Abstract
GABAergic dysfunction has been strongly implicated in the pathophysiology of schizophrenia. In this study, we analyzed the expression levels of several GABAergic genes in the anterior cingulate cortex (ACC) of postmortem subjects with schizophrenia (n=21) and a comparison group of individuals without a history of psychiatric illness (n=18). Our analyses revealed a significant sex by diagnosis effect, along with significant differences in GABAergic gene expression based on medication status. Analyses revealed that in male groups, the expression of GABAergic genes was generally lower in schizophrenia cases compared to the controls, with significantly lower expression levels of GABA-Aα5, GABA-Aβ1, and GABA-Aε. In females, the expression of GABAergic genes was higher in the schizophrenia cases, with significantly higher expression of the GABA-Aβ1 and GAD67 genes. Analysis of the effect of medication in the schizophrenia subjects revealed significantly higher expression of GABA-Aα1–3, GABA-Aβ2, GABA-Aγ2, and GAD67 in the medicated group compared to the unmedicated group. These data show that sex differences in the expression of GABAergic genes occur in the ACC in schizophrenia. Therefore our data support previous findings of GABAergic dysfunction in schizophrenia and emphasize the importance of considering sex in analyses of the pathophysiology of schizophrenia. Sex differences in the GABAergic regulation of ACC function may contribute to the differences observed in the symptoms of male and female patients with schizophrenia. In addition, our findings indicate that antipsychotic medications may alter GABAergic signaling in the ACC, supporting the potential of GABAergic targets for the development of novel antipsychotic medication.
Keywords: antipsychotic, γ-aminobutyric acid, receptor, mRNA, gender, postmortem brain
1. Introduction
Schizophrenia is a widespread and debilitating disorder, with a lifetime risk of approximately 0.7% (Saha et al., 2005). Several hypotheses for the pathophysiology of schizophrenia have been proposed. These focus on the neurotransmitter systems implicated by pharmacological evidence, particularly the dopamine system (Kuepper et al., 2012; Seeman, 2013), the serotonin (5-HT) system (Meltzer et al., 2012), and more recently the glutamate system (Coyle et al., 2012; Javitt, 2012; Moghaddam and Krystal, 2012; Sodhi et al., 2008). Inadequate inhibition of these systems due to dysfunctional γ-aminobutyric acid (GABA) neurotransmission has also been proposed, and accumulating data support the GABAergic hypothesis of schizophrenia (Guidotti et al., 2005; Stan and Lewis, 2012).
The combined effect of nature (genes) and nurture (a stressful environment) is considered to underpin the causes of schizophrenia (Brown, 2011; Gejman et al., 2011; Owen et al., 2010; Roth et al., 2009; Uher, 2014). Gene expression provides a readout of both the genetic and the environmental factors that contribute to the pathophysiology of schizophrenia. Analysis of human postmortem brain is a powerful approach with which to elucidate the pathophysiological mechanisms of schizophrenia, because unlike studies of living patients, detailed molecular analyses can be performed directly in the critical brain regions of interest.
Accumulating data indicate that GABAergic function is disrupted in schizophrenia. Significant associations have been detected between variation of several GABAergic genes and schizophrenia, including the genes encoding the 67 kilodalton isoform of glutamic acid dehydrogenase (GAD67) (Straub et al., 2007; Zhao et al., 2007), and the GABA-A receptor subunits GABA-Aα1, GABA-Aα6 (Petryshen et al., 2005), GABA-Aβ2 (Lo et al., 2007; Lo et al., 2004; Yu et al., 2006; Zhao et al., 2007) and GABA-Aγ2 (Zai et al., 2009). Data from postmortem gene expression analyses have revealed reduced expression of GAD67 in several brain regions in schizophrenia, including the dorsolateral prefrontal cortex (DLPFC) (Akbarian et al., 1995; Curley et al., 2011; Duncan et al., 2010; Guidotti et al., 2000; Hashimoto et al., 2008a; Hashimoto et al., 2008b; Hashimoto et al., 2005; Kimoto et al., 2014; Veldic et al., 2005; Volk et al., 2000; Woo et al., 2008), and the anterior cingulate cortex (ACC) (Guidotti et al., 2000; Hashimoto et al., 2008b; Thompson et al., 2009; Woo et al., 2004). Moreover, differences in the expression of GABA-A receptor genes have been detected in the DLPFC in schizophrenia, such as the GABA-A receptor subunits α1 (Beneyto et al., 2011; Hashimoto et al., 2008a; Hashimoto et al., 2008b; Impagnatiello et al., 1998; Ishikawa et al., 2004; Ohnuma et al., 1999), α2 (Beneyto et al., 2011; Volk et al., 2002), α5 (Beneyto et al., 2011; Duncan et al., 2010; Impagnatiello et al., 1998), β2 (Beneyto et al., 2011), and δ (Hashimoto et al., 2008a; Hashimoto et al., 2008b). Studies of neutrotransmitter-to-receptor binding indicate that increased GABA-A receptor binding occurs in the neurons of layers II and III of the ACC (Benes et al., 1992) and in the prefrontal cortex in schizophrenia (Benes et al., 1996; Hanada et al., 1987). These data indicate that there is dysregulation of GABAergic signalling in schizophrenia. Moreover, reduced numbers of GABAergic cells expressing the glutamate receptor subunits GRIN2A and GRIK1 have been reported to occur in the ACC in schizophrenia, indicating that glutamatergic inputs may fail to adequately activate inhibitory GABAergic interneurons in schizophrenia (Woo et al., 2007; Woo et al., 2004).
The ACC is a critical component of the cortico-limbic circuitry in the brain, which is considered to be disrupted in schizophrenia (Benes, 2010; Beneyto and Lewis, 2011). Abnormal function of the ACC would be predicted to disrupt several aspects of emotional and cognitive processing, which are important components of the clinical presentation of schizophrenia. Data indicate altered morphological, metabolic, and neurotransmitter-related abnormalities in the ACC in schizophrenia (Clark et al., 2006). Furthermore, structural changes of the ACC have been detected using brain imaging of patients with schizophrenia (reviewed in Fornito et al., 2009). Functional imaging reveals deficits in the activation of the ACC during tests of executive function in schizophrenia patients (Minzenberg et al., 2009). For example, schizophrenia patients show impaired performance of the Stroop task, which measures information processing skills that require activation of the ACC (Krabbendam et al., 2009).
In the current study we have tested the hypothesis that there is abnormal GABAergic gene expression in the ACC in schizophrenia. We investigated the expression levels of several GABAergic genes in the ACC of postmortem subjects with schizophrenia and a comparison (control) group of subjects without a history of psychiatric illness. Genes were prioritized for analysis if they were previously associated with schizophrenia. We report sex differences in GABAergic gene expression in the ACC in schizophrenia which may contribute to the differences observed in the symptoms and pathophysiology of the disorder in males and females. Furthermore, we report differential GABAergic gene expression in medicated and unmedicated patients with schizophrenia, indicating that these medications may alter ACC function in schizophrenia through the GABAergic system.
2. Materials and Methods
2.1 Tissue used for study
Subjects were recruited at the Mount Sinai/Bronx Veterans Administration Medical Center Department of Psychiatry Brain Bank. Postmortem brain tissue was taken from individuals with schizophrenia diagnosed by DSM-IV criteria and comparison (control) subjects with no history of psychiatric or neurological disorders (Table 1). All subjects died of natural causes, without a history of alcoholism and/or substance abuse. All subjects had thorough neurological characterization to rule out neurodegenerative disorders including Alzheimer’s disease, as described previously (Oni-Orisan et al., 2008). This included evaluation for National Institute of Neurological and Communicative Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINCDS-AIREN) criteria for a diagnosis of vascular dementia; NINCDS, DSMIV and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) diagnosis of dementia; Consensus criteria for Lewy body disease; unified Parkinson's disease rating scale (UPDRS); clinical criteria for frontotemporal dementia; and tests of cognitive function including the mini-mental state examination (MMSE) and clinical dementia rating (CDR). The brain tissue also underwent neuropathological examination macro- and microscopically using CERAD guidelines. All assessment, consent, and postmortem procedures were conducted as required by the Institutional Review Boards of Pilgrim Psychiatric Center, Mount Sinai School of Medicine, and the Bronx Veterans Administration Medical Center. Tissue collection was as described previously (Oni-Orisan et al., 2008). Frozen tissue was placed on slides in 20µm sections including the anterior cingulate cortex, and stored at −80°C.
Table 1.
Demographic variables of postmortem subjects included in the study
| N | Age | pH | RIN | PMI | ||
|---|---|---|---|---|---|---|
| Control | Male | (0) | 72.8 (14.1) | 6.41 (0.27) | 6.91 (0.60) | 8.2 (6.7) |
| Female | 13 (0) | 85.5 (8.5) | 6.41 (0.25) | 6.71 (0.84) | 7.3 (6.5) | |
| Total | 18 (0) | 81.9 (11.5) | 6.41 (0.25) | 6.76 (0.77) | 7.6 (6.3) | |
| Schizophrenia | Male | 14 (8) | 80.0 (10.6) | 6.60 (0.24) | 6.39 (0.78) | 11.9 (4.6) |
| Female | 7(5) | 75.4 (5.0) | 6.30 (0.41) | 6.61 (1.09) | 13.0 (6.0) | |
| Total | 21 (13) | 78.5 (9.3) | 6.50 (0.33) | 6.46 (0.87) | 12.2 (5.0) | |
Values shown are mean with standard deviation for continuous variables in parentheses.
Abbreviations: Age, age at death in years; N, number of subjects (number on antipsychotic medication at time of death shown in parentheses); PMI, postmortem interval in hours; RIN, RNA integrity number.
2.2 RNA extraction and cDNA synthesis
Tissue sections were stained in order to differentiate grey and white matter by incubation in 1% cresyl violet acetate for 2 minutes, submersion in 95% ethanol, 100% ethanol for 30 seconds, followed by immersion in xylene for 5 minutes. Grey matter was collected from the slides and RNA was extracted using an RNeasy mini kit (Qiagen, Hilden, Germany). The RNA integrity number (RIN) was determined using an Agilent BioAnalyzer (Agilent Technologies, Santa Clara, California) to provide a measure of RNA quality at the UIC DNA Services Core Facility.
All RNA samples were diluted to 20ng per microliter, followed by reverse transcription using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, California). Preamplification of cDNA was necessary due to the low starting concentration of mRNA. Equal volumes of each TaqMan assay to be used for expression analysis were combined for the preamplification reaction. 40µl cDNA, together with 4µl pooled assays and 44µl TaqMan JumpStart (Sigma-Aldrich, St Louis, Missouri), was preamplified for 14 cycles as previously described (Mengual et al., 2008; Sodhi et al., 2011).
2.3 qPCR
Gene expression was measured using commercial TaqMan assays (Applied Biosystems; Table 2). The expression of GAD67 and selected GABA-A receptor subunits (Table 2) were measured in preamplified cDNA derived from the gray matter of the ACC from each subject. In addition the expression levels of the housekeeping genes glyceraldehyde-3-phosphate dehydrogenase (GAPDH), cyclophilin (PPIA), and glucuronidase beta (GUSB) were measured. Assays for each target gene were performed in duplicate in 96-well optical plates using a Stratagene MX3000P instrument (Stratagene, La Jolla, California) and Sequence Detector Software (SDS version 1.6; PE Applied Biosystems). The relative standard curve method was used for these analyses as described previously (Sodhi et al., 2011). Briefly, standard curves were generated for each target assay and for each endogenous control assay using a calibration curve and the geometric mean of GAPDH, PPIA, and GUSB expression was used for normalization of the target genes according to Applied Biosystems instructions (Guide to Performing Relative Quantitation of Gene Expression Using Realtime Quantitative PCR, Applied Biosystems).
Table 2.
Analyses of GABAergic gene expression in the ACC in schizophrenia.
| Gene | Protein | Locus | Assay ID# |
|---|---|---|---|
| GABRA1 | GABA-Aα1 | 5q34 | Hs00168058_m1 |
| GABRA2 | GABA-Aα2 | 4p12 | Hs00168069_m1 |
| GABRA3 | GABA-Aα3 | Xq28 | Hs00168073_m1 |
| GABRA5 | GABA-Aα5 | 15q12 | Hs00181291_m1 |
| GABRB1 | GABA-Aβ1 | 4p12 | Hs00181306_m1 |
| GABRB2 | GABA-Aβ2 | 5q34 | Hs00241451_m1 |
| GABRD | GABA-Aδ | 1p36 | Hs00181309_m1 |
| GABRE | GABA-Aε | Xq28 | Hs00608332_m1 |
| GABRG1 | GABA-Aγ1 | 4p12 | Hs00381554_m1 |
| GABRG2 | GABA-Aγ2 | 5q34 | Hs00168093_m1 |
| GABRG3 | GABA-Aγ3 | 15q12 | Hs00264276_m1 |
| GAD1 | GAD67 | 2q31 | Hs01065893_m1 |
| GUSB | GUSB | 7q21 | Hs99999908_m1 |
| GAPDH | GAPDH | 12p13 | Hs99999905_m1 |
| PP1A | Cyclophilin | 7p13 | Hs99999904_m1 |
Relative gene expression was measured using commercially designed assays (Applied Biosystems).
2.4 Statistical Analysis
Statistical analysis was conducted using SPSS version 20. Any subjects with RIN < 5.0 were excluded from the analyses. For α= 0.05, and n= 39, our observed power (one-tailed hypothesis) was 0.8 for a large effect size of 0.84, as determined by an online statistical calculator (www.danielsoper.com). Shapiro-Wilk tests were used to determine if data were normally distributed. Normally distributed data were analysed using multivariate ANCOVA to investigate main effects, and post-hoc analyses of individual genes were performed using univariate ANCOVA. Postmortem interval (PMI), age at death, and brain pH were included as covariates due to their correlation with gene expression and/or differences in their values between diagnostic groups (data not shown). Analyses included relative gene expression as the independent variable and both diagnosis and sex as the dependent variables. Data that were not normally distributed were analysed using the non-parametric Mann-Whitney U test. Due to the potential confounding effects of antipsychotic medications, and the reports of changes in GABAergic gene expression in rodents treated with haloperidol or clozapine (Zink et al., 2004a; Zink et al., 2004b), univariate ANCOVAs were also conducted to investigate the effect of medication status, including PMI, age at death, and brain pH as covariates.
3. Results
3.1 Effect of diagnostic group
Data were normally distributed for all target gene transcripts except GABA-Aε. Analysis of normally distributed target genes by multivariate ANOVA indicated no significant effect of diagnosis (F11,22=0.644, p>0.05) or sex (F11,22=1.454, p>0.05), but revealed a significant sex by diagnosis interaction for GABAergic gene expression (F=11,22=3.049, p=0.013). To further investigate this sex by diagnosis effect, post-hoc analyses were conducted on males and females separately.
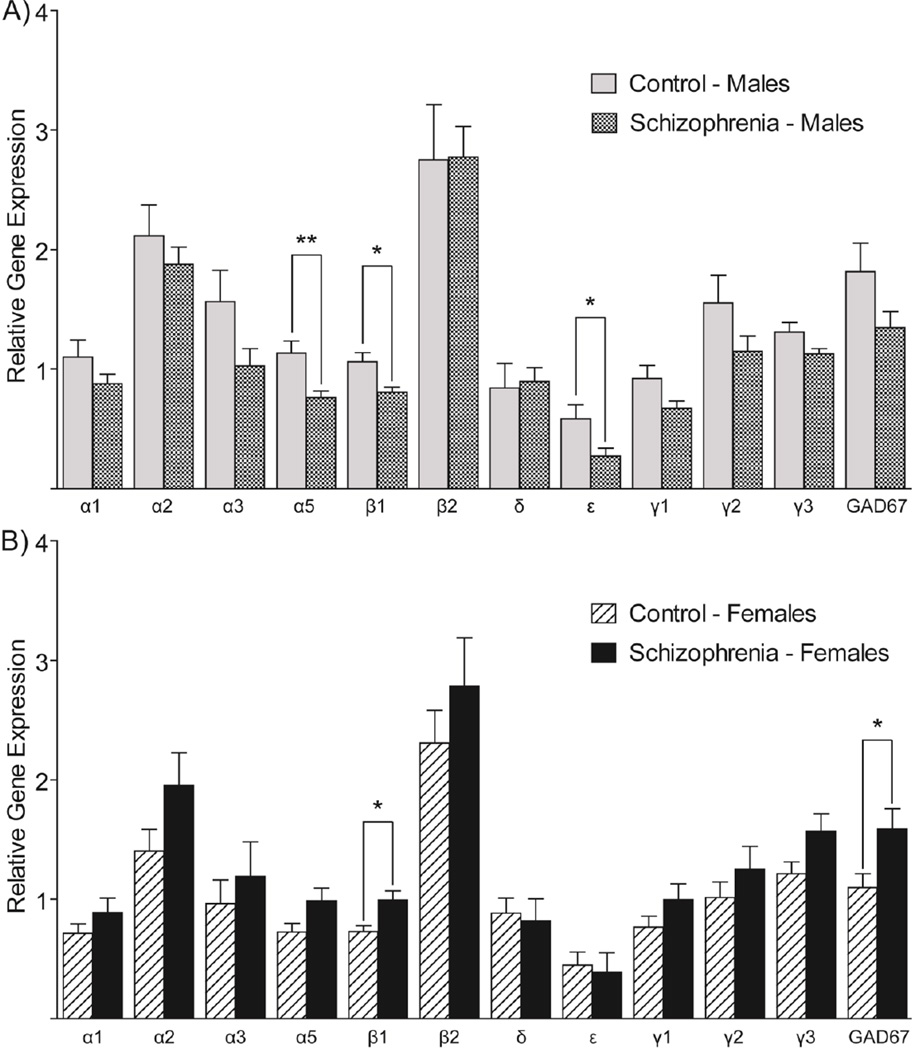
Expression data in male subjects were normally distributed for every gene tested. Multivariate ANCOVA indicated no significant association between GABAergic gene expression and diagnosis in the male group (F12,3=1.521, p>0.05), but post-hoc analyses of individual genes revealed significantly decreased expression of GABA-Aα5 (F1,14=9.504, p=0.008), GABA-Aβ1 (F1,14=7.427, p=0.016), and GABA-Aε (F1,14=4.843, p=0.045) (Figure 1A). In the female subjects, all gene expression data were normally distributed except for GABA-Aε. Multivariate ANOVA of normally distributed data showed no effect of diagnosis (F11,5=1.233, p>0.05), but post-hoc tests indicated significant increases in the expression of GABA-Aβ2 (F1,15=7.197, p=0.017) and GAD67 (F1,15=4.545, p=0.05) (Figure 1B). The Mann-Whitney U test indicated no significant differences in the expression of GABA-Aε between the diagnostic groups (p>0.05).
Figure 1. Differential GABAergic gene expression occurs in the ACC of males and females with schizophrenia.
The relative expression of each GABA-A receptor subunit and GAD67 is compared in schizophrenia and control subjects. (A) Male schizophrenia subjects have a generalized decrease in the expression of almost every gene tested relative to male controls. (B) Female schizophrenia subjects appear to have a generalized increase in the expression in almost every GABAergic gene tested. Post-hoc analyses reveal significant alterations in the expression levels of specific genes, and are summarized in the graphs. Values shown are mean ± SEM of the relative expression of each target gene normalized to the geometric mean of three housekeeping genes (GUSB, PPIA, and GAPDH), measured by qPCR. * indicates p≤0.05; ** indicates p≤0.01.
3.2 Effect of medication status
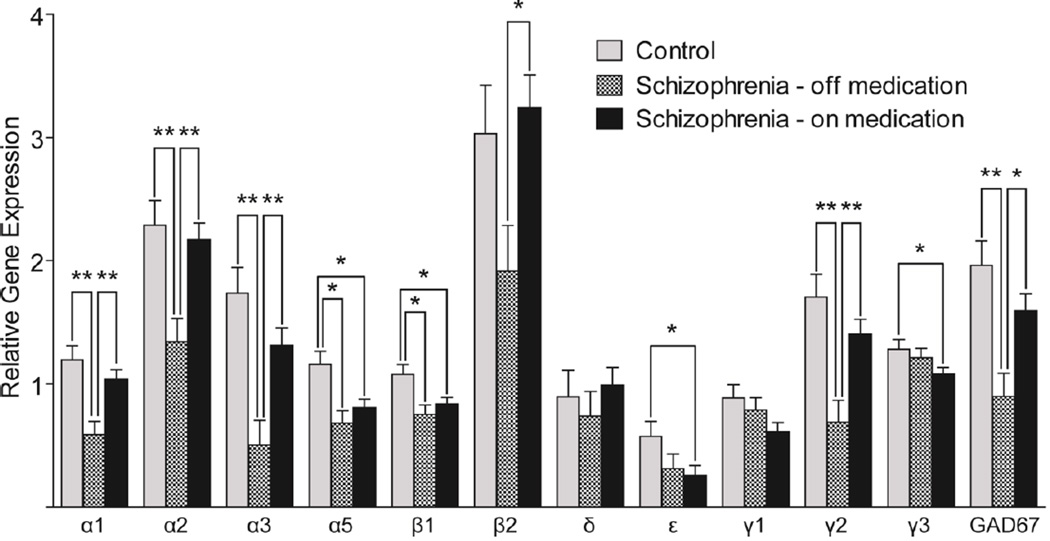
The patient group was dichotomized into cases that were reported to be on antipsychotic medication at time of death and those who were not on antipsychotic medication. Analysis of gene expression by medication status was conducted in the male schizophrenia group only, due to the small number of females (<5) in the ‘off medication’ group. ANCOVA showed significant differences between the on- and off- medication groups for the expression of several GABAergic genes (Figure 2, Table 3). Within the male subjects the off-medication group had significantly lower expression compared to the on-medication group for the genes encoding GABA-Aα1, GABA-Aα2, GABA-Aα3, GABA-Aβ2, GABA-Aγ2, and GAD67 (Figure 2).
Figure 2. Medication appears to ‘correct’ GABAergic deficits in the ACC in male patients with schizophrenia.
The relative expression of each GABA-A receptor subunit and GAD67 is compared in schizophrenia subjects on and off medication at the time of death. Male schizophrenia subjects on medication have a generalized increase in the expression of the majority of genes tested relative to the patients off medication. Post-hoc analyses reveal significant alterations in the expression levels of specific genes; the off medication group had significantly lower expression compared with both the controls and the on medication groups for the genes encoding GABA-Aα1 (p=0.005 and 0.006 respectively), GABA-Aα2 (p=0.010 and 0.005), GABA-Aα3 (p=0.003 and 0.007), GABA-Aγ2 (p=0.004 and 0.008), and GAD67 (p=0.005 and 0.013); expression in the control group was higher than both the on and off medication schizophrenia groups for GABA-Aα5 (p=0.012 for both) and GABA-Aβ1 (p=0.023 and 0.024 respectively), medicated schizophrenia cases showed higher expression of GABA-Aβ2 than the off medication group (p=0.015), the control group had higher expression of GABA-Aγ3 (p=0.050) and GABA-Aε (p=0.049) than the medicated schizophrenia group, and there were no changes of expression for GABA-Aδ and GABA-Aγ1. Values shown are mean + SEM of the expression of each target gene normalized to the geometric mean of three housekeeping genes (GUSB, PPIA, and GAPDH), measured by qPCR. * indicates p≤0.05; ** indicates p≤0.01.
Table 3.
GABAergic gene expression in the ACC – effects of medication in male subjects
| Control N=5 |
Schizophrenia Off Medication N=6 |
Schizophrenia On Medication N=8 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Protein | Mean | SEM | Mean | SEM | Mean | SEM | F2,13 | P |
| GABRA1 | GABA-Aα1 | 1.20 | 0.11 | 0.59 | 0.11 | 1.04 | 0.08 | 6.67 | 0.010* |
| GABRA2 | GABA-Aα2 | 2.29 | 0.20 | 1.34 | 0.19 | 2.17 | 0.14 | 6.33 | 0.012* |
| GABRA3 | GABA-Aα3 | 1.74 | 0.21 | 0.50 | 0.20 | 1.31 | 0.15 | 7.40 | 0.007* |
| GABRA5 | GABA-Aα5 | 1.16 | 0.10 | 0.68 | 0.10 | 0.80 | 0.07 | 5.19 | 0.022* |
| GABRB1 | GABA-Aβ1 | 1.08 | 0.08 | 0.75 | 0.08 | 0.83 | 0.06 | 3.98 | 0.045* |
| GABRB2 | GABA-Aβ2 | 3.03 | 0.39 | 1.92 | 0.37 | 3.24 | 0.27 | 3.95 | 0.046* |
| GABRD | GABA-Aδ | 0.90 | 0.21 | 0.74 | 0.20 | 0.99 | 0.15 | 0.49 | 0.625 |
| GABRE | GABA-Aε | 0.57 | 0.13 | 0.31 | 0.12 | 0.25 | 0.09 | 2.36 | 0.134 |
| GABRG1 | GABA-Aγ1 | 0.89 | 0.11 | 0.79 | 0.10 | 0.61 | 0.08 | 2.84 | 0.095 |
| GABRG2 | GABA-Aγ2 | 1.70 | 0.19 | 0.69 | 0.18 | 1.40 | 0.13 | 6.67 | 0.010* |
| GABRG3 | GABA-Aγ3 | 1.28 | 0.08 | 1.21 | 0.08 | 1.08 | 0.05 | 2.96 | 0.088 |
| GAD1 | GAD67 | 1.96 | 0.20 | 0.90 | 0.19 | 1.59 | 0.14 | 6.14 | 0.013* |
Control subjects have no history of psychiatric disorder and no antipsychotic medication treatment; schizophrenia subjects are grouped based on whether or not they were on medication at time of death. Data were analyzed by univariate ANOVA with PMI, pH, and age of death included as covariates.
= p≤0.05.
4. Discussion
This is the first report of sex-specific changes of GABAergic gene expression in the ACC in schizophrenia. While the majority of GABAergic genes had reduced expression in the ACC of the male schizophrenia group relative to the male comparison subjects, there appeared to be an overall increase in the female schizophrenia group relative to female comparison group. Statistically significant reductions in the expression levels of GABA-Aα5, β1, and ε genes (GABRA5, GABRB1, and GABRE respectively) were observed in the male schizophrenia group (Figure 1A). In contrast, gene expression was significantly increased for GABA-Aβ1 and GAD67 (encoded by GABRB1and GAD1 respectively) in the female patients relative to the female comparison group (Figure 1B). Therefore our data support previous findings of GABAergic dysfunction in schizophrenia and emphasize the importance of considering sex when investigating the pathophysiology of schizophrenia by revealing significant sex differences in the relative expression of these GABAergic genes in the ACC of males and females with schizophrenia.
Although the differential expression of specific GABA-A receptor subunits has been previously reported in schizophrenia, these differences have not been consistent between the studies conducted. For example, our findings in the ACC in males of decreased expression of the GABA-Aα5 subunit in schizophrenia have previously been reported in the prefrontal cortex in schizophrenia (Duncan et al., 2010), specifically in layer 4 (Beneyto et al., 2011). Conversely, an increase in GABA-Aα5 expression has been reported in the same region (Impagnatiello et al., 1998). Targeting this GABA receptor subunit may be therapeutic in schizophrenia. Indeed, a GABA-A receptor positive allosteric modulator that binds to the GABA-Aα5 subunit, has been shown to reverse dopaminergic hyperactivation in a rodent model of schizophrenia (Gill et al., 2011).
The abnormal expression of GABA-Aα5 may contribute to the cognitive symptoms experienced by patients with schizophrenia. The GABA-Aα5 subunit is reported to play a role in spatial memory (Collinson et al., 2002), although global ablation of this subunit in GABRA5 knockout mice resulted in improved performance in memory and learning (Olsen and Sieghart, 2008). These data indicate that altered expression of GABA-Aα5 may disrupt the pathways necessary for optimal cognitive function.
The GABA-A receptors containing the α5 subunit are located at extrasynaptic sites (Brunig et al., 2002), and these extrasynaptic GABA-A receptors are required for optimal tonic inhibitory function (Brickley and Mody, 2012; Yamada et al., 2007). Impaired tonic inhibition has previously been implicated in schizophrenia. The δ subunit of the GABA-A receptor which, like GABA-Aα5, is also predominantly within extrasynaptic GABA receptors (Zheleznova et al., 2009), is also reported to have lower expression in schizophrenia (Maldonado-Aviles et al., 2009). Moreover our data indicated reduced levels of GABA-Aε expression in the ACC of males with schizophrenia. The role of the GABA-Aε subunit in behavior is relatively unknown, but this subunit appears to confer insensitivity to anesthesia (Thompson et al., 2002) and to treatment with benzodiazepine drugs (Belujon et al., 2009; Kasparov et al., 2001; Thompson et al., 1998). GABA-A receptors containing the GABA-Aε subunit are unusual because they exhibit spontaneous activity (Neelands et al., 1999). Furthermore, there have been indications that receptors containing GABA-Aε are located extrasynaptically and contribute to tonic inhibition (Li et al., 2013). Therefore our GABA-Aα5 and GABA-Aε findings lend further support to the notion that altered tonic inhibition by GABA may contribute to the pathophysiology of schizophrenia (Maldonado-Aviles et al., 2009).
Reduced GAD67 expression is a consistent finding in schizophrenia (Akbarian et al., 1995; Curley et al., 2011; Duncan et al., 2010; Guidotti et al., 2000; Hashimoto et al., 2008a; Hashimoto et al., 2008b; Hashimoto et al., 2005; Kimoto et al., 2014; Thompson et al., 2009; Volk et al., 2000; Woo et al., 2004), however, we observed increased GAD67 expression in the ACC of the female schizophrenia group. It is possible that this difference may be due to medication, because we observed that the expression of GAD67 was significantly higher in patients who were on medication compared with those who were off medication (Figure 2; Table 3). Indeed, elevated GAD67 expression has been detected in the ACC of rodents after treatment with antipsychotic drugs (Zink et al., 2004a; Zink et al., 2004b).
The differential gene expression in males and females with schizophrenia may contribute to the sex differences observed in this disorder. While the frequency of schizophrenia is similar for both sexes (Saha et al., 2005), male patients have an earlier age of onset compared with female patients (Eranti et al., 2013; Faraone et al., 1994; Goldstein et al., 1990; Leung and Chue, 2000; Szymanski et al., 1995; van der Werf et al., 2014). Sex differences have also been noted in response to antipsychotic treatment (Smith, 2010; Usall et al., 2007), perhaps because male schizophrenia patients exhibit a greater level of negative symptoms which are relatively unresponsive to current antipsychotic treatments (Chue and Lalonde, 2014). The increased levels of GABA-Aβ1 (and perhaps also GAD67) in the ACC of female patients may contribute to their increased vulnerability to depressive symptoms compared with male schizophrenia patients (Andia et al., 1995; Goldstein and Link, 1988; Hafner, 2003).
As with any study using postmortem human tissue, there are limitations to this work. Firstly, while the sample size has sufficient power to detect relatively large effect sizes when the entire group was considered, this statistical power would be reduced after subgrouping the subjects by sex. This postmortem cohort differs from others previously tested because there is very little substance abuse in these subjects, and a relatively high average age (Table 1). It is beyond the scope of the current study to determine whether the differences reported here are also present in the earlier stages of schizophrenia. The results were also not corrected for multiple comparisons. Therefore these findings may require further validation through replication in other postmortem schizophrenia cohorts.
Potential confounding effects of antipsychotic medication in the schizophrenia group were explored by comparing male patients who were medicated with male patients who were unmedicated at their time of death. Treatment of male rats with haloperidol and clozapine leads to increased expression of GAD67 and GABA-A receptors in the ACC (Zink et al., 2004a; Zink et al., 2004b). Our data indicate that a similar effect may occur in human subjects treated with antipsychotic drugs (Figure 2). Higher expression levels of GAD67 and the GABA-A subunits α1, α2, α3, β2, and γ2 were detected in patients who were on-medication while male patients who were off-medication had lower levels of GABAergic gene expression. Therefore, antipsychotic medication may ’correct’ the expression levels of GABAergic genes to the levels observed in the male controls (Figure 2; Table 3). It was beyond the scope of this study to fully determine whether medication was the sole cause of differences in GABAergic gene expression observed, and we were also unable to determine the effect of medication in females because only two female schizophrenia cases were off medication at time of death. Therefore the extent to which GABAergic targets are modulated by currently prescribed antipsychotic medication requires further investigation. Given the sex differences reported here, future investigations of the effects of medication on GABAergic gene expression in females could reveal interesting data.
Our ongoing studies will determine whether the differences in GABAergic gene expression we have observed are specific to particular cell types or cortical layers. The current data provided an overview of GABAergic gene expression across the cortex because we included all gray matter layers in our analyses. Previous studies have indicated differential GABA receptor binding in schizophrenia in specific cortical layers of the ACC (Benes et al., 1992).
In summary, our data show that sex differences in the expression of GABAergic genes occur in the anterior cingulate cortex in schizophrenia, and that antipsychotic medications may influence the regulation of GABAergic genes in this critical brain region. These data indicate that molecular pathways including the GABAergic genes contribute to the pathophysiology and treatment of schizophrenia, and provide further support to the notion that the GABAergic system contains novel targets for antipsychotic drug development.
Acknowledgements
The authors thank Dr. Dennis Grayson for his feedback on the manuscript.
Role of the funding source
This work was funded by the UIC Collaborative Engagement in Novel Therapeutic Research and Enterprise award to MS, and NIH R01 awards MH066392 and MH064673 to VH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MS designed the study, VH provided the post-mortem tissue used in the study, JB performed the experimental procedures and wrote the initial draft of the manuscript. GB conducted the statistical analyses, GB and MS wrote the manuscript, and VH provided critical comments. All authors approve the final manuscript.
Conflicts of Interest
All authors declare they have no conflicts of interest
References
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Andia AM, Zisook S, Heaton RK, Hesselink J, Jernigan T, Kuck J, Morganville J, Braff DL. Gender differences in schizophrenia. J Nerv Ment Dis. 1995;183(8):522–528. doi: 10.1097/00005053-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Belujon P, Baufreton J, Grandoso L, Boue-Grabot E, Batten TF, Ugedo L, Garret M, Taupignon AI. Inhibitory transmission in locus coeruleus neurons expressing GABAA receptor epsilon subunit has a number of unique properties. J Neurophysiol. 2009;102(4):2312–2325. doi: 10.1152/jn.00227.2009. [DOI] [PubMed] [Google Scholar]
- Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology. 2010;35(1):239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci. 1992;12(3):924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Marie A, Khan Y. Up-regulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75(4):1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21(5):999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Lewis DA. Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 2011;29(3):295–304. doi: 10.1016/j.ijdevneu.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73(1):23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443(1):43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Chue P, Lalonde JK. Addressing the unmet needs of patients with persistent negative symptoms of schizophrenia: emerging pharmacological treatment options. Neuropsychiatr Dis Treat. 2014;10:777–789. doi: 10.2147/NDT.S43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol Psychiatry. 2006;11(5):459–470. 423. doi: 10.1038/sj.mp.4001806. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22(13):5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012;(213):267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168(9):921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44(10):673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Eranti SV, MacCabe JH, Bundy H, Murray RM. Gender difference in age at onset of schizophrenia: a meta-analysis. Psychol Med. 2013;43(1):155–167. doi: 10.1017/S003329171200089X. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Chen WJ, Goldstein JM, Tsuang MT. Gender differences in age at onset of schizophrenia. Br J Psychiatry. 1994;164(5):625–629. doi: 10.1192/bjp.164.5.625. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35(5):973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejman PV, Sanders AR, Kendler KS. Genetics of schizophrenia: new findings and challenges. Annu Rev Genomics Hum Genet. 2011;12:121–144. doi: 10.1146/annurev-genom-082410-101459. [DOI] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel alpha5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Link BG. Gender and the expression of schizophrenia. J Psychiatr Res. 1988;22(2):141–155. doi: 10.1016/0022-3956(88)90078-7. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Santangelo SL, Simpson JC, Tsuang MT. The role of gender in identifying subtypes of schizophrenia: a latent class analytic approach. Schizophr Bull. 1990;16(2):263–275. doi: 10.1093/schbul/16.2.263. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180(2):191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hanada S, Mita T, Nishino N, Tanaka C. [3H]muscimol binding sites increased in autopsied brains of chronic schizophrenics. Life Sci. 1987;40(3):259–266. doi: 10.1016/0024-3205(87)90341-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008a;13(2):147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008b;165(4):479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25(2):372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95(26):15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Hidaka S, Asada T. Immunohistochemical and immunoblot study of GABA(A) alpha1 and beta2/3 subunits in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci Res. 2004;50(1):77–84. doi: 10.1016/j.neures.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull. 2012;38(5):911–913. doi: 10.1093/schbul/sbs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparov S, Davies KA, Patel UA, Boscan P, Garret M, Paton JF. GABA(A) receptor epsilon-subunit may confer benzodiazepine insensitivity to the caudal aspect of the nucleus tractus solitarii of the rat. J Physiol. 2001;536(Pt 3):785–796. doi: 10.1111/j.1469-7793.2001.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto S, Bazmi HH, Lewis DA. Lower Expression of Glutamic Acid Decarboxylase 67 in the Prefrontal Cortex in Schizophrenia: Contribution of Altered Regulation by Zif268. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbendam L, O'Daly O, Morley LA, van Os J, Murray RM, Shergill SS. Using the Stroop task to investigate the neural correlates of symptom change in schizophrenia. Br J Psychiatry. 2009;194(4):373–374. doi: 10.1192/bjp.bp.108.055459. [DOI] [PubMed] [Google Scholar]
- Kuepper R, Skinbjerg M, Abi-Dargham A. The dopamine dysfunction in schizophrenia revisited: new insights into topography and course. Handb Exp Pharmacol. 2012;(212):1–26. doi: 10.1007/978-3-642-25761-2_1. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Li ZX, Yu HM, Jiang KW. Tonic GABA inhibition in hippocampal dentate granule cells: its regulation and function in temporal lobe epilepsies. Acta Physiol (Oxf) 2013;209(3):199–211. doi: 10.1111/apha.12148. [DOI] [PubMed] [Google Scholar]
- Lo WS, Harano M, Gawlik M, Yu Z, Chen J, Pun FW, Tong KL, Zhao C, Ng SK, Tsang SY, Uchimura N, Stober G, Xue H. GABRB2 association with schizophrenia: commonalities and differences between ethnic groups and clinical subtypes. Biol Psychiatry. 2007;61(5):653–660. doi: 10.1016/j.biopsych.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Lo WS, Lau CF, Xuan Z, Chan CF, Feng GY, He L, Cao ZC, Liu H, Luan QM, Xue H. Association of SNPs and haplotypes in GABAA receptor beta2 gene with schizophrenia. Mol Psychiatry. 2004;9(6):603–608. doi: 10.1038/sj.mp.4001461. [DOI] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O'Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166(4):450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW, Horiguchi M. Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr Pharm Biotechnol. 2012;13(8):1572–1586. doi: 10.2174/138920112800784880. [DOI] [PubMed] [Google Scholar]
- Mengual L, Burset M, Marin-Aguilera M, Ribal MJ, Alcaraz A. Multiplex preamplification of specific cDNA targets prior to gene expression analysis by TaqMan Arrays. BMC Res Notes. 2008;1:21. doi: 10.1186/1756-0500-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Krystal JH. Capturing the angel in "angel dust": twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38(5):942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelands TR, Fisher JL, Bianchi M, Macdonald RL. Spontaneous and gammaaminobutyric acid (GABA)-activated GABA(A) receptor channels formed by epsilon subunit-containing isoforms. Mol Pharmacol. 1999;55(1):168–178. doi: 10.1124/mol.55.1.168. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93(2):441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered vesicular glutamate transporter expression in the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2008;63(8):766–775. doi: 10.1016/j.biopsych.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O'Donovan MC. Suggestion of roles for both common and rare risk variants in genome-wide studies of schizophrenia. Arch Gen Psychiatry. 2010;67(7):667–673. doi: 10.1001/archgenpsychiatry.2010.69. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Middleton FA, Tahl AR, Rockwell GN, Purcell S, Aldinger KA, Kirby A, Morley CP, McGann L, Gentile KL, Waggoner SG, Medeiros HM, Carvalho C, Macedo A, Albus M, Maier W, Trixler M, Eichhammer P, Schwab SG, Wildenauer DB, Azevedo MH, Pato MT, Pato CN, Daly MJ, Sklar P. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol Psychiatry. 2005;10(12):1074–1088. 1057. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Sodhi M, Kleinman JE. Epigenetic mechanisms in schizophrenia. Biochim Biophys Acta. 2009;1790(9):869–877. doi: 10.1016/j.bbagen.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Schizophrenia and dopamine receptors. Eur Neuropsychopharmacol. 2013;23(9):999–1009. doi: 10.1016/j.euroneuro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Smith S. Gender differences in antipsychotic prescribing. Int Rev Psychiatry. 2010;22(5):472–484. doi: 10.3109/09540261.2010.515965. [DOI] [PubMed] [Google Scholar]
- Sodhi M, Wood KH, Meador-Woodruff J. Role of glutamate in schizophrenia: integrating excitatory avenues of research. Expert Rev Neurother. 2008;8(9):1389–1406. doi: 10.1586/14737175.8.9.1389. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Simmons M, McCullumsmith R, Haroutunian V, Meador-Woodruff JH. Glutamatergic gene expression is specifically reduced in thalamocortical projecting relay neurons in schizophrenia. Biol Psychiatry. 2011;70(7):646–654. doi: 10.1016/j.biopsych.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AD, Lewis DA. Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr Pharm Biotechnol. 2012;13(8):1557–1562. doi: 10.2174/138920112800784925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12(9):854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, Chakos M, Koreen A, Jody D, Kane J, et al. Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry. 1995;152(5):698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43(11):970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Bonnert TP, Cagetti E, Whiting PJ, Wafford KA. Overexpression of the GABA(A) receptor epsilon subunit results in insensitivity to anaesthetics. Neuropharmacology. 2002;43(4):662–668. doi: 10.1016/s0028-3908(02)00162-4. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Bonnert TP, Whiting PJ, Wafford KA. Functional characteristics of recombinant human GABA(A) receptors containing the epsilon-subunit. Toxicol Lett. 1998;100–101:233–238. doi: 10.1016/s0378-4274(98)00190-8. [DOI] [PubMed] [Google Scholar]
- Uher R. Gene-environment interactions in severe mental illness. Front Psychiatry. 2014;5:48. doi: 10.3389/fpsyt.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usall J, Suarez D, Haro JM. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153(3):225–231. doi: 10.1016/j.psychres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- van der Werf M, Hanssen M, Kohler S, Verkaaik M, Verhey FR, van Winkel R, van Os J, Allardyce J. Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol Med. 2014;44(1):9–16. doi: 10.1017/S0033291712002796. [DOI] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102(6):2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12(10):1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Woo TU, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Shrestha K, Amstrong C, Minns MM, Walsh JP, Benes FM. Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr Res. 2007;96(1–3):46–61. doi: 10.1016/j.schres.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Yamada J, Furukawa T, Ueno S, Yamamoto S, Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb Cortex. 2007;17(8):1782–1787. doi: 10.1093/cercor/bhl087. [DOI] [PubMed] [Google Scholar]
- Yu Z, Chen J, Shi H, Stoeber G, Tsang SY, Xue H. Analysis of GABRB2 association with schizophrenia in German population with DNA sequencing and one-label extension method for SNP genotyping. Clin Biochem. 2006;39(3):210–218. doi: 10.1016/j.clinbiochem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zai CC, Tiwari AK, King N, De Luca V, Mueller DJ, Shaikh S, Wong GW, Meltzer HY, Lieberman JA, Kennedy JL. Association study of the gamma-aminobutyric acid type a receptor gamma2 subunit gene with schizophrenia. Schizophr Res. 2009;114(1–3):33–38. doi: 10.1016/j.schres.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Zhao X, Qin S, Shi Y, Zhang A, Zhang J, Bian L, Wan C, Feng G, Gu N, Zhang G, He G, He L. Systematic study of association of four GABAergic genes: glutamic acid decarboxylase 1 gene, glutamic acid decarboxylase 2 gene, GABA(B) receptor 1 gene and GABA(A) receptor subunit beta2 gene, with schizophrenia using a universal DNA microarray. Schizophr Res. 2007;93(1–3):374–384. doi: 10.1016/j.schres.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Zheleznova NN, Sedelnikova A, Weiss DS. Function and modulation of delta-containing GABA(A) receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S67–S73. doi: 10.1016/j.psyneuen.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M, Schmitt A, May B, Muller B, Braus DF, Henn FA. Differential effects of long-term treatment with clozapine or haloperidol on GABA transporter expression. Pharmacopsychiatry. 2004a;37(4):171–174. doi: 10.1055/s-2004-827173. [DOI] [PubMed] [Google Scholar]
- Zink M, Schmitt A, May B, Muller B, Demirakca T, Braus DF, Henn FA. Differential effects of long-term treatment with clozapine or haloperidol on GABAA receptor binding and GAD67 expression. Schizophr Res. 2004b;66(2–3):151–157. doi: 10.1016/S0920-9964(03)00088-4. [DOI] [PubMed] [Google Scholar]