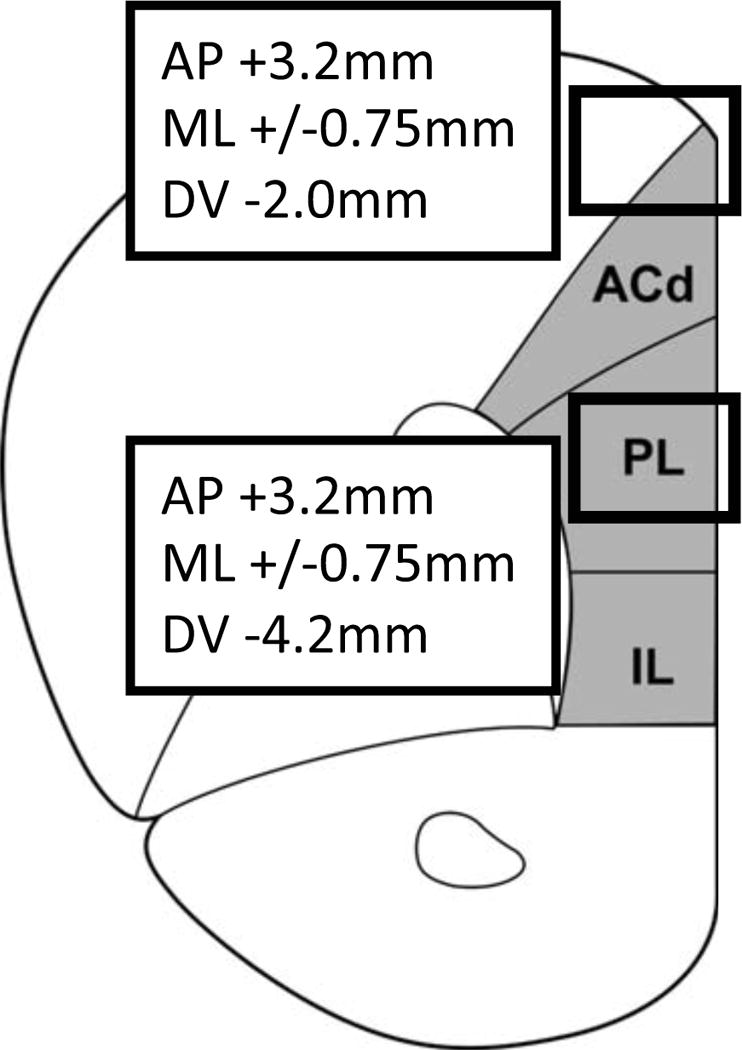
Drug infusions into rat prelimbic PFC.
Rats were implanted with chronic infusion cannulae directed above prelimbic PFC (PL) (AP +3.2mm; ML +/−0.75mm; DV −4.2mm) or dorsal anterior cingulate cortex (ACd) (AP +3.2mm; ML +/−0.75mm; DV −2.0mm), as indicated by boxes. IL: infralimbic cortex.
Experiments used a within-subjects design, with at least a one-week washout period between treatments.
In the first experiment, we tested whether blocking HCN channels with ZD7288 would prevent the impairing effects of D1R agonist infusion into prelimbic PFC. Each rat (N = 6) received the following 4 treatments in pseudo-random order: 1) ZD7288+SKF81297, 2) ZD7288+vehicle, 3) vehicle+SKF81297, and 4) vehicle+vehicle. 0.001μg/0.5μL ZD7288 was infused into PFC 30min. before testing, as described in Wang et al. (22), as this dose had no effect on performance on its own in pilot studies. Thus, any effect of the drug on working memory performance would be due to an interaction between ZD7288 and SKF81297, rather than to non-specific additive effects of the two treatments. Pilot studies determined a dose of SKF81297 (0.001–0.5μg/0.5μL) for each rat that impaired accuracy. Previous research showed that SKF81297 must be infused into prelimbic PFC to be effective (4). SKF81297 and ZD7288 were made daily by dissolving them in sterile saline to the appropriate concentrations.
In the second experiment, we examined whether ZD7288 would also block working memory impairments induced by a pharmacological stressor, FG7142. FG7142 is a benzodiazepine partial inverse agonist, which mimics stress (39). This pharmacological stressor was used instead of physical stressors (e.g. restraint stress), as it minimizes habituation that occurs with repeated physical stressors (40). Pilot studies determined an FG7142 dose (3.0–7.5mg/kg; Tocris) for each rat that impaired accuracy, but allowed task performance. Each rat (N = 5) received the following four treatments in pseudo-random order: 1) ZD7288+FG7142, 2) ZD7288+vehicle, 3) vehicle+FG7142, and 4) vehicle+vehicle. FG7142 was suspended in solution (Supplementary Methods) and injected IP 30min. before testing, as described in Birnbaum et al. (41). ZD7288 (0.001μg/0.5μL) was infused into PFC 30min. before testing, as described above.
Finally, an anatomical control experiment was performed to verify that ZD7288 improved performance via actions in prelimbic PFC, rather than via drug diffusion up the cannulae. This study followed the above methods, except that ZD7288 was infused into ACd (N = 7).
Histological verification of cannula positions
Following completion of the experiments, the locations of the cannulae were verified by histological examination (Supplementary Methods). Only data from rats with correctly placed cannulae were analyzed (Supplementary Fig. S1).
Behavioral data analysis
Data were analyzed using 2-way ANOVA with repeated measures with within-subjects factors of HCN channel blockade (ZD7288 vs. vehicle) and D1R stimulation (SKF81297 vs. vehicle) or stress (FG7142 vs. vehicle). User-defined contrasts then compared performance between pairs of drug conditions. Statistical analyses were performed using Systat.
Monkey behavior
Parallel behavioral experiments were performed in a monkey performing the ODR task with unilateral infusions of vehicle (10 or 20μL) or SKF81297 (10mM; 10 or 20μL) into DLPFC (Supplementary Methods). Previous research showed that unilateral lesions of DLPFC impaired working memory for cues in the visual field contralateral to the lesion site (42).
Results
HCN channels and D1Rs colocalize in dendritic spines of monkey DLPFC
Dual immunoelectron microscopy revealed co-expression of D1Rs and HCN channels in pyramidal neurons, as well as spatial interaction on spine membranes in layer III monkey DLPFC. Both proteins were localized in the synthetic machinery in the soma (Fig. 3A), and colocalized at the plane of the plasma membrane of dendritic spines (Figs. 3B–E). Although HCN channels are prominent along the distal pyramidal dendrites, D1Rs are not present at this location (26). Thus, co-localization was found in the spine head near the synapse (Figs. 3B, C, E) and in the spine neck (Fig. 3D), where D1Rs could generate cAMP next to HCN channels and regulate their open state. These findings built on previous work revealing HCN channel interaction with key cAMP-regulating molecules (26).
Figure 3.
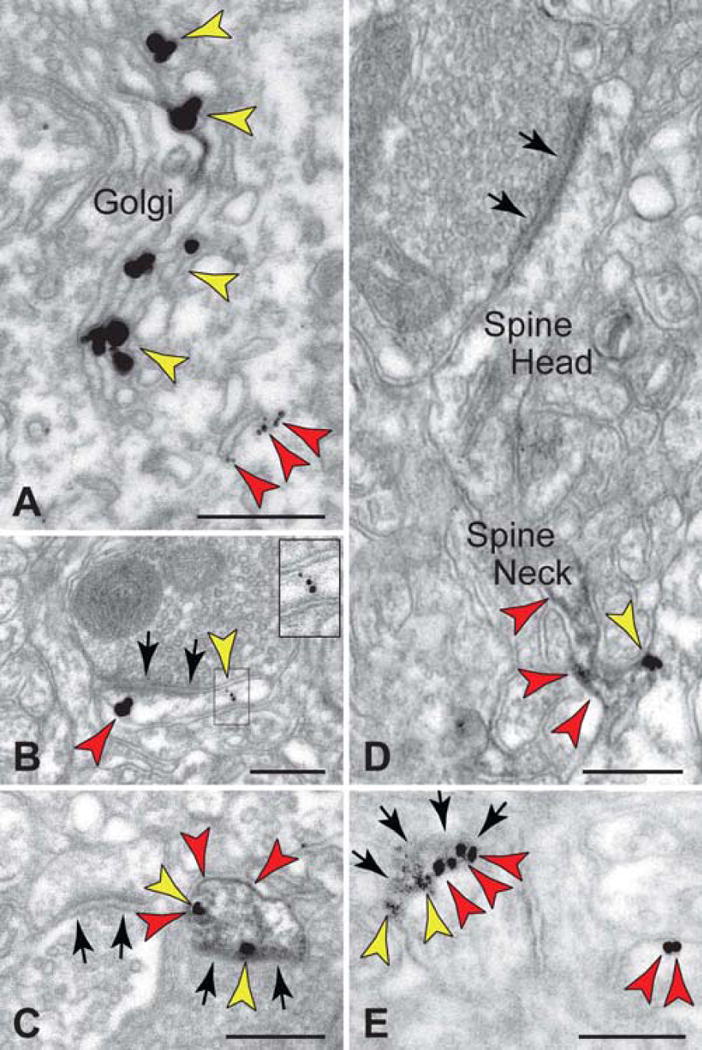
Dual immunoelectron microscopy for HCN1 channels and dopamine (DA) D1 receptors (D1Rs) in monkey DLPFC.
A. HCN1 channels (red arrows) and D1Rs (yellow arrows) were co-expressed in layer III pyramidal neurons in the Golgi and reticular endomembranes, showing that D1R are manufactured by layer III pyramidal cells.
B–E. In the neuropil, HCN1 channels and D1Rs were colocalized in dendritic spines, as shown by gold-gold (B) or peroxidase-gold (C–E) labeling. HCN1 channels were localized at the elongated spine neck with D1Rs positioned at the base of the emerging spine (D). An oblique section through a synapse (the synaptic disk is indicated by multiple arrows) demonstrated perisynaptic localization for both proteins (E); lead-contrasting was omitted from E to facilitate visualization of D1R-immunoperoxidase. Black arrows point to axospinous synapses. Scale bars: 200nm.
D1R stimulation suppressed PFC delay-related firing via opening of HCN channels in monkeys performing a working memory task
This experiment examined whether the HCN channel blocker, ZD7288, could block the impairing effect of the D1R agonist, SKF38393, on delay-related firing in DLPFC. Two monkeys (C and P) were trained to perform an ODR spatial working memory task (Fig. 1A), while 42 delay-related cells (25 from monkey C; 17 from monkey P) with varying degrees of spatial tuning were recorded in DLPFC. For tuned neurons, the preferred direction was determined by circular regression, whereas for neurons without significant tuning, the direction with the highest mean firing during the delay period was selected as the “preferred” direction for further analyses. Neurons were only included in the analyses if their activity during the ZD7288+SKF38393 condition did not change significantly with time or trial number. As a result, 1 neuron from monkey C (r = −0.90, p = 0.014 vs. time; r = −0.81, p = 0.052 vs. trial number) and 1 neuron from monkey P (r = −0.86, p = 0.0003 vs. time; r = −0.87, p = 0.0002 vs. trial number) were excluded. As Vijayraghavan et al. (5) already established that D1R stimulation suppressed PFC firing, the remaining 16 cells (8 from monkey C; 8 from monkey P) that showed reduced delay-related firing with SKF38393 were analyzed. Of these, 8 cells (4 from monkey C; 4 from monkey P) showed spatial tuning during Control, as determined by circular regression.
As shown in Figure 4A, drug application significantly altered neuronal firing relative to the Control condition (F[2, 30] = 30.671, p < 0.0005; N = 16 neurons). User-defined contrasts revealed that iontophoresis of SKF38393 alone significantly reduced firing relative to Control (F[1, 15] = 84.151, p < 0.0005). However, co-application of ZD7288 and SKF38393 led to significantly higher firing than with SKF38393 alone (F[1, 15] = 26.364, p < 0.0005), and which was similar to Control (F[1, 15] = 2.365, p = 0.145). Thus, blockade of HCN channels prevented the suppression of firing caused by D1R stimulation. Figure 4B shows the drug effects on population firing across all task epochs.
Figure 4.
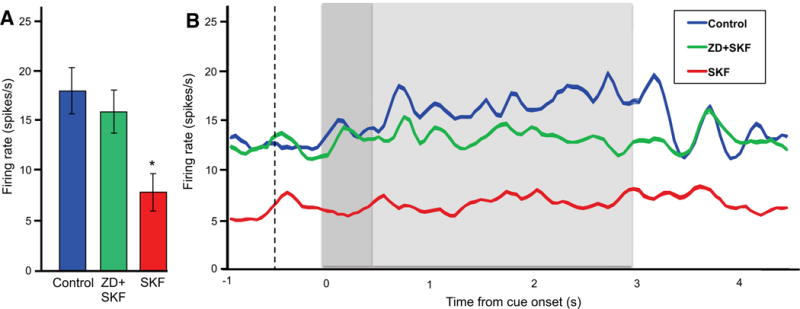
The D1R agonist, SKF38393 (SKF), suppressed delay-related firing, and this suppression was blocked by the HCN channel blocker, ZD, in monkeys performing the ODR task (N = 16 neurons).
A. Drug effects on the mean firing rate during the delay period. Error bars represent SEM. *p < 0.0005 vs. Control, ZD+SKF.
B. Drug effects on population firing across all task epochs. The dotted line indicates onset of the fixation period, the dark grey background indicates the cue period, and the light grey background indicates the delay period.
A subset of neurons was sufficiently stable over long testing sessions to assess ZD7288 alone, in addition to the other conditions. These neurons showed a significant main effect of drug treatment (F[4, 20] = 6.017, p = 0.002; N = 6 neurons) (Fig. 5A). User-defined contrasts showed that there was no significant effect of ZD7288 alone relative to Control (F[1, 5] = 0.621, p = 0.467), and that Control levels of firing were maintained following co-application of ZD7288 and SKF38393 (F[1, 5] = 1.351, p = 0.298). However, removal of ZD7288 while continuing iontophoresis of SKF38393 significantly reduced firing relative to Control (F[1, 5] = 193.632, p < 0.0005) and to ZD7288+SKF38393 (F[1, 5] = 16.607, p = 0.010). Figure 5B shows the drug effects on population firing across all task epochs. Figure 6 shows sample data from an individual neuron.
Figure 5.
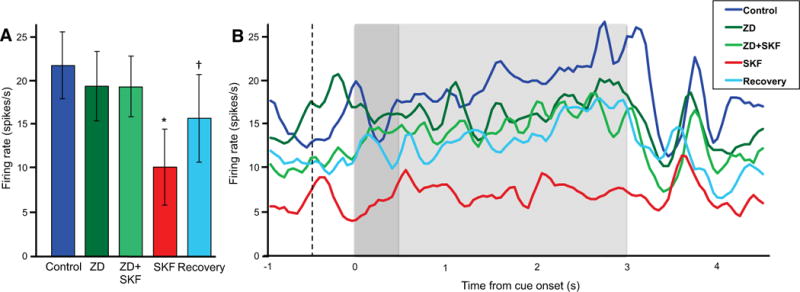
In the subset of neurons in which we assessed ZD alone and Recovery, SKF38393 (SKF) suppressed delay-related firing, and this suppression was blocked by a non-improving dose of ZD in monkeys performing the ODR task (N = 6). Firing increased during Recovery, following the removal of SKF.
A. Drug effects on the mean firing rate during the delay period. Error bars represent SEM. *p < 0.0005 vs. Control, ZD+SKF; †p = 0.030 vs. SKF.
B. Drug effects on population firing across all task epochs. The dotted line indicates onset of the fixation period, the dark grey background indicates the cue period, and the light grey background indicates the delay period.
Figure 6.
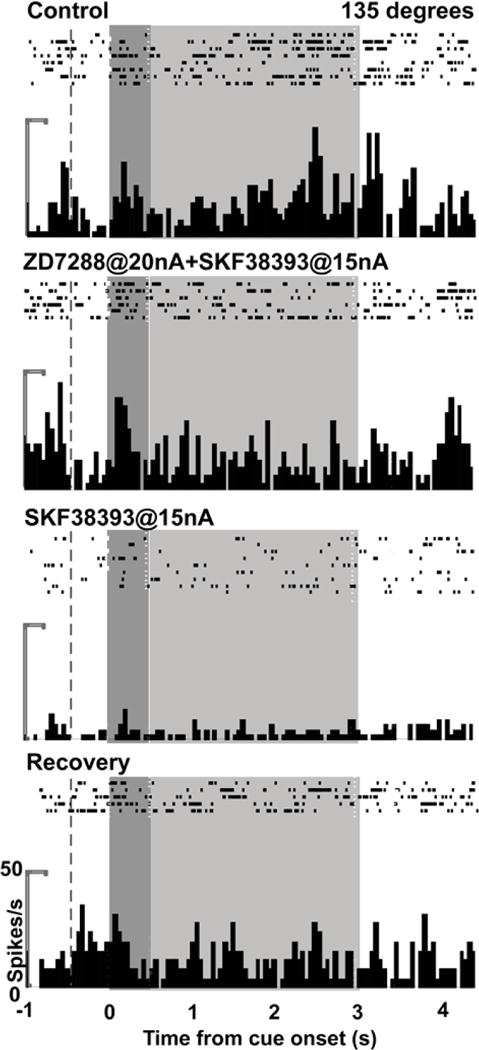
The effects of SKF38393 (SKF) and ZD on neuronal firing in an individual neuron recorded in DLPFC while a monkey performed the ODR task. For the 135° direction, the neuron showed enhanced delay-related firing (p = 0.0020 vs. fixation) during Control. The delay firing was maintained with ZD+SKF (p > 0.05 vs. Control), but was then reduced with SKF (p = 0.00017 vs. Control; 0.0015 vs. ZD+SKF). The delay firing returned during Recovery (p = 0.00013 vs. SKF). The ZD condition was not performed in this neuron. The dotted line indicates onset of the fixation period, the dark grey background indicates the cue period, and the light grey background indicates the delay period.
D1R stimulation opened HCN channels in mouse PFC slices
Whole-cell recordings from layer V pyramidal neurons in mouse PFC slices were performed to verify that D1R stimulation increased Ih. The D1R agonist, SKF81297, significantly enhanced the hyperpolarization-induced sag in membrane potential corresponding to Ih (p = 0.038 vs. Control; N = 7 neurons; Fig. 7).
Figure 7.
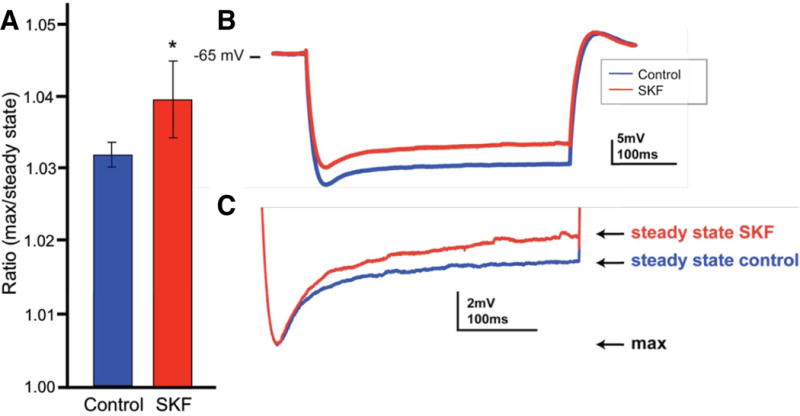
The effects of the D1R agonist, SKF81297 (SKF), on the HCN channel current (Ih) in layer V pyramidal neurons in mouse PFC slices.
A. SKF increased the ratio of the maximum change in membrane potential to the subsequent steady state potential during the hyperpolarizing pulse (*p = 0.038 vs. Control; N = 7). Error bars represent SEM.
B. A sample trace showing that SKF induced a “sag” in the membrane potential relative to control conditions, which indicated an increase in Ih.
C. The trace in B scaled to highlight the difference in the membrane potential response between SKF and control conditions.
D1R stimulation in PFC or stress impaired working memory performance via opening of HCN channels in rats
Effects of D1R stimulation and HCN channel blockade on working memory
Rats were trained in a T-maze spatial working memory task to examine the behavioral effects of D1R-HCN channel interactions. Infusions of ZD7288 into rat medial PFC (Fig. 2) significantly reversed the impairing effects of SKF81297 on working memory (Fig. 8A). There was a significant main effect of ZD7288 (F[1, 5] = 26.03, p = 0.004), and trends towards a main effect of SKF81297 (F[1, 5] = 4.33, p = 0.092) and interaction between ZD7288 and SKF81297 (F[1,5] = 6.231, p = 0.055). User-defined contrasts revealed that SKF81297 significantly impaired performance relative to vehicle (F[1, 5] = 15.92, p = 0.010), in agreement with previous findings (4, 5). Co-infusion of ZD7288 reversed this impairment (ZD7288+SKF81297 vs. SKF81297: F[1, 5] = 40.00, p = 0.001) to performance levels similar to vehicle (F[1, 5] = 0.12, p = 0.74). ZD7288 alone did not significantly affect performance relative to vehicle (F[1, 5] = 0.36, p = 0.57), which verified that the effects observed with ZD7288+SKF81297 were not due to non-specific additive effects.
Figure 8.
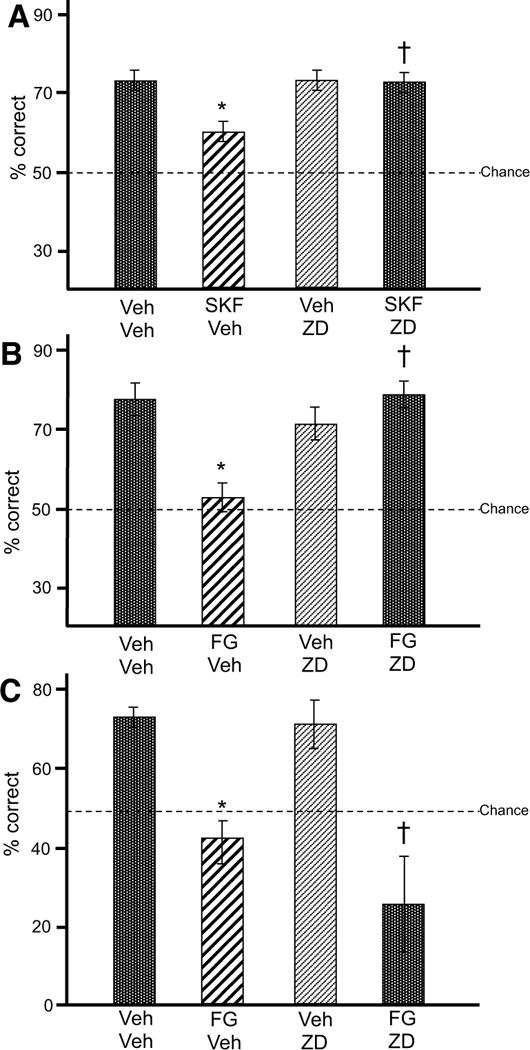
The effects of SKF81297 (SKF) and stress on spatial working memory performance in rats.
A. SKF impaired spatial working memory performance in rats (N = 6) relative to vehicle (Veh) (*p = 0.010 vs. Veh+Veh), and this impairment was blocked by PFC infusions of ZD (†p=0.001 vs. SKF+Veh). Results represent % correct out of 10 trials. Error bars represent SEM.
B. The pharmacological stressor, FG7142 (FG) impaired spatial working memory performance in rats (N = 5) relative to vehicle (Veh) (*p = 0.004 vs. Veh+Veh), and this impairment was blocked by PFC infusions of ZD (†p = 0.006 vs. FG+Veh). Results represent % correct out of 10 trials. Error bars represent SEM.
C. FG impaired spatial working memory performance in rats (N = 7) relative to vehicle (Veh) (*p = 0.002 vs. Veh+Veh). However, this impairment was not blocked by ZD infusions dorsal to PFC (†p = 0.008 vs. Veh+Veh). Results represent % correct out of 10 trials. Error bars represent SEM.
Effects of pharmacological stress and HCN channel blockade on working memory
Infusions of ZD7288 into rat medial PFC significantly reversed the impairing effects of the pharmacological stressor, FG7142, on working memory (Fig. 8B). There was a significant main effect of ZD7288 (F[1, 4] = 10.39, p = 0.032), a non-significant main effect of FG7142 (F[1, 4] = 3.55, p = 0.133), and a significant interaction between ZD7288 and FG7142 (F[1, 4] = 25.76, p = 0.007). User-defined contrasts revealed that FG7142+vehicle significantly impaired performance relative to vehicle (F[1, 4] = 35.71, p = 0.004), in agreement with previous findings (43). Infusion of ZD7288 reversed this impairment (ZD7288+FG7142 vs. FG7142: F[1, 4] = 28.24, p = 0.006), returning performance to vehicle levels (F[1, 4] = 0.074, p = 0.80). ZD7288 alone did not significantly affect performance relative to vehicle (F[1, 4] = 2.25, p = 0.21), which verified that the effects observed with ZD7288+FG7142 were not due to non-specific additive effects.
Anatomical control experiments verified that ZD7288 improved working memory via actions in prelimbic PFC, rather than via drug diffusion up the cannulae (Fig. 8C). When ZD7288 was infused into ACd (Fig. 2), there was no significant main effect of ZD7288 (F[1, 6] = 1.23, p = 0.309), a significant main effect of FG7142 (F[1, 6] = 45.14, p = 0.001), and no significant interaction between ZD7288 and FG7142 (F[1, 6] = 2.87, p = 0.14). User-defined contrasts revealed that FG7142 significantly impaired performance relative to vehicle (F[1, 6] = 28.90, p = 0.002). Co-infusion of ZD7288 did not reverse this impairment (ZD7288+FG7142 vs. FG7142: F[1, 6] = 2.21, p = 0.19), and performance following ZD7288+FG7142 was still significantly impaired relative to vehicle (F[1, 6] = 15.28, p = 0.008). Again, ZD7288 alone did not significantly affect performance relative to vehicle (F[1, 6] = 0.092, p = 0.772).
Finally, we verified that the changes in performance following D1R stimulatin or stress were due to working memory impairments, and not to general motor impairments, by analyzing the rats’ response times for each trial during vehicle and SKF81297 or FG7142 conditions (Supplementary Results).
D1R stimulation in PFC impaired working memory performance in a monkey
Unilateral infusions of SKF81297 into monkey DLPFC impaired working memory performance in the ODR task for spatial cues contralateral to the infusion sites (p < 0.05 vs. Control; Supplementary Figs. S4–6). Infusions of vehicle had no effect (p > 0.05 vs. Control; Supplementary Fig. S6). In addition, infusions of SKF81297 did not impair performance under visually guided control conditions, when working memory was not needed (p > 0.05 vs. Control; Supplementary Figs. S6–7). Further details are provided in the Supplementary Results.
Discussion
The current study showed that the impairment in PFC function during stress involves D1R opening of HCN channels to weaken PFC network connections. Combining anatomical, physiological and behavioral evidence, we found that 1) D1Rs and HCN channels colocalize and spatially interact at dendritic spines in monkey layer III DLPFC, 2) stimulation of D1Rs increases Ih in mouse PFC slices, 3) suppression of neuronal firing by D1R-cAMP signaling can be prevented by blocking HCN channels in monkey DLPFC, and 4) working memory impairment induced by D1R stimulation or pharmacological stress can be prevented by blocking HCN channels in rat PFC.
While cAMP is known to be involved in a multitude of intracellular pathways, e.g. (44–46), the current study demonstrated that suppression of DLPFC function by D1Rs involves cAMP-mediated increase in Ih, thus revealing an ionic component to this important physiological response. It is of interest that D1R stimulation suppressed firing throughout all epochs of task-related firing, replicating earlier findings with high doses of D1R stimulation (5). As the firing of Delay cells is thought to be driven by recurrent excitation from neurons with shared network properties (2), D1R weakening of these network inputs may alter all phases of task-related firing.
Lower levels of D1R stimulation likely engage several mechanisms that would also enhance spatially tuned persistent firing (5), some of which would not involve HCN channels, e.g. increasing NMDA receptor insertion into the postsynaptic density (47, 48), and enhancing GABAergic lateral inhibition (49–51). However, HCN channel opening likely contributes as well (Supplementary Figs. S3C, S8).
Rodent vs. monkey PFC homology
Although there are many differences between the rodent medial PFC and the monkey DLPFC (29, 31), almost two decades of research suggest that some mechanisms do extend across species. For example, DA depletion in these regions impairs working memory in both rodents (52) and monkeys (52–54), while excessive D1R stimulation in PFC impairs cognitive function in rats and reduces neuronal firing in monkeys via increased cAMP signaling (5). Our in vitro recordings in rodent PFC were made from layer V pyramidal neurons, which have properties of both layer III and layer V pyramidal neurons in primates (55), e.g. they respond to both D1R and D2R agonists. These neurons are often used for intracellular recordings, and have been essential for direct examination of ionic mechanisms. The current data indicate that D1R impairment in PFC working memory function involves HCN channel opening in both rodents and monkeys. However, the precise contributions of these channels to neuronal physiology may differ across species, particularly as HCN channels play a variety of roles depending upon their ultrastructural localization and molecular interactions.
Excitatory vs. inhibitory nature of D1R-HCN channel signaling
D1Rs have been shown to enhance excitability of rodent PFC pyramidal neurons in vitro, both via cAMP (21, 56–58) and independently of cAMP (59, 60), through increase in sodium and reduction in potassium currents (21, 59, 61, 62), and possibly NMDA receptors (63–65). These basic, excitatory influences may be saturated in a behaving animal, with additional inhibitory effects being observed with higher levels of stimulation (5), or the inhibitory effects in vivo may override these excitatory mechanisms (3, 66).
HCN channels also show both excitatory and inhibitory influences on membrane potential, likely depending on the laminar position of the neuron, and whether the recording is from a highly active neuron in vivo vs. a hyperpolarized neuron in a PFC slice. It should be noted that Ih does not necessarily require hyperpolarization to open, as HCN channels have a tonically active leak current component (67–71) that is blocked by ZD7288 (67, 72). Furthermore, HCN channels and D1Rs are found near a constellation of cAMP signaling proteins at dendritic spines, whereas HCN channels on dendrites have few cAMP signaling proteins nearby (26). While speculative, these findings suggest that HCN channels at spines may open primarily in response to cAMP, and reduce firing by shunting network inputs and/or reducing temporal summation, e.g. (73–75). Finally, HCN channels may also interact with other potassium channels to alter dendritic excitability, e.g. KCNQ (Kv7) channels (76), Kir2.2/2.3 and potassium-selective leak (Kleak) channels (77).
While the current study and previous work (26) indicate that HCN channels on spines can co-localize with D1Rs, a future, dual quantitative analysis of HCN1 and D1Rs in the PFC neuropil will be necessary to determine the extent of this co-expression. Low doses of ZD7288 may be especially potent in blocking HCN channels on spines, due to D1R-mediated phosphorylation of channels keeping them open (78–81), and/or because channel blockade may be more efficacious in a thin spine, given its very small volume compared to that of a large dendrite.
Relevance to psychiatric disorders
These mechanisms are likely relevant to a range of psychiatric disorders associated with dysregulated DA signaling, in which patients often show precipitation or exacerbation of symptoms with stress (3, 12). For example, D1Rs are upregulated in DLPFC of patients with schizophrenia (82–84), especially in young, drug-naïve patients (11), and this increase correlates with poor working memory (82, 83). The current data suggest that some of this impairment may arise from D1R-HCN channel weakening of PFC network firing.
Supplementary Material
Acknowledgments
The authors thank Lisa Ciavarella, Tracy Sadlon, Sam Johnson, Michelle Wilson and Jessica Thomas Ebbett for their invaluable technical expertise, and Benny Brunson and others at the Yale Animal Resources Center for their superb care of our animals.
This work was supported by NINDS NS07224 to NJG, PHS RL1AA017536 to AFTA within Consortium U54RR024350, NARSAD Young Investigator Grant to YY and NIMH MH099045 and a Smith Family Award for Excellence in Biomedical Research to MJH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in abstract form at the Society for Neuroscience meetings in 2007 (San Diego, CA) and 2011 (Washington, DC), the DISC1 2010 meeting in 2010 (Edinburgh, UK), and the New York Academy of Sciences Advancing Drug Discovery for Schizophrenia meeting in 2011 (New York, NY).
Financial Disclosures: All authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 3.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 6.Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: Immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- 7.Cai JX, Arnsten AF. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;283:183–189. [PubMed] [Google Scholar]
- 8.Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 11.Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, et al. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 2012;26:794–805. doi: 10.1177/0269881111409265. [DOI] [PubMed] [Google Scholar]
- 12.Breier A, Wolkowitz O, Pickar D. Stress and schizophrenia: advances in neuropsychiatry and psychopharmacology. In: Tamminga C, Schult S, editors. Schizophrenia research. New York: Raven Press Ltd.; 1991. [Google Scholar]
- 13.Goldman-Rakic PS. Circuitry of the frontal association cortex and its relevance to dementia. Arch Gerontol Geriatr. 1987;6:299–309. doi: 10.1016/0167-4943(87)90029-x. [DOI] [PubMed] [Google Scholar]
- 14.Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 1995;359:131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA Receptors Subserve Persistent Neuronal Firing during Working Memory in Dorsolateral Prefrontal Cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- 17.Wilson FA, O’Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci U S A. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn Sci. 2010 doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci U S A. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- 25.Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- 26.Paspalas CD, Wang M, Arnsten AF. Constellation of HCN Channels and cAMP Regulating Proteins in Dendritic Spines of the Primate Prefrontal Cortex: Potential Substrate for Working Memory Deficits in Schizophrenia. Cereb Cortex. 2013 doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 28.Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 30.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- 34.Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 35.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, W C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- 37.Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 38.Taylor JR, Birnbaum S, Ubriani R, Arnsten AF. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- 40.Meller E, Shen C, Nikolao TA, Jensen C, Tsimberg Y, Chen J, et al. Region-specific effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. 2003;979:57–64. doi: 10.1016/s0006-8993(03)02866-x. [DOI] [PubMed] [Google Scholar]
- 41.Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 42.Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- 44.Dash PK, Karl KA, Colicos MA, Prywes R, Kandel ER. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 46.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pei L, Lee FJ, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G, Greengard P, Yan Z. Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:2596–2600. doi: 10.1073/pnas.0308618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 50.Kroner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- 51.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- 53.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 54.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II–III pyramidal neurons in rat prefrontal cortex. J Neurosci. 2003;23:867–875. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Feng XQ, Zheng P. Activation of presynaptic D1 dopamine receptors by dopamine increases the frequency of spontaneous excitatory postsynaptic currents through protein kinase A and protein kinase C in pyramidal cells of rat prelimbic cortex. Neuroscience. 2002;112:499–508. doi: 10.1016/s0306-4522(02)00113-6. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, O’Donnell P. D(1) dopamine receptors potentiate nmda-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- 59.Gorelova NA, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol. 2000;84:75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- 60.Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- 62.Shi WX, Smith PL, Pun CL, Millet B, Bunney BS. D1–D2 interaction in feedback control of midbrain dopamine neurons. J Neurosci. 1997;17:7988–7994. doi: 10.1523/JNEUROSCI.17-20-07988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maguire G, Werblin F. Dopamine enhances a glutamate-gated ionic current in OFF bipolar cells of the tiger salamander retina. J Neurosci. 1994;14:6094–6101. doi: 10.1523/JNEUROSCI.14-10-06094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith DO, Lowe D, Temkin R, Jensen P, Hatt H. Dopamine enhances glutamate-activated currents in spinal motoneurons. J Neurosci. 1995;15:3905–3912. doi: 10.1523/JNEUROSCI.15-05-03905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 68.Macri V, Accili EA. Structural elements of instantaneous and slow gating in hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2004;279:16832–16846. doi: 10.1074/jbc.M400518200. [DOI] [PubMed] [Google Scholar]
- 69.Proenza C, Angoli D, Agranovich E, Macri V, Accili EA. Pacemaker channels produce an instantaneous current. J Biol Chem. 2002;277:5101–5109. doi: 10.1074/jbc.M106974200. [DOI] [PubMed] [Google Scholar]
- 70.Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, et al. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vemana S, Pandey S, Larsson HP. Intracellular Mg2+ is a voltage-dependent pore blocker of HCN channels. Am J Physiol Cell Physiol. 2008;295:C557–65. doi: 10.1152/ajpcell.00154.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Proenza C, Yellen G. Distinct populations of HCN pacemaker channels produce voltage-dependent and voltage-independent currents. J Gen Physiol. 2006;127:183–190. doi: 10.1085/jgp.200509389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- 74.Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, et al. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- 76.George MS, Abbott LF, Siegelbaum SA. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nat Neurosci. 2009;12:577–584. doi: 10.1038/nn.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vargas G, Lucero MT. Modulation by PKA of the hyperpolarization-activated current (Ih) in cultured rat olfactory receptor neurons. J Membr Biol. 2002;188:115–125. doi: 10.1007/s00232-001-0178-y. [DOI] [PubMed] [Google Scholar]
- 79.Arinsburg SS, Cohen IS, Yu HG. Constitutively active Src tyrosine kinase changes gating of HCN4 channels through direct binding to the channel proteins. J Cardiovasc Pharmacol. 2006;47:578–586. doi: 10.1097/01.fjc.0000211740.47960.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu X, Duan KL, Shang CF, Yu HG, Zhou Z. Calcium influx through hyperpolarization-activated cation channels (I(h) channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci U S A. 2004;101:1051–1056. doi: 10.1073/pnas.0305167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, et al. A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem. 2005;280:34224–34232. doi: 10.1074/jbc.M506544200. [DOI] [PubMed] [Google Scholar]
- 82.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abi-Dargham A. Probing cortical dopamine function in schizophrenia: what can D1 receptors tell us? World Psychiatry. 2003;2:166–171. [PMC free article] [PubMed] [Google Scholar]
- 84.Hirvonen J, van Erp TG, Huttunen J, Aalto S, Nagren K, Huttunen M, et al. Brain dopamine d1 receptors in twins discordant for schizophrenia. Am J Psychiatry. 2006;163:1747–1753. doi: 10.1176/ajp.2006.163.10.1747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


