Abstract
Background
Sleep disturbance is associated with activation of systemic and cellular inflammation, as well as pro-inflammatory transcriptional profiles in circulating leukocytes. Whether treatments that target insomnia-related complaints might reverse these markers of inflammation in older adults with insomnia is not known.
Methods
In this randomized trial, 123 older adults with insomnia were randomly assigned to cognitive behavioral therapy for insomnia (CBT-I), tai chi chih (TCC), or sleep seminar education active control condition (SS) for two hour sessions weekly over 4 months with follow-up at 7- and 16-months. We measured C-reactive protein (CRP) at baseline, month 4 and 16, Toll-like receptor-4 (TLR-4)-activated monocyte production of proinflammatory cytokines at baseline, month 2, 4, 7, and 16, and genome-wide transcriptional profiling at baseline and month 4.
Results
As compared to SS active control, CBT-I reduced levels of CRP (month 4, 16, P’s<0.05), monocyte production of proinflammatory cytokines (month 2 only, P<0.05), and pro-inflammatory gene expression (month 4, P<0.01). TCC marginally reduced CRP (month 4, P=0.06), and significantly reduced monocyte production of proinflammatory cytokines (month 2, 4, 7, 16, all P’s<0.05) and proinflammatory gene expression (month 4, P<0.001). In CBT and TCC, TELIS promoter-based bioinformatics analyses indicated reduced activity of nuclear factor (NF)-κB and AP1.
Conclusions
Among older adults with insomnia, CBT-I reduced systemic inflammation, TCC reduced cellular inflammatory responses, and both treatments reduced expression of genes encoding proinflammatory mediators. The findings provide an evidence-based molecular framework to understand the potential salutary effects of insomnia treatment on inflammation, with implications for inflammatory disease risk.
Keywords: Insomnia, Inflammation, Cognitive behavioral therapy, Tai Chi, Gene expression, Aging
INTRODUCTION
Insomnia, diagnosed by difficulties in initiating sleep, frequent awakenings, or inability to return to sleep, which are associated with daytime impairments (1), occurs in over 15% of older adults (2). Given that poor sleep prospectively predicts depression (3–5), chronic disease risk (6), and mortality (7), increasing attention has focused on the association between sleep disturbance and inflammation (8). Activation of cellular signals that initiate the production of inflammatory cytokines and markers of systemic inflammation (i.e., C-reactive protein, CRP) are associated with risk of depression (9), and a wide spectrum of medical conditions (10–14).
The causal relationship between insomnia and inflammation remains unclear. Exogenously triggered activation of inflammation induces depressive symptoms (15, 16) and also alters sleep in humans (17, 18). Conversely, insomnia is associated with elevated levels of proinflammatory cytokines (8, 19). Indeed, decreases in sleep duration are prospectively associated with increases in CRP (20), and experimental sleep disruption induces increases in CRP)(21), increases in cellular inflammation (22, 23), and increases in the expression of inflammatory response genes (23) via activation of the transcription factor, nuclear factor (NF)-κB (24). In the present study, we sought to determine whether two experimental interventions that improve insomnia symptoms (25), might reduce systemic and cellular markers of inflammation and reverse inflammatory gene expression and activation of transcriptional signaling.
In persons experiencing significant life adversity, cognitive behavioral stress management, as well as meditation, can at least partially reverse the pattern of leukocyte proinflammatory transcriptional alterations associated with stress (26–28). However, these small randomized controlled trials have not targeted patients with insomnia, nor comprehensively captured a vertically integrated assessment of inflammation including systemic levels (e.g., CRP), upstream cellular production of proinflammatory cytokines (e.g, Toll-like receptor (TLR)-4 activation of monocytic production of proinflammatory cytokines), and gene expression with promoter based bioinformatics analyses of several specific transcription factors (TF). TLR-4 activation mediates innate immune responses to common pathogens (29), and aberrant increases of TLR-4 activity are linked to inflammatory (30) and cardiovascular disease (31).
Cognitive behavioral therapy for insomnia (CBT-I), a multi-component behavioral intervention that provides sleep education, stimulus control (strengthening associations between bed and sleep), and therapy for anxiety-provoking beliefs about sleep, primarily targets sleep behaviors with effects on arousal mechanisms. CBT is an effective treatment for insomnia in older adults (25, 32) and adults (33), with an efficacy that is better sustained than pharmacotherapy (34). As a comparison to CBT-I, Tai Chi Chih (TCC), a westernized version of Tai Chi (35–37), is thought primarily to target arousal mechanisms with secondary effects on insomnia (38–40) which in turn decreases sympathetic activation and related inflammation (41, 42). TCC improves sleep quality (43–45), and reduces inflammation in older adults (25, 46–49).
In a randomized controlled, comparative efficacy trial over 4 months with follow-up at 7 and 16 months in 123 older adults with insomnia, we previously reported that CBT and TCC were associated with improvements in sleep quality, fatigue, and depressive symptoms as compared to an active control, sleep seminar (SS) (25). In addition, remission of insomnia was associated with reduced proportion of having high CRP (>3.0 pg/ml) at month 16. SS controlled for non-specific factors (e.g., expectation, group, and attention). Given evidence linking sleep disturbance, as well as related arousal mechanisms to inflammatory dynamics (8), we hypothesize that both CBT-I and TCC, in this same sample (n=123), will reverse increases in levels of CRP, increases in TLR-4 induced activation of monocyte production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF), and increases in pro-inflammatory gene expression programs and the specific pattern of bioinformatically inferred TF activation (i.e., increased activity of NF-κB/Rel and AP1 TFs) relative to SS. Because levels of CRP are relatively stable and changes in CRP in response to behavioral interventions (i.e., exercise) are found after year-long, not months, administration (50–52), CRP was measured at baseline, month 4 (post-intervention), and month 16. In contrast, because changes in TLR4 induced monocytic production in IL-6 and TNF have been found immediately after sleep disturbance (23), this marker was measured at baseline, month 2 (mid-intervention), and month 4, 7, and 16. Post-intervention differences in the expression of a priori defined pro-inflammatory gene programs and bioinformatically inferred TF activation were tested in a subsample.
METHODS
Participants
This randomized controlled trial was conducted from April 2006 to August 2011 with UCLA IRB approval. As described (25), 123 community-dwelling adults older than 55 years of age who fulfilled criteria for primary insomnia in Diagnostic and Statistical Manual (Fourth Edition, Text Revision)(DSMIV-TR)(53) and for general insomnia in the International Classification of Sleep Disorders (Second Edition)(54) were randomly assigned to CBT-I, TCC, or SS (2:2:1). Complete inclusion criteria are provided in Supplement.
Interventions
Each group participated in 120-minutes of class time weekly for 4 months with 7- and 16 months follow-up. CBT-I was modified to teach behavioral strategies for management of daytime activity levels and enhancement of mood (33). TCC, a movement meditation, emphasized control over arousal mechanisms, which are thought to contribute to insomnia (55, 56). Sleep Seminar (SS) provided sleep hygiene information and education about physical, medical, and psychosocial factors in relation to aging and insomnia. Supplement provides information about the treatments, acceptance, credibility, and expectation for change (25).
Outcomes
Insomnia outcomes included remission of insomnia diagnosis by DSM-IV-TR criteria using a structured interview and checklist, and improvements in patient-reported outcomes of insomnia symptom severity and daily sleep diaries (25). In this study, assessment of inflammation included three levels of analysis: systemic (i.e. CRP levels), cellular (i.e., TLR-4 activation of monocytic production of inflammatory cytokines TNF and IL-6) and genomic (i.e., gene expression and bioinformatics analyses of transcription pathways). Prior to each blood sampling, subjects were queried about recent (i.e., last month) infection, illness, or vaccination, and sampling was re-scheduled if subjects reported any one of these issues. All blood samples were non-fasting and collected between 8–10 a.m.
CRP levels were measured (25). To clarify the functional basis for altered CRP, production of proinflammatory cytokines by monocytes following ligation of the Toll-like receptor 4 (TLR4) with lipopolysaccharide (LPS) was assessed (23). To evaluate the upstream sources of cellular inflammatory cytokine expression, RNA from peripheral blood mononuclear cells (PBMC) were collected for evaluation of gene expression profiling and bioinformatic analysis in a random subsample at 4 months for comparison of CBT-I or TCC, relative to SS (n=78). Additionally, we determined whether gene expression profiles were comparable in three groups at baseline prior to intervention (n=24), given the effects of sleep on inflammatory gene expression (23). RNA was extracted (Qiagen PAXgene Blood RNA Kit; Valencia CA), and subject to genome-wide transcriptional profiling using Illumina HT-12 v4 BeadArrays following the manufacturer’s standard protocol (Illumina Inc., San Diego CA). Quantilenormalized gene expression values were log2-tranformed and subject to general linear model analysis to provide maximum likelihood point estimates of differential transcript abundance across conditions, which provide maximally replicable inputs into the 2 higher-order set-based bioinformatics analyses (57–59). TELiS promoter-based bioinformatics analyses tested the hypothesis that PMBC from older adults with insomnia who were randomized to either CBT or TCC, relative to SS, would show alterations in global gene expression profiles consistent with decreased activity of the pro-inflammatory transcription factors NF-κB (assessed by prevalence of the TRANSFAC V$NFKAPPAB65_01 nucleotide motif in differentially expressing promoters) and AP-1 (V$AP1FJ_Q2). We also explored whether these treatments increased activity of Type I interferon signaling pathways (V$ISRE_01, V$IRF2_01); increased activity of the anti-inflammatory glucocorticoid receptor (GR) (V$GR_Q6), and decreased activity of CREB transcription factors involved in β-adrenergic signaling by the sympathetic nervous system (SNS) (V$CREB_01), because sleep is implicated in the regulation of these pathways (60). The ratio of response element frequencies in the promoters of up- vs. down-regulated genes was taken as a measure of differential activity of transcription control pathways, and (log) ratios were averaged over 9 different parametric combinations of promoter length (−300, −600, and −1000 to +200 bp upstream of RefSeq-designated transcription start site) and motif detection stringency (TRANSFAC mat_sim values of .80, .90, and .95) to ensure robust results (61). To identify the primary cellular sources of differentially expressed genes, we carried out Transcript Origin Analysis (62). In both TELiS and Transcript Origin Analyses, standard errors were estimated by 2000 cycles of bootstrap resampling of residual vectors from the linear models used to estimate differential gene expression across groups (controlling for correlated expression across genes).
Because body mass index (BMI) and physical activity (63) are related to inflammation, changes were evaluated.
Sample size
For circulating markers of inflammation (i.e., CRP), mind-body treatments reduced circulating markers of inflammation with an effect size 0.91 (46, 48, 49), 40 per treatment group provides statistical power of 80% (α = 0.05). For TLR-4 activation of monocytic production of cytokines, TCC reduced production of proinflammatory cytokines with an effect size of .92 (64); 40 per treatment group provides statistical power of greater than 80% (α = 0.05). For the gene expression outcome, mind-body treatments altered transcriptional profiling with an effect size .98 (26, 27, 65); 15 per treatment group provides statistical power of greater than 80%. Sleep disturbance altered gene expression of IL-6 and TNF with effect sizes of 2.06 and 2.59 (α = 0.05)(23); 5 per treatment group provides statistical power of greater than 80% for baseline comparison.
Statistical Methods
Intervention effects on CRP levels and TLR-4 activation of monocyte production of proinflammatory cytokines were tested on an intention-to-treat basis using a mixed model analysis of variance (ANOVA) approach without a covariance structure assumption given the variability in time between measures; data from all randomized participants were included with no imputation of missing data. The mixed model approach utilizes all available data and generates unbiased estimates under the assumption that data are missing completely at random (MCAR). In cases where there were any significant or trend (P<0.10) pairwise differences at baseline among the three groups, the model was adjusted for baseline to better assess relative differences at during and after treatment; this applied to TLR-4 activation of monocytic inflammatory cytokines production values but not CRP values. A priori contrasts (Least Significant Difference [LSD] test) tested group differences in CRP at month 4 and 16, whereas group differences in TLR-4 activation of monocytic production of proinflammatory cytokines were tested at month 2, 4, 7, and 16 Differentially expressed genes were identified based on 1.2 fold magnitude difference in average gene expression between treatment groups at baseline (n=24; CBT, n=8; TCC, n=8; SS, n=8) and at 4 months (n=78; CBT-I, n=31; TCC, n=32; SS, n=15). Data were available on >95% of the subjects at all timepoints among those who completed follow-up assessments. Analyses were carried out with IBM SPSS for Windows, version 22.
RESULTS
Baseline Characteristics of the Patients
A total of 294 subjects underwent baseline assessment, 207 were eligible, and 123 completed baseline (Figure S1; Supplement). Treatment groups were comparable with regards to background characteristics (Table 1); none of these variables was included as a covariate. A total of 112 (92%) participants completed the assigned interventions (month 4), and 108 (89%) completed follow-up (month 16). Those who did not complete the intervention were younger (t(121)=2.12; p<0.05), and had higher scores on the PSQI (t(121)=1.72; p=0.09) and MDFSI (t(121)=1.78; p=0.05), whereas those who did not complete the follow-up had higher scores on the IDS-C (t(110)=2.82; p=0.05) and MDFSI (t(110)=3.23; p<0.001). Other demographic and outcome variables did not differ between the completers and noncompleters at months 4 or 16. Retention rate was similar between groups (χ2(2)=3.16; p=0.21). The observed pattern of missing data did not violate the MCAR assumption (χ2(394)=308.7; p=0.68). Average rate of session attendance was similar (F(2,120)=0.99; p=0.38), and the three interventions were perceived as similarly acceptable at baseline (χ2(2)=0.58; p=0.74) and month 4 (χ2(2)=3.58; p=0.17). Among the TCC participants, frequency of practice for >30 minutes decreased from 3.3 (SD, 2.2) days to 2.3 (SD, 2.0) days (t(33)=3.16; p=0.004) from months 4 to 16. There were no significant changes from baseline to month 4 in BMI (F(2,90.9)=1.71; p=0.19), or physical activity (i.e., metabolic equivalents per week (F(2,104.8)=1.79; p = 0.17). Only two subjects reported antidepressant medications use.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of Participants*
| Variable | CBT (N=50) |
TCC (N=48) |
SS (N=25) |
F or χ2; p |
|---|---|---|---|---|
| Age (55 to 85 years), mean (SD) | 64.4 (6.1) | 66.3 (7.4) | 66.4 (7.7) | 1.16; 0.32 |
| Female, No. (%) | 39 (78.0) | 31 (64.6) | 18 (72.0) | 2.17; 0.34 |
| Ethnicity, Hispanic, No. (%) | 3 (6.0) | 3 (6.4) | 3 (12.5) | 1.12; 0.57 |
| Race, Non-white, No. (%) | 6 (12.2) | 7 (14.9) | 4 (16.7) | 0.29; 0.86 |
| Marital status, Married, No. (%) | 25 (50.0) | 21 (43.8) | 8 (32.0) | 2.19; 0.33 |
| Employment | ||||
| Working, No. (%) | 21 (42.0) | 20 (41.7) | 10 (40.0) | 0.03; 0.98 |
| Work Hrs/wk, mean (SD) | 13.6 (18.5) | 11.7 (18.6) | 11.4 (15.3) | 0.18; 0.84 |
| Education (years), mean (SD) | 15.8 (1.4) | 15.7 (1.5) | 15.3 (1.5) | 1.26; 0.29 |
| Body mass index (kg/m2), mean (SD) | 25.4 (3.3) | 26.4 (4.0) | 26.3 (4.5) | 0.95; 0.39 |
| Co-morbidity | ||||
| Medical, No. (%)# | 19 (39.6) | 22 (48.9) | 14 (56.0) | 1.93; 0.38 |
| Depression history, No. (%) | 12 (24.0) | 12 (25.0) | 8 (32.0) | 0.60; 0.74 |
| Other psychiatric history, No. (%) | 9 (18.0) | 6 (12.5) | 3 (12.5) | 0.71; 0.70 |
| Prior hypnotic drug usage, number (%) | 12 (24.0) | 7 (14.6) | 2 (8.0) | 3.36; 0.19 |
All values are mean (sd) unless noted with percentage
Between-group differences were tested with a chi-square test for categorical variables and one-way analysis of variance for continuous variables
Among those who reported medical co-morbidity, 95% reported cardiovascular disease
Outcome of systemic inflammation
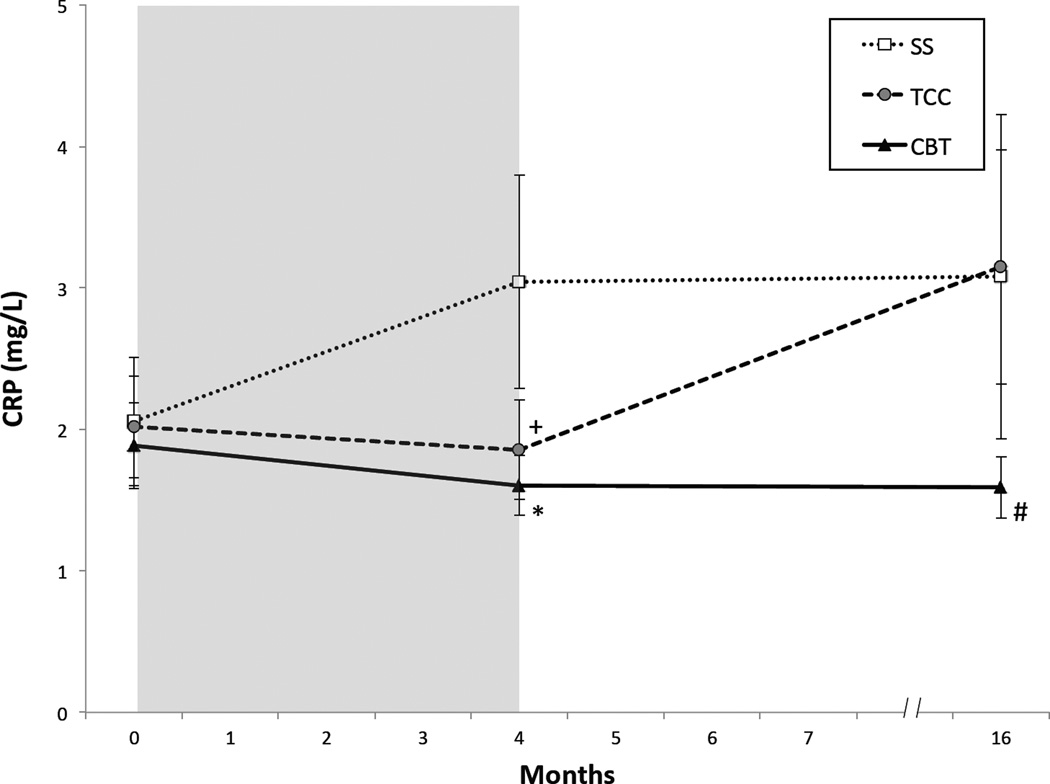
Change in mean levels of CRP in the three intervention groups from baseline to month 4 (i.e., post-intervention) and month 16 (i.e., one year follow-up) showed an overall statistical trend (group × time: F(2.108.1)=2.43, p=0.09) (Figure 1,). As compared to SS, CBT resulted in overall lower levels of CRP (t(105.4)=2.08 p=0.04). As compared to SS, TCC tended to have lower levels of CRP, but these differences were not significant at either month 4 (t(103.1)=1.92 p=0.06) or month 16 (t(97.2)=0.13 p=0.90). Whereas CBT and TCC showed similar low levels of CRP at month 4 (t(104.3)=0.59 p=0.56), CRP levels diverged at month 16; CRP levels remained low in CBT but increased in TCC (t(97.1)=1.77 p=0.08), along with sustained insomnia remission in CBT but not TCC (25).
Figure 1.
Circulating Levels of CRP from Baseline to Month 16, by Treatment Group. Values are mean (SEM). Shaded area indicates period of administration of intervention following baseline assessment. Significant pairwise comparisons: *CBT vs SS P=0.02; +TCC vs SS P=0.06, #CBT vs TCC P=0.08
Outcome of cellular inflammation
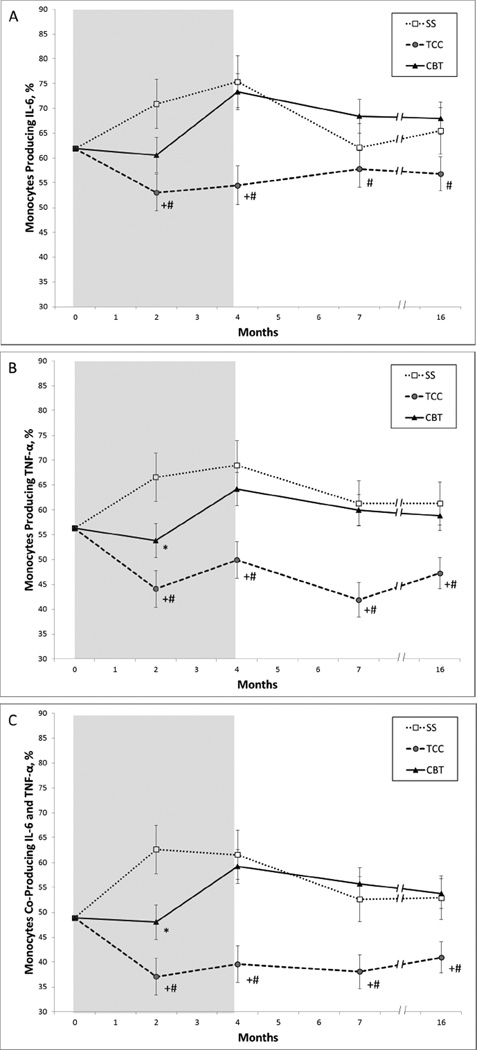
Change in mean levels of TLR-4 activated monocyte production of proinflammatory cytokines in the three intervention groups from baseline to month 2 (i.e., mid-intervention), month 4 (i.e., post-intervention), month 7 (i.e., 3 month follow-up), and month 16 (i.e., one year follow-up) was statistically significant for the percentage of monocytes producing IL-6 only (group × time F(8,91.8)=3.23, p<0.01; Figure 2A). As compared to SS, TCC resulted in overall lower levels of percentage of monocytes producing IL-6 only (t(208.3)=2.72 p<0.01), with significant differences at months 2 (t(98.0)=2.87 p=0.005) and 4 (t(97.8)=3.18 p=0.002) but not 16 (t(125.5)=1.49 p=0.14). As compared to SS, CBT-I showed a trend for lower levels of percentage of monocytes producing IL-6 at month 2 only (t(97.3)=1.70 p=0.09), but not at months 4, 7, or 16 (all P’s>0.30). As compared to CBT-I, TCC also resulted in lower levels at months 4 (t(100.3)=3.39 p=0.001), 7 (t(89.5)=2.13 p=0.04), and 16 (t(126.5)=2.38 p=0.02).
Figure 2.
Toll-like 4 Receptor Stimulated Monocytic Production from Baseline to Month 16, by Treatment Group. Values are mean (SEM) percentage of monocytes producing IL-6 (A), TNF(B); or both IL-6 and TNF(C). Shaded area indicates period of administration of intervention following baseline assessment. Significant pairwise comparisons: *CBT vs SS P<0.05; +TCC vs SS P<0.05, #CBT vs TCC P<0.05
The profile of change over time between the three groups was statistically significant for the percentage of monocytes producing TNF (group × time F(8,100.2)=3.29, p<0.01; Figure 2B). As compared to SS, TCC resulted in lower levels of percentage of monocytes producing TNF (t(271.6)=3.58 p<0.001), with significant differences at months 2 (t(100.1)=3.75 p<0.001), 4 (t(97.4)=3.18 p=0.005), 7 (t(103.2)=3.17 p<0.001) and 16 (t(125.4)=2.30 p=0.03). As compared to SS, CBT-I also resulted in lower levels of percentage of monocytes producing TNF at month 2 (t(98.9)=2.10 p=0.04), but not at months 4, 7, or 16 (all p’s>0.43). As compared to CBT-I, TCC resulted in lower levels of percentage of monocytes producing TNF (t(276.6)=3.33 p<0.001), with non significant differences at month 2 (t(104.6)=1.91 p<0.06), but significant differences at months 4 (t(99.8)=2.87 p=0.005), 7 (t(107.9)=3.66 p<0.001), and 16 (t(125.4)=2.34 p=0.03).
Finally, the profile of change over time between the three groups was statistically significant for the percentage of monocytes co-producing TNF and IL-6 (group × time F(8,101.2)=4.63, p<0.01; Figure 2C). As compared to SS, TCC resulted in lower levels of percentage of monocytes co-producing TNF and IL-6 (t(207.8)=3.79 p<0.001), with significant differences at months 2 (t(102.3)=3.74 p=0.001), 4 (t(100.9)=3.39 p=0.001), 7 (t(93.0)=2.56 p=0.02) and 16 (t(137.4)=2.24 p=0.03). As compared to SS, CBT-I also resulted in lower levels of percentage of monocytes co-producing TNF and IL-6 at month 2 (t(100.5)=2.16 p=0.02), but not at months 4, 7, or 16 (all p’s>0.57). As compared to CBT, TCC-I resulted in lower levels of percentage of monocytes co-producing TNF and IL-6 (t(213.9)=3.97 p<0.001), with significant differences at months 2 (t(109.9)=2.16 p<0.04), 4 (t(103.2) = 3.63 p<0.001), 7 (t(97.8)=3.72 p<0.001), and 16 (t(138.2)=2.93 p<0.01).
IDS-C scores as a time varying covariate did not change the results for CRP or cellular inflammation.
Outcome of gene expression
Genome-wide transcriptional profiling was carried out using blood samples in a random subsample at month 4 (n=78), with bioinformatic evaluation of genes showing a 1.2-fold up- or down-regulation in CBT-I vs. SS, or in TCC vs. SS (differentially expressed genes listed in Supplement). For CBT-I, a total of 347 gene transcripts showed a 1.2-fold down-regulation, and a total of 191 gene transcripts showed a 1.2-fold up-regulation relative to SS. Prominent among down-regulated genes for CBT-I were transcripts in involved in inflammation (e.g., TLR-1, TNF, REL, JUN, FOSL2, IL-6, FOSB, IFNG, JUNB, IL-8, IL-1B, PTGS2). Among CBT-I up-regulated genes were those involved interferonand antibody responses (e.g., CD19, MX1, ISG15, OAS2, IGLL1, IFNAR1, IFITI, OASI, IFI44L, IGS). For TCC, a total of 202 gene transcripts showed a 1.2-fold down-regulation and 52 gene transcripts showed a 1.2-fold up-regulation relative to SS. Prominent among the TCC-down-regulated genes were transcripts involved in immunological activation and inflammation (e.g., IL-6, IFNGR1, CD69, FOSB, FOS, IFNG, JUNB, IL-8, IL-1B, PTGS2). Comparisons across groups at baseline showed no difference in inflammatory gene expression.
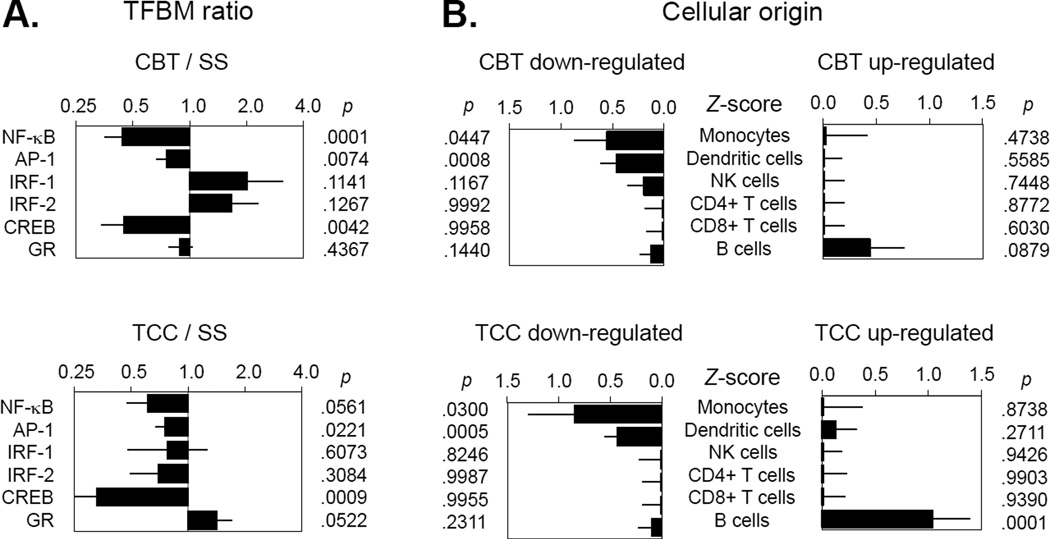
For CBT-I vs. SS, TELiS promoter-based bioinformatic analyses of genes showed differential change in expression (Figure 3A). Results indicated reduced activity of NF-κB (mean prevalence ratio = 0.44 ± standard error = 0.09, p < .0001), reduced activity of AP-1 (0.75 ± 0.08, p = .0074), and reduced activity of CREB (0.45 ± 0.11, p = .0042). Results also showed non-significant trends toward upregulation of interferon-activated transcription factors (IRF-1: 2.01 ± 0.71, p = .1141; IRF-2: 1.66 ± 0.46, p = .1267). Transcript origin analyses identified genes down-regulated in CBT-I as originating primarily from monocytes (p = .0447) and dendritic cells (p = .0008) (Figure 3B). No specific leukocyte subpopulation was identified as predominantly contributing to CBT-I up-regulated genes (although B cells showed a non-significant trend).
Figure 3.
Transcription factor activity as measured by TELiS promoter-based bioinformatic analyses of genes at 4 months (post-intervention), showing differential change in gene expression for comparisons of CBT vs. SS, and TCC vs. SS
For TCC vs. SS TELiS analyses showed results similar to those for CBT-I. Results (Figure 3A indicated a near-significant trend toward reduced activity of NF-κB (0.61 ± 0.14, p = .0561) and significantly reduced activity of AP-1 (0.76 ± 0.09, p = .0221) and CREB (0.33 ± 0.09, p = .0009). Results also indicated a trend toward up-regulation of GR activity (1.42 ± 0.28, p = .0522). As with CBT, transcript origin analyses identified genes down-regulated in TCC participants as originating primarily from monocytes (p = .0447) and dendritic cells (p = .0008) (Figure 3B). B cells were identified as predominantly contributing to CBT-I up-regulated genes (p < .0001).
DISCUSSION
Given mounting evidence that insomnia patients are at greater risk for depression, medical comorbidities, and mortality (8); that sleep disturbance is associated with inflammation (6, 8, 20, 21); and that inflammation can lead to increased risk of depression (9, 66), cardiovascular disease (11), diabetes mellitus (14), and certain cancers (67–69), this study is significant by examining for the first time the efficacy of insomnia treatment on inflammation. These novel results link sleep disturbance to increased levels of systemic and cellular inflammation, and to increased leukocyte expression of pro-inflammatory genes in older adults with insomnia. Indeed, CBT-I reduces insomnia symptoms (25), reduces levels of CRP, and reverses activation of molecular inflammatory signaling pathways. CBT-I-induced reduction of systemic inflammation as indexed by CRP was maintained during follow-up at 7 and 16 months, consistent with maintenance of sleep improvements (25). In contrast, TCC targets stress effector mechanisms that drive insomnia complaints, reduces TLR-4-activated monocyte production of IL-6 and TNF, and reverses activation of inflammatory signaling pathways. TCC-induced reduction of cellular inflammation was maintained during follow-up at 7 and 16 months and this reversal of cellular inflammation occurred even though improvements in sleep disturbance were not maintained in the long-term, suggesting that these changes are independent of improvements in sleep. The ultimate duration of transcriptional impact of CBT-I and TCC remains to be established. Importantly, these effects were identified from a controlled, randomized intervention trial analyzed by intent-to-treat, which was not confounded by differences in patient characteristics.
CBT-I effects on CRP may have implications for aging-related inflammatory disease, in which the maintenance of sleep improvements afforded by CBT-I appears to be critical for the persistent reduction of systemic inflammation. We have previously reported that CBT treatment was associated with a significant 50% decrease in the proportionate risk of high CRP (25), comparable to the benefit reported with vigorous physical activity (70) or weight loss (71). Moreover, TCC-induced changes in the cellular production of proinflammatory cytokines, as well as the effects of both CBT-I and TCC on proinflammatory gene expression in the basal leukocyte transcriptome, may also have significant implications for inflammatory disease risk, as well as cancer-related disease processes. Pro-inflammatory signaling has been linked breast cancer progression and recurrence (72, 73), whereas Type I interferon activity has been linked to reduced progression (74). Indeed, CBT-I, but not TCC, had the additional effect of increasing activity of interferon-responsive transcription factors, consistent with prior findings that CBT-I increases ex vivo production of interferon (75).
Other types of behavioral interventions including exercise and stress reduction approaches have immunomodulatory effects. Exercise, as compare to health education control, attenuates age-related increases in CRP in older individuals (50). Similarly, a meta-analyses of 34 studies, employing varying types of mind-body interventions (e.g., meditation, yoga, tai chi), showed a moderate effect to reduce CRP (49). Whereas TCC incorporates a component of physical activity, neither physical activity nor BMI changed in any of the groups. The pattern of pro-inflammatory transcriptional bias observed in this study of older adults with insomnia is similar to that reported in breast cancer survivors (26, 64), as well as in different populations who are reporting psychological distress (27, 28, 65).
Diverse behavioral and psychological challenges may activate a conserved transcriptional response due to sympathetic nervous system activation of pro-inflammatory transcription factors such as NF-κB (42). We speculate that TCC induces a relaxation response, which reduces sympathetic outflow (41) with effects on inflammation (42). In the present study, bioinformatic analyses indicated reduced activity of CREB family transcription factors in both CBT-I and TCC, which is consistent with reduced sympathetic nervous system signaling through β-adrenergic receptors. Further, we found that TCC marginally increased the expression of genes bearing GR response elements, indicating potentially increased glucocorticoid signal transduction or glucocorticoid receptor sensitivity to the antiinflammatory effects of cortisol. Finally, as support for the hypothesis that these behavioral treatments target a conserved transcriptional response, bioinformatic inferences of the specific cell types mediating the CBT-I and TCC alterations implicated the same myeloid lineage antigen presenting cells (i.e., monocytes), similar to transcriptional shifts in four other independent studies (26–28, 65). Direct evaluation of these transcription factors is necessary to confirm indirect inferences of transcription factor activity based on bioinformatics analyses.
Several limitations require consideration. Although physical activity did not change in any of the groups, TCC incorporated low levels of physical activity not found in CBT-I. Second, although expectancy for benefit was similar, participants were aware of their intervention assignment. Third, this study focused on older adults, and findings may not generalize to younger populations who have lower levels of inflammation. Fourth, women were over-represented, and those with medical co-morbidity were excluded. Fifth, although subjects were required to have a regular sleep wake cycle, it is possible that circadian re-alignment might have contributed to effects. Sixth, this study used one set of therapists to deliver TCC intervention, and another set to deliver CBT and SS treatments; differences between therapists might have contributed to treatment effects. Finally, the point estimates of differential gene expression are not subject to individual hypothesis testing and serve only as intermediate effect size inputs into higher-order gene set-based bioinformatics analyses testing a priori hypotheses regarding shared transcription factor promoter motifs (i.e., inflammation-related NF-κB, GR, and CREB factors) and shared cellular origin (i.e., pro-inflammatory monocytes). This study was not designed or powered for discovery-based analyses of statistically reliable associations between experimental conditions and specific individual gene transcripts. A longer-term follow-up is needed to determine the persistence of effects on transcriptional dynamics.
This study demonstrates that CBT-I primarily targets sleep behaviors and reduces systemic inflammation, whereas TCC, which is thought to target stress effector mechanisms, reduces cellular inflammation. The components of CBT-I vs TCC, which drive these differential effects on inflammation, require further clarification. Both treatments reverse some of the major changes in immune system gene expression previously observed in association with sleep disturbance (23). Given the links between insomnia, depression, and inflammatory disease risk, these findings provide an evidence-based molecular framework to understand how behavioral interventions that target sleep may reduce inflammation and represent a third pillar, along with diet and physical activity, to promote health.
Supplementary Material
ACKNOWLEDGMENTS
Supported by grant R01-AG034588 from the National Institute of Aging, other grant support from the National Institutes of Health to MRI including R01-CA119159; R01-HL079955; R01 HL095799; P30-AG028748; P30-AG017265; UL RR 033176; the Cousins Center for Psychoneuroimmunology, and UCLA Claude D. Pepper Older Americans Independence Center.
We thank all the study participants for their support and dedication to this research project; the physicians for providing medical clearance (Drs. Lara Kierlin, Hyong Jin Cho, Marissa Caudill); the intervention instructors (Kate Hollister, Jennifer Levin; Roberta Taggert); the members of the institutional review board at UCLA, and data safety monitoring board members (Drs. Annette Stanton and Julienne Bower).
The National Institutes of Health had no role in the design and conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-V. Fifth Edition ed. Washington, D.C.: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Lee E, Cho HJ, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685–1691. doi: 10.5665/sleep.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165:1543–1550. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disorders. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, Ma J, Gao X. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation. 2014;129:737–746. doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Ann Rev Psychol. 2015;66:2.1–2.30. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GD, Rumley A, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. NEJM. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 12.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 13.Barzilay JI, Forsberg C, Heckbert SR, Cushman M, Newman AB. The association of markers of inflammation with weight change in older adults: the Cardiovascular Health Study. Int J Obes (Lond) 2006;30:1362–1367. doi: 10.1038/sj.ijo.0803306. [DOI] [PubMed] [Google Scholar]
- 14.Brunner EJ, Kivimaki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP, Shah T, Humphries SE, Hingorani AD, Marmot MG, Timpson NJ, Kumari M. Inflammation, insulin resistance, and diabetes--Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5:e155. doi: 10.1371/journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, Miller AH. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, Pollmächer T. Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- 19.Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–253. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Ferrie JE, Kivimaki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, Kumari M, Davey Smith G, Shipley MJ. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013;178:956–961. doi: 10.1093/aje/kwt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 22.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 24.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, Yokomizo M, Lavretsky H, Carroll JE, Motivala SJ, Bootzin R, Nicassio P. Cognitive Behavioral Therapy vs. Tai Chi for Late Life Insomnia and Inflammatory Risk: A Randomized Controlled Comparative Efficacy Trial. Sleep. 2014;37:1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JM, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, Nazarian N, Khalsa DS, Lavretsky H. Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinol. 2013;38:348–355. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Arevalo J, Cole SW. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinol. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 30.Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250–265. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 31.Satoh M, Shimoda Y, Maesawa C, Akatsu T, Ishikawa Y, Minami Y, Hiramori K, Nakamura M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int J Cardiol. 2005 doi: 10.1016/j.ijcard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 33.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 34.Morin CM, Vallieres A, Guay B, Ivers H, Savard J, Merette C, Bastien C, Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.G R, Zhang Y, VJ M, D'Ambrosio C, Wang C. Tai Chi Improves Sleep Quality in Healthy Adults and Patients with Chronic Conditions: A Systematic Review and Meta-analysis. J Sleep Disorders Ther. 2013;141:2–6. doi: 10.4172/2167-0277.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, Lee Y, McAlindon T. A randomized trial of tai chi for fibromyalgia. NEJM. 2010;363:743–754. doi: 10.1056/NEJMoa0912611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164:493–501. doi: 10.1001/archinte.164.5.493. [DOI] [PubMed] [Google Scholar]
- 38.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 39.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–267. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 40.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 41.Motivala SJ, Sollers J, Thayer J, Irwin MR. Tai chi chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci. 2006;61:1177–1180. doi: 10.1093/gerona/61.11.1177. [DOI] [PubMed] [Google Scholar]
- 42.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. Sleep. 2008;31:1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 44.Li F, Fisher KJ, Harmer P, Irbe D, Tearse R, Weimer C. Tai Chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geratr Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Fisher KJ, Weimer C, Shirai M. The effects of Tai Chi training on self-rated sleep quality in older adults: a randomized controlled trial. Sleep. 2003;26:A423. [Google Scholar]
- 46.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20:764–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–517. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 48.Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, Irwin MR. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19:839–850. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One. 2014;9:e100903. doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, Castillo MC, Reighard AE, Vanderah E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, IL-6 independent of beta-blockers BMI, psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Campbell PT, Campbell KL, Wener MH, Wood BL, Potter JD, McTiernan A, Ulrich CM. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med Sci Sports Exerc. 2009;41:1533–1539. doi: 10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSMIV- TR) 4th Ed. Washington, DC: American Psychiatric Association; 2000. Text Revision ed. [Google Scholar]
- 54.A.Ao.S. Medicine, editor. Medicine AAoS. The International Classificaiton of Sleep Disorders: Diagnostic and Coding Manual. 2nd Ed. Darien, IL: American Academy of Sleep Medicine; 2005. ed, ed. [Google Scholar]
- 55.Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Cole SW, Galic Z, Zack JA. Controlling false-negative errors in microarray differential expression analysis: a PRIM approach. Bioinformatics. 2003;19:1808–1816. doi: 10.1093/bioinformatics/btg242. [DOI] [PubMed] [Google Scholar]
- 58.Shi L, Campbell G, Jones WD, Campagne F, Wen Z, Walker SJ, Su Z, Chu TM, Goodsaid FM, Pusztai L, Shaughnessy JD, Jr, Oberthuer A, Thomas RS, Paules RS, Fielden M, Barlogie B, Chen W, Du P, Fischer M, Furlanello C, Gallas BD, Ge X, Megherbi DB, Symmans WF, Wang MD, Zhang J, Bitter H, Brors B, Bushel PR, Bylesjo M, Chen M, Cheng J, Cheng J, Chou J, Davison TS, Delorenzi M, Deng Y, Devanarayan V, Dix DJ, Dopazo J, Dorff KC, Elloumi F, Fan J, Fan S, Fan X, Fang H, Gonzaludo N, Hess KR, Hong H, Huan J, Irizarry RA, Judson R, Juraeva D, Lababidi S, Lambert CG, Li L, Li Y, Li Z, Lin SM, Liu G, Lobenhofer EK, Luo J, Luo W, McCall MN, Nikolsky Y, Pennello GA, Perkins RG, Philip R, Popovici V, Price ND, Qian F, Scherer A, Shi T, Shi W, Sung J, Thierry-Mieg D, Thierry-Mieg J, Thodima V, Trygg J, Vishnuvajjala L, Wang SJ, Wu J, Wu Y, Xie Q, Yousef WA, Zhang L, Zhang X, Zhong S, Zhou Y, Zhu S, Arasappan D, Bao W, Lucas AB, Berthold F, Brennan RJ, Buness A, Catalano JG, Chang C, Chen R, Cheng Y, Cui J, Czika W, Demichelis F, Deng X, Dosymbekov D, Eils R, Feng Y, Fostel J, Fulmer-Smentek S, Fuscoe JC, Gatto L, Ge W, Goldstein DR, Guo L, Halbert DN, Han J, Harris SC, Hatzis C, Herman D, Huang J, Jensen RV, Jiang R, Johnson CD, Jurman G, Kahlert Y, Khuder SA, Kohl M, Li J, Li L, Li M, Li QZ, Li S, Li Z, Liu J, Liu Y, Liu Z, Meng L, Madera M, Martinez-Murillo F, Medina I, Meehan J, Miclaus K, Moffitt RA, Montaner D, Mukherjee P, Mulligan GJ, Neville P, Nikolskaya T, Ning B, Page GP, Parker J, Parry RM, Peng X, Peterson RL, Phan JH, Quanz B, Ren Y, Riccadonna S, Roter AH, Samuelson FW, Schumacher MM, Shambaugh JD, Shi Q, Shippy R, Si S, Smalter A, Sotiriou C, Soukup M, Staedtler F, Steiner G, Stokes TH, Sun Q, Tan PY, Tang R, Tezak Z, Thorn B, Tsyganova M, Turpaz Y, Vega SC, Visintainer R, von Frese J, Wang C, Wang E, Wang J, Wang W, Westermann F, Willey JC, Woods M, Wu S, Xiao N, Xu J, Xu L, Yang L, Zeng X, Zhang J, Zhang L, Zhang M, Zhao C, Puri RK, Scherf U, Tong W, Wolfinger RD, Consortium M. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat Biotechnol. 2010;28:827–838. doi: 10.1038/nbt.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, Mei N, Chen T, Herman D, Goodsaid FM, Hurban P, Phillips KL, Xu J, Deng X, Sun YA, Tong W, Dragan YP, Shi L. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat Biotechnol. 2006;24:1162–1169. doi: 10.1038/nbt1238. [DOI] [PubMed] [Google Scholar]
- 60.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 64.Irwin M, Olmstead R, Breen E, Witarama T, Carrillo C, N S, Arevalo J, Ma J, Nicassio P, Ganz P, Bower J, Cole S. Tai Chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. J Natl Cancer Inst. 2014 doi: 10.1093/jncimonographs/lgu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irwin MR, Miller AH. Depressive disorders and immunity 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Touvier M, Fezeu L, Ahluwalia N, Julia C, Charnaux N, Sutton A, Mejean C, Latino-Martel P, Hercberg S, Galan P, Czernichow S. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol. 2013;177:3–13. doi: 10.1093/aje/kws359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105:1871–1880. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 70.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13:561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 72.Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–3419. doi: 10.1200/JCO.2009.21.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bielenberg DR, McCarty MF, Bucana CD, Yuspa SH, Morgan D, Arbeit JM, Ellis LM, Cleary KR, Fidler IJ. Expression of interferon-beta is associated with growth arrest of murine and human epidermal cells. J Invest Dermatol. 1999;112:802–809. doi: 10.1046/j.1523-1747.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- 75.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitivebehavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. J Clin Oncol. 2005;23:6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.