SUMMARY
Rheb, a ubiquitous small GTPase, is well known to bind and activate mTOR, which augments protein synthesis. Inhibition of protein synthesis is also physiologically regulated. Thus, with cell stress the unfolded protein response system leads to phosphorylation of the initiation factor eIF2α and arrest of protein synthesis. We now demonstrate a major role for Rheb in inhibiting protein synthesis through enhancing the phosphorylation of eIF2α by protein kinase-like endoplasmic reticulum kinase (PERK). Interplay between the stimulatory and inhibitory roles of Rheb may enable cells to modulate protein synthesis in response to varying environmental stresses.
INTRODUCTION
Protein synthesis is orchestrated by an array of proteins, which activate or inhibit translation based on their phosphorylation status. The mTOR system enhances protein synthesis in response to nutrients and growth factors. Cellular stress, on the other hand, triggers processes that inhibit translation in order to conserve cellular resources. Phosphorylation of eIF2α (eukaryotic translation initiation factor 2α) is a major mediator of this inhibitory system and involves four discrete kinases - protein kinase-like endoplasmic reticulum kinase (PERK), general control nonderepressible 2 (GCN2), Protein kinase RNA-activated also known as protein kinase R (PKR), and The heme-regulated inhibitor (HRI) (Donnelly et al., 2013).
There exists an inverse relationship between mTOR signaling and phospho-eIF2α. Under pathological conditions, such as apoptosis, hypoxia, serum and nutrient deprivation, mTOR activity is downregulated, whereas phospho-eIF2αis upregulated leading to diminished global protein synthesis (Deng et al., 2002; Hara et al., 1998; Kim et al., 2002; Koumenis et al., 2002; Liu et al., 2006; Schneider et al., 2008; Tee and Proud, 2001). In some instances mTOR and eIF2α may act in parallel. For example, deletion of TSC2, an inhibitor of mTOR signaling, augments signaling both by mTOR and PERK (Ozcan et al., 2008). Pharmacologic and genetic studies suggest that mTOR signaling does influence phospho-eIF2α. For instance, the mTOR inhibitor, rapamycin, is known to upregulate phospho-eIF2α in some cells (Anand and Gruppuso, 2006; Kato et al., 2012; Kubota et al., 2003; Matsuo et al., 2005). Similarly, heat inactivation of mTOR potentiates phospho-eIF2α in yeast cells, whereas the genetic deletion of PTEN, a negative regulator of mTOR, reduces phospho-eIF2α in cancerous cells (Mounir et al., 2009; Valbuena et al., 2012).
The GTPase Rheb is well established as an inducer of mTOR thereby augmenting protein synthesis. Here we demonstrate that Rheb plays a major role in inhibiting protein synthesis by enhancing PERK-mediated phospho-eIF2α levels. This action may underlie, in part, the reciprocal relationship of mTOR and eIF2α signaling.
RESULTS
GTPase Rheb inhibits protein synthesis
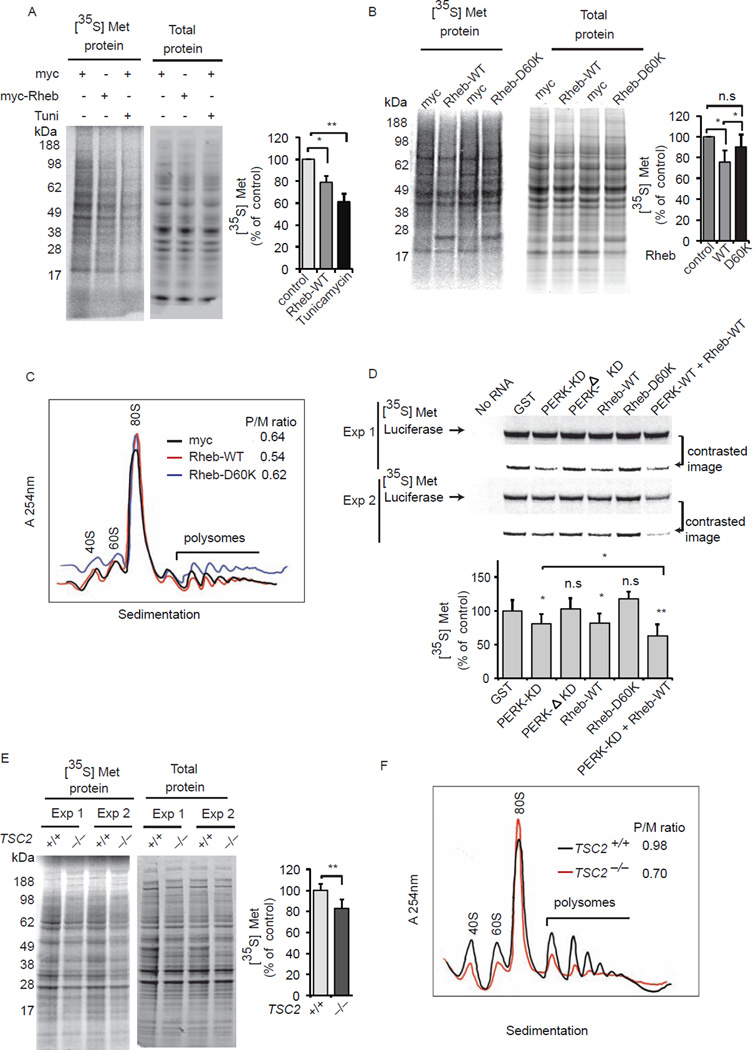
The canonical mTOR pathway involves TSC1/2 binding to and inhibiting Rheb, preventing activation of mTOR signaling by Rheb (Inoki et al., 2003). Rheb, acting via mTOR, is generally regarded as a physiologic stimulant of protein synthesis (Hall et al., 2007; Wang et al., 2008). By contrast, in HEK293 cells, we observe that overexpression of Rheb is associated with diminished protein synthesis, as measured by incorporation of [35S]-Met (Figure. 1A). Tunicamycin, an ER stressor, elicits cell stress and inhibited protein synthesis (Figure 1A). We confirm that overexpressing Rheb augments phospho-S6 kinase, an index of mTOR signaling (Figure S1). The marked stimulation by tunicamycin of CHOP, an apoptotic protein, confirms its stressor actions (Figure S1). However, Rheb overexpression does not affect CHOP levels (Figure S1), indicating that diminished incorporation of [35S]-Met by Rheb (Figure 1A) may not be due to cellular stress. Inhibition of protein synthesis by Rheb is dependent upon its guanine-nucleotide binding, as it is absent with Rheb-D60K (Figure 1B), which cannot bind GTP or GDP (Aspuria and Tamanoi, 2004). Polysome profiles revealed the Rheb WT overexpressing cells showed reduced polysome/monosome ratio (Figure 1C). We next assessed whether Rheb can directly modulate protein synthesis in vitro (Figure 1D). Rheb WT, but not Rheb D60K, markedly decreases luciferase mRNA translation, an effect also elicited by active PERK kinase, a known inhibitor of protein synthesis (Harding et al., 1999). Rheb overexpression increased the viability of the HEK293 cells using MTS assay (which measures the mitochondrial activity) but it did not significantly alter the cell number (counted using hemocytometer) (Figure S2). In TSC2 depleted fibroblasts, which possess high Rheb–mTOR activity (Inoki et al., 2003; Zhang et al., 2003), we observe diminished protein synthesis (Figure 1E), consistent with previous report compared to TSC2 intact cells (Auerbach et al., 2011). TSC2 depleted fibroblasts also exhibited reduced polysome/monosome ratio (Figure 1F) and diminished cell numbers, compared to TSC2 intact cells (Figure S3). Thus, GTPase Rheb can act as a negative regulator of protein synthesis.
Figure 1. Rheb inhibits protein synthesis.
(A) Autoradiography of [35S]-Met incorporation and total proteins (ponceu staining) of HEK293 cells were transfected with myc or myc-Rheb WT. Tunicamycin (100 nM) treated cells used as control. (B) Autoradiography and total proteins (ponceu staining) of HEK293 cells expressing myc, myc-Rheb WT and myc-Rheb D60K. (C) Polysome profiles of myc, Rheb WT and Rheb D60K expressing HEK293 cells. (D) Autoradiography of rabbit reticulocyte-based in vitro translation assay in presence of recombinant proteins, GST (500 ng), GST-PERK-kinase domain (KD) (50 ng), GST-PERK-ΔKD (50 ng) or GST-Rheb WT (500 ng) or D60K (500 ng). (E) Autoradiography and total protein of TSC2+/+ and TSC2−/− MEFs pulsed with [35S]-Met as in A. (F) Polysome profiles of TSC2+/+ and TSC2−/− MEFs. (**p<0.01: *p<0.05, Student t test). Data are means ± SEM from 3 experiments.
Rheb enhances the phosphorylation of eIF2α, which inhibits protein synthesis
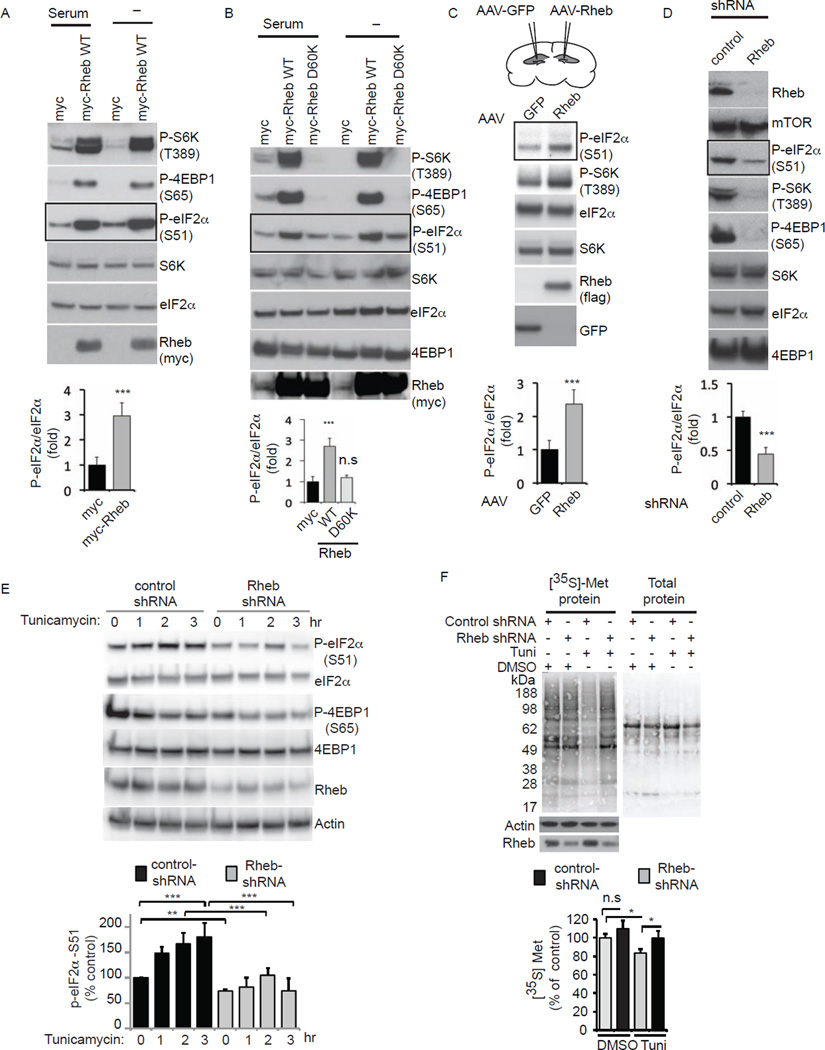
By what mechanism might Rheb inhibit protein synthesis? We examined regulation of eIF2α, whose phosphorylation is associated with inhibition of protein synthesis (Harding et al., 1999). Overexpressing Rheb (2–4 folds more than endogenous Rheb) in the absence or presence of serum markedly stimulates phosphorylation of eIF2α at serine-51 with an increase comparable to the stimulation of mTOR signaling monitored as phospho-S6 kinase/phospho-4EBP1 (Figure 2A). This action of Rheb is dependent upon its guanine-nucleotide binding, as levels of phospho-eIF2α, like those of phospho-S6 kinase and phospho-4EBP1, are markedly reduced in the presence of Rheb-D60K (Figure 2B).
Figure 2. Rheb promotes the phosphorylation of eIF2α (P-eIF2α.
(A) Western blotting of indicated proteins in HEK293 cells expressing myc or myc-Rheb WT constructs in DMEM with serum (+) or without serum (−). (B) Western blotting of indicated proteins in HEK293 cells transfected with myc, myc-Rheb-WT or Rheb-D60K and processed as in A. (C) Western blotting of indicated proteins in the hippocampus of adult mice overexpressing of AAV-Rheb or AAV GFP. (D) Western blotting of indicated proteins in HEK293 cells expressing Rheb shRNA or control shRNA. (E) Western blotting of tunicamycin (100 nM) treated control or Rheb shRNA expressing HEK293 cells. (F) Autoradiography of [35S]-Met incorporation and total proteins (ponceu) of tunicamycin (100 nM) or DMSO (0.5%) treated control or Rheb shRNA expressing cells. (***p<0.001: **p<0.01: *p<0.05, Student t test). Data are means ± SEM from 3 experiments. Graphs in A and B are quantified from serum conditions.
Rheb influences phospho-eIF2α levels in intact animals. Thus, injections of adeno associated viral particles (AAV) expressing Rheb directly into the hippocampus elicit a substantial increase in phospho-eIF2α and phospho-S6 kinase (Figure 2C). Further evidence that Rheb is required for phosphorylation of eIF2α comes from experiments depleting Rheb by shRNA leading to a profound reduction in phospho-eIF2α as well as phospho-S6 kinase/phospho-4EBP1 (Figure 2D). Rheb also altered the protein expressions of eEF2, the target of mTORC1 signaling (Browne and Proud, 2002; Hay and Sonenberg, 2004), and ATF-4, the target of phospho-eIF2α signaling (Lu et al., 2004). While Rheb-WT overexpression increased the eEF2/ATF-4 protein levels, Rheb shRNA treatment attenuated those levels (Figure S4A–D). Note although ATF-4 levels go up, the CHOP, one of the ATF-4 target genes is not increased (Figure S1). Although mechanisms are unclear, CHOP can also be induced through ATF-4 independent pathways. It has been shown that ATF-4−/− MEFs elicits CHOP expression induced by thapsigargin (Ma and Hendershot, 2004), and that ATF-4 is necessary but not sufficient for the induction of CHOP expression under certain conditions (Harding et al., 2000). Thus, Rheb might selectively induce ATF-4, but not its downstream target, CHOP, under the experimental conditions used in this study.
We then tested whether Rheb is necessary for ER-stress-mediated phospho-eIF2α and protein synthesis. As expected, ER stressor, tunicamycin, increased the phospho-eIF2α levels and decreased the phosphorylation of mTORC1 target, 4EBP1, in a time-dependent manner, consistent with previous reports (Deldicque et al., 2011; Li et al., 2008) and that increase is markedly reduced in Rheb shRNA-treated cells (Figure 2E). While Rheb depletion (~50%) did not markedly affect the [35S] Met-incorporation in control conditions, it prevented the loss of [35S] Met-incorporation induced by tunicamycin (Figure 2F). Thus, Rheb physiologically regulates ER stress-induced phospho-eIF2α levels and protein synthesis.
Rheb induces phosphorylation of eIF2α independent of mTORC1, PI3K and MAPK signaling
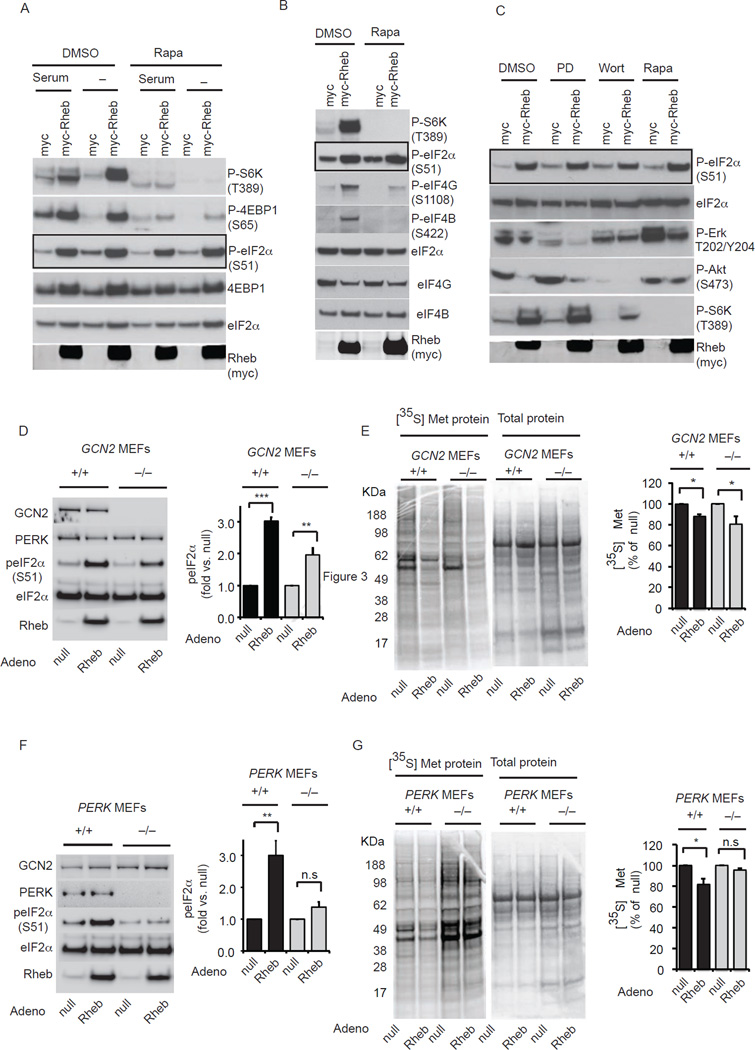
Experiments with rapamycin rule out mTOR as mediating Rheb’s effects on phospho-eIF2α. Rapamycin virtually abolishes stimulation by Rheb of phospho-S6 kinase and phospho-4EBP1 but has negligible effects on phospho-eIF2α (Figure 3A). This finding buttresses the conclusion that phosphorylation of eIF2α does not involve mTOR, consistent with previous reports (Bunpo et al., 2009; O'Connor et al., 2008). In contrast to phosphorylation of eIF2α, which is not mediated by mTOR, phosphorylation of other elongation factors does involve mTOR. Thus, rapamycin prevents phosphorylation of eIF4G and eIF4B, targets of S6K (Shahbazian et al., 2006), but not eIF2α (Figure 3B).
Figure 3. Rheb induces P- eIF2α independent of mTORC1 and promotes P-eIF2α predominantly through PERK.
(A) Western blotting of indicated proteins of HEK293 expressing cDNAs of myc (control) or myc-Rheb WT were grown in DMEM with serum (+) or without serum (–) or pretreated with the inhibitors rapamycin (250 nM) or DMSO (0.5%, control). (B) Western blotting of indicated proteins in HEK293 cells expressing myc or myc-Rheb and pretreated with rapamycin or DMSO as in A (C). Western blotting of indicated proteins in HEK293 cells expressing myc or myc-Rheb and pretreated with inhibitors of MAPK [PD98059, 50 µM] or PI3K [wortmannin, 100 nM], rapamycin or DMSO. (D) Western blotting of indicated proteins in GCN2−/− cells and their isogeneic wild-type (+/+) MEFs were infected with adenoviral-null or adenoviral-Rheb. (E) Autoradiography of [35S]-Met incorporation and total proteins (ponceu) in GCN2 MEF cells infected with adenoviral-null or adenoviral-Rheb (F) Western blotting of indicated proteins in PERK−/− cells and their isogeneic wild-type (+/+) MEFs were infected with adenoviral-null or adenoviral-Rheb. GTPase Rheb fails to induce P-eIF2α in PERK−/− MEF cells. (G) Autoradiography of [35S]-Met incorporation and total proteins (ponceu) in PERK MEF cells infected with adenoviral-null or adenoviral-Rheb (***p<0.001: **p<0.01: *p<0.05, Student t test). Data are means ± SEM from 3 experiments.
Kinase inhibitors provide additional evidence of specificity (Figure 3C). Thus, wortmannin, which inhibits PI3 kinase, abolishes phosphorylation of Akt and greatly reduces phospho-S6 kinase but does not alter phosphorylation of eIF2α or Erk. PD98095, which inhibits the MAP kinase pathway, greatly reduces phospho-Erk but does not influence phospho-eIF2α, phospho-Akt or phospho-S6 kinase. By contrast, rapamycin, as expected, abolishes phospho-S6 kinase but fails to influence phospho-eIF2α, phospho-Erk or phospho-Akt (Figure 3C). Thus Rheb induces phosphorylation of eIF2α independent of mTORC1, PI3K and MAPK signaling.
Rheb elicits phosphorylation of eIF2α via PERK
Four protein kinases are known to phosphorylate eIF2α: GCN2, PERK, PKR and HRI (Wek et al., 2006). Of these, GCN2 and PERK account for the great bulk of eIF2α phosphorylation and so have been the focus of our attention. Adenoviral-mediated Rheb expression enhances phosphorylation of eIF2α, or reduces [35S] Met-labeling, comparable levels both in GCN2 WT and GCN2 deleted MEFs (Figures 3D, 3E). By contrast, the Rheb-mediated increase in the phospho-eIF2α levels or suppression of [35S] Met-labeling is markedly reduced in PERK deleted MEFs (Figures 3F, 3G). Rheb overexpression also increased the viability and reduced the polysome/monosome ratio in PERK intact MEFs, compared to PERK deleted MEFs (Figure S5A, S5B). In these overexpression studies, we found exogenously expressed Rheb levels are 2–4 fold more than endogenous Rheb. Interestingly, Rheb overexpression had no significant effect on cell number, but decreased the proliferation of PERK deleted MEFs, indicating that loss of PERK might interfere with Rheb’s effect on cell proliferation (Figure S5C). We also confirmed importance of PERK in Rheb-mediated phospho–eIF2α, in HEK293 cells transiently transfected with PERK shRNA (Figure S6). Thus, Rheb modulates phospho-eIF2α and affects protein synthesis predominantly via PERK.
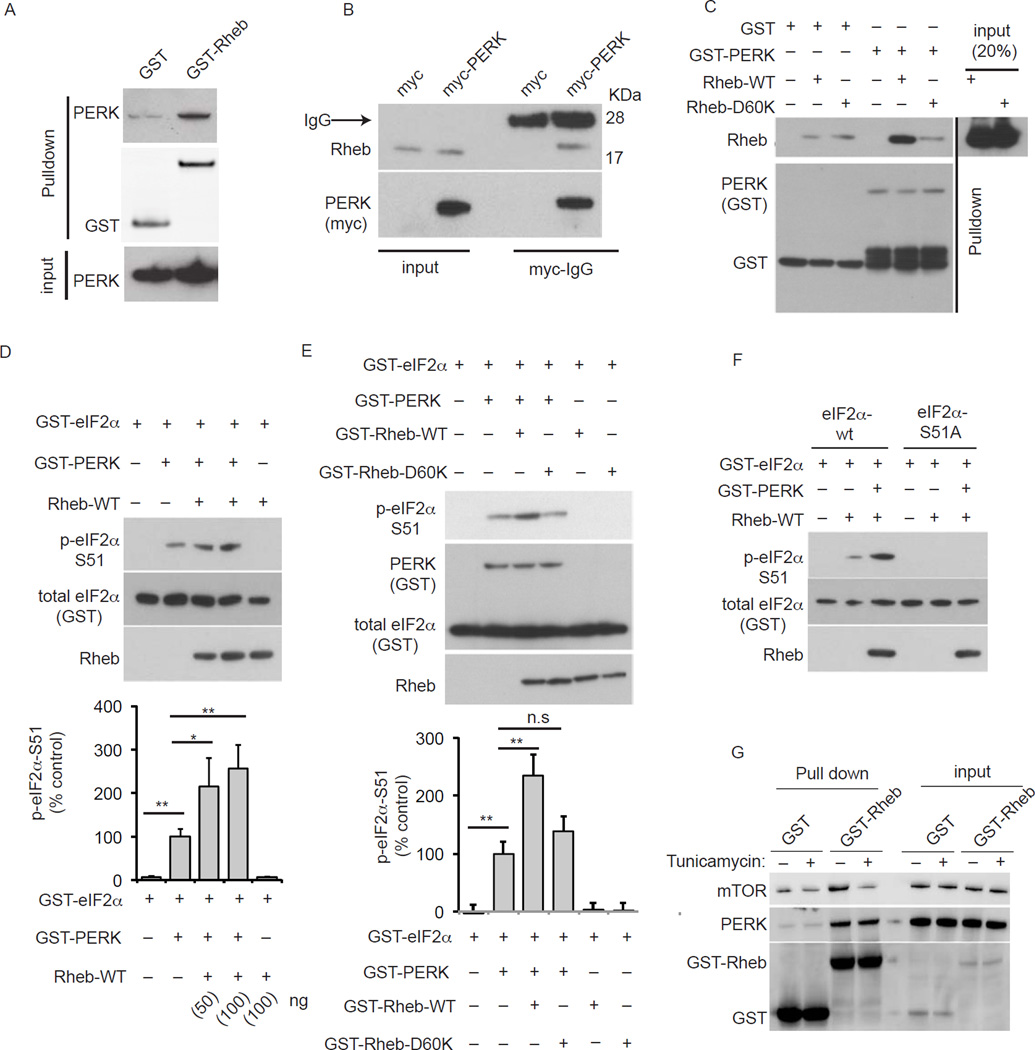
We assessed the physical association of Rheb and PERK, and how Rheb modulates PERK activity in vitro. An intimate association of PERK and Rheb is evident in the robust binding of overexpressed PERK with endogenous Rheb, or overexpressed Rheb with endogenous PERK, in HEK293 cells (Figures 4A, 4B). Binding between these two proteins is direct. Rheb WT, compared to Rheb D60K, binds strongly to PERK, as demonstrated with purified proteins in vitro (Figure 4C).
Figure 4. Rheb GTPase directly binds and activates PERK in vitro.
(A) Western blotting/glutathione-binding affinity analysis of indicated proteins in HEK293 cells expressing GST or GST-Rheb. (B) Western blotting analysis of immunoprecipitated (myc-IgG) proteins in HEK293 expressing myc or myc-PERK, and corresponding inputs. (C) Western blotting of glutathione-GST affinity purified recombinant proteins (and corresponding input) containing equimolar concentrations of untagged Rheb WT or Rheb D60K, and GST-PERK-KD (kinase domain). (D) Western blotting indicated proteins of in vitro PERK kinase activity containing PERK-KD with eIF2α in the absence or presence of untagged Rheb (50 and 100ng). (E) Western blotting indicated proteins of in vitro PERK kinase activity containing PERK-KD with eIF2α in the presence of GST-Rheb WT or GST-Rheb D60K (100ng). (F) Western blotting indicated proteins of in vitro PERK kinase activity with GST-eIF2α WT and GST-eIF2α S51A. (G) Western blotting/glutathione-binding affinity analysis of indicated protein in HEK293 cells transfected with GST or GST-Rheb, in presence of DMSO (−, 0.5%) to tunicamycin (+, 100 nM).
Further evidence for the importance of Rheb in phosphorylation of eIF2α comes with PERK kinase experiments showing a concentration-response relationship for Rheb’s stimulation of eIF2α phosphorylation in vitro (Figure 4D). This activity is GTP-dependent, as Rheb D60K does not elicit PERK-kinase activity in vitro (Figure 4E). This phosphorylation is selective for eiF2α-S51, as it is abolished with S51A mutations (Figure 4F). Since ER-stress upregulates phosphorylation of eIF2α but downregulates mTORC1 signaling (Figure 2E), consistent with previous reports (Deldicque et al., 2011; Li et al., 2008), we wondered whether this inverse signaling might occur due to an enhanced Rheb interaction with PERK during ER stress. We found tunicamycin treatment has abolished the Rheb–mTOR interaction but the Rheb–PERK interactions remains unaffected (Figure 4G). Thus, depending upon the cellular status, such as ER stress, Rheb-mTOR interaction would be dismantled, but Rheb-PERK interactions might be stabilized. Though this interaction may not be increased further by ER stress, we cannot rule out the possibility that this Rheb-PERK complex is more active under stress conditions. Thus, under ER stress conditions, Rheb might selectively associate with PERK to facilitate reduction of protein synthesis through eIF2α signaling.
DISCUSSION
A canonical cascade for protein synthesis involves interactions of Rheb with mTOR to activate the mTORC1 protein synthesis pathway (Wang and Proud, 2006). In the present study we demonstrate an alternative pathway in which Rheb inhibits protein synthesis by stimulating the phosphorylation of eIF2α. In terms of the overall protein synthetic activity of cells, inhibition of protein synthesis by Rheb may be its principal role, as overexpressing Rheb decreases cellular protein synthesis.
The mechanism whereby Rheb inhibits protein synthesis appears to involve enhancement of phosphorylation of eIF2α predominantly by the PERK protein kinase. Thus, deletion of PERK but not another protein kinase, GCN2, markedly abolishes Rheb-mediated enhancement of phospho-eIF2α. Rheb overexpression appears to decrease Extracellular Regulated Kinase (Erk) phosphorylation, a signaling linked to protein synthesis (Kelleher et al., 2004). This is consistent with previous findings in which deletion of mTOR or deletion of Rheb, elicits phosphorylation of Erk (Carracedo et al., 2008; Kelleher et al., 2004; Li et al., 2011). Whether Rheb mediated reduction of Erk signaling is a feedback loop to regulate protein synthesis remains unclear. Nevertheless, rapamycin which increases Erk signaling (Carracedo et al., 2008) actually inhibits rather than promotes, protein synthesis in certain cells (Huo et al., 2011). Thus the significance of crosstalk between Rheb and Erk signaling and what role they play in protein synthesis remains unclear.
Inhibition of protein synthesis in response to stressful stimuli is a major mechanism to protect cells from stress-elicited cell death. Phosphorylation of eIF2α by PERK is regarded as a principal means whereby cell stress down-regulates protein synthesis. In the absence of cell stress eIF2α forms a ternary complex with transfer RNA and the 43S ribosome, which initiates translation (Kimball, 1999). With cell stress, phosphorylation of eIF2α prevents the formation of the ternary complex, abrogating new protein formation.
Though Rheb is well known to activate mTOR, the physiologic rationale for this action has not been altogether clear. Our findings suggest that Rheb may functions as a molecular switch between stimulation and inhibition of protein synthesis (see graphical abstract). Thus, by binding to mTOR kinase, Rheb stimulates protein synthesis. On the other hand, when it binds to PERK to enhance phosphorylation of eIF2α, protein synthesis is inhibited. Under what cellular contexts these opposite functions of Rheb are involved remains less clear. When cells are actively growing, Rheb–mTOR circuitry might promote protein synthesis, and when cells are under stress, for example during uncontrolled growth, the Rheb-PERK circuitry might reduce protein synthesis. This later notion is supported by our binding experiments in which we found that under ER-stress conditions (where mTORC1 activation is reduced and P-eIF2α signaling is increased) Rheb losses it’s interaction with mTOR but remain bound to PERK (Figure 4G). As cell growth and ER stress is intimately connected (Tsang et al., 2010), such dynamic binding of Rheb may regulate normal physiology to reciprocally alter mTOR and P-eIF2α signaling, as observed in various cell types (Anand and Gruppuso, 2006; Deng et al., 2002; Hara et al., 1998; Kato et al., 2012; Kim et al., 2002; Koumenis et al., 2002; Kubota et al., 2003; Liu et al., 2006; Matsuo et al., 2005; Schneider et al., 2008; Tee and Proud, 2001).
Our model raises the possibility that a hitherto unidentified post-translational modification of Rheb – perhaps phosphorylation– mediates the reciprocity of Rheb’s capacities to stimulate or inhibit protein synthesis. Bioinformatic analysis (GPS phosphorylation prediction software) indicates that Rheb possesses several consensus sequences for phosphorylation, for example, threonine 38 for protein kinase B, serine 130 for ribosomal S6 kinase, serine 179 for CAMkinase, and tyrosine 67 for tyrosine kinase. Phosphorylation of Rheb at serine 130 by p38 regulated kinase inhibits Rheb-mediated mTORC1 activation (Zheng et al., 2011). Whether phosphorylation or other post-translational modifications, such as acetylation, differentiate Rheb’s interactions with mTOR vs. PERK remains to be seen.
The Rheb/PERK/eIF2α system may influence major organismic activities. Recently we found Rheb GTPase can regulate BACE1, a principle enzyme implicated in Alzheimer’s disease (AD) pathology (Shahani et al., 2013). PERK and phospho-eIF2α levels are linked to BACE1 regulation and AD-related behavioral deficits (Devi and Ohno, 2010; Ma et al., 2013; O'Connor et al., 2008). We also found huntingtin serves as a new effector of Rheb GTPase to activate mTORC1 to modulate Huntington disease (HD)-related symptoms (Pryor et al., 2014). Though the role of P-eIF2α signaling in HD-symptoms is not clear, a recent study implicated p-eIF2α in HD cellular toxicity (Leitman et al., 2014). Moreover, Rheb through phospho-eIF2α may regulate ER stress—a feature linked to all major age-related diseases, such as diabetes, Parkinson’s disease, AD, and HD (Chang et al., 2002; Harding et al., 1999; Hoozemans et al., 2007; Kang et al., 2011; Kouroku et al., 2007). Thus, Rheb–eIF2α circuitry may have multiple cellular functions with implications in physiological as well as pathological conditions.
EXPERIMENTAL PROCEDURES
Reagents, Plasmids and Antibodies
Most of the reagents were purchased from Sigma, unless mentioned otherwise. The details of cDNA, antibodies, viral particles and other reagents used can be found in the Supplementary Experimental Procedures.
Cell Culture, Transfections and Amino Acid Treatments
HEK293 cells were grown in DMEM (Gibco 11965-092) with 10% FBS (fetal bovine serum), 1% pen-strep and 5 mM glutamine. Briefly, cells were seeded in 3.5 or 6 cm-plates. After 18–24hr the cells were transfected with cDNA constructs using polyfect (Qiagen) as per the manufacturer’s instructions. For the serum starvation protocol, after 48hr the growth media was replaced with either DMEM with or without 10% FBS. Cells were kept in these media for 1–2hr and lysed followed by Western blotting.
Immunoblotting, Co-immunoprecipitation, and in vitro Binding
Glutathione-affinity and Western blotting, and in vitro binding protocols were carried out essentially as before (Shahani et al., 2013; Subramaniam et al., 2010; Subramaniam et al., 2009). More details can be found in the Supplementary Experimental Procedures.
Cell viability and proliferation assay
HEK293 cells (transfected with indicated vectors) or MEFs (infected with null or Rheb adeno viruses) grown in 24 well plate, and after 48hrs, the viability was assessed using MTS assay, as per the manufacturer protocol (Promega). For proliferation assay, an equal number (1 × 104) of HEK cells or MEFs were seeded in 24 well plate that are transfected or infected with Rheb, and after 48hrs cell numbers were counted using hemocytometer.
Recombinant protein purification
Recombinant proteins are produced essentially as indicated before (Tyagi et al., 2009). More information can be found in the Supplementary Experimental Procedures.
PERK in vitro kinase assay
In vitro kinase assays with recombinant bacterially expressed GST-PERK-KD or GST-PERK-ΔKD (10 ng) were performed at 30°C for 30 min in 30 µl of kinase buffer (20 mM Hepes, pH 7.5, 50 mM KCl, 1 mM DTT, 2 mM MgCl2, 0.1 mM ATP) containing 1 µg of purified GST-eIF2α-WT or GST-eIF2α-S51A proteins as substrate. Phosphorylation of eIF2α was detected using Western blotting with phospho-eIF2α antibody. Where indicated, purified Rheb WT or Rheb D60K protein (50 ng or 100 ng) was added to the in vitro kinase reaction.
In vitro Translation Assay
In vitro translation assays were performed as described earlier (Harding et al., 1999). Briefly, to measure translational inhibition by Rheb and PERK, purified 50 ng GST–PERK-KD or GSTPERK-ΔKD and 500 ng of GST-Rheb WT or GST-Rheb-D60K were pre-incubated for 10 min at room temperature with 12 µl of an in vitro translation mixture (Promega) before addition of 0.5 µg in vitro-transcribed capped luciferase mRNA. The [35S] Met labelled luciferase protein was resolved by 4–12% Bis-Tris Gel (Invitrogen).
Intrahippocampal AAV injection
Adeno-associated virus (AAV)-Rheb was stereotaxically injected into the hippocampus adult (P90) male C57BL/6 mice and tiisue/protein extraction and Western blotting was carried out as described before (Shahani, 2013).
L-[35S]-Met labeling of HEK293 cells
Myc (control), myc-Rheb WT or myc-Rheb D60K vectors (1 or 2µg each) were transfected, using polyfect, into HEK293 cells in DMEM plus 10% serum, grown in 6-well plates, (Invitrogen). After 2 days the media was replaced with labeling media (methionine/cysteine free DMEM media, Invitrogen) and 100 µCi/well of [35S]-Met for 30 min, and cells were either directly lysed in 2× Laemmli buffer with β-mercaptoethanol or in Tris buffer with 100µg of cycloheximide, before loading onto the gels. The gels were blotted onto PVDF membrane, then proceed either for autoradiography (Bio-Rad), protein staining (ponceu), or to detect phosphorylation of eIF2α and other indicated proteins by Western blotting. For tunicamycin (Tu) experiments, Tu (100 nM) was added three hour before changing the medium to labeling media with [35S]-Met.
Experiments with Mouse Embryonic Fibroblasts
TSC2−/− and TSC2+/+ MEFs were from David J. Kwiatkowski (Brigham and Women's Hospital, Boston). PERK−/− and PERK+/+ MEFs were from David Ron (University of Cambridge, Cambridge) GCN2−/− and GCN2+/+ MEFs were from ATCC. More details can be found in the Supplementary Experimental Procedures.
Polysome profiling
Polysome profiling was essentially carried out as described before (Thoreen et al., 2012). For more details see the Supplementary Experimental Procedures.
Statistical Analysis
Data were expressed as means ± SEM as indicated. All experiments were performed at least in triplicate and repeated twice. Statistical analysis was performed using Student's t-test (MS Excel).
Supplementary Material
ACKOWLEDGMENTS
We thank Melissa Benilous, Nancy Norton, and Trina Miles for administrative support. We thank Micheal Farzan and Charles Bailey for sharing their spectrophotometer. This work was supported by the Scripps startup funds (to S. Subramaniam) and O'Keeffe Neuroscience Scholar Award (to W. P.) and United States Public Health Service grant DA000266 (S.H.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Su. S initiated the project and conducted initial cell culture/biochemical work. R.T carried out binding, in vitro translation, in vitro kinase and TSC2 [35S]-Met work. N.S carried out PERK/GCN2 MEFs work, cell viability and proliferation work and polysome profiling. L. G assisted with MEF-related work. M.F carried out polysome profiling. M.F and K. K shared the polysome-related reagents, provided helpful discussion. W.P carried out hippocampal injections work with the help of S. Sw. P.F.W and P.Y.C provided reagents and helpful discussion. Su.S and S.H.S wrote the manuscript with input from co-authors.
None of the authors of this manuscript have financial interests related this work.
REREFENCES AND CITATIONS
- Anand P, Gruppuso PA. Rapamycin inhibits liver growth during refeeding in rats via control of ribosomal protein translation but not cap-dependent translation initiation. The Journal of nutrition. 2006;136:27–33. doi: 10.1093/jn/136.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspuria PJ, Tamanoi F. The Rheb family of GTP-binding proteins. Cellular signalling. 2004;16:1105–1112. doi: 10.1016/j.cellsig.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem. 2009;284:32742–32749. doi: 10.1074/jbc.M109.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of clinical investigation. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RC, Wong AK, Ng HK, Hugon J. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport. 2002;13:2429–2432. doi: 10.1097/00001756-200212200-00011. [DOI] [PubMed] [Google Scholar]
- Deldicque L, Bertrand L, Patton A, Francaux M, Baar K. ER stress induces anabolic resistance in muscle cells through PKB-induced blockade of mTORC1. PloS one. 2011;6:e20993. doi: 10.1371/journal.pone.0020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D, Kaufman RJ, Ron D, Sonenberg N. Activation of GCN2 in UV-irradiated cells inhibits translation. Current biology : CB. 2002;12:1279–1286. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- Devi L, Ohno M. Phospho-eIF2alpha level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PloS one. 2010;5:e12974. doi: 10.1371/journal.pone.0012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: their structures and functions. Cellular and molecular life sciences : CMLS. 2013;70:3493–3511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DJ, Grewal SS, de la Cruz AF, Edgar BA. Rheb-TOR signaling promotes protein synthesis, but not glucose or amino acid import, in Drosophila. BMC biology. 2007;5:10. doi: 10.1186/1741-7007-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson's disease. Biochemical and biophysical research communications. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Huo Y, Iadevaia V, Proud CG. Differing effects of rapamycin and mTOR kinase inhibitors on protein synthesis. Biochemical Society transactions. 2011;39:446–450. doi: 10.1042/BST0390446. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Lu MK, Guan KL. The TSC1 and TSC2 tumor suppressors are required for proper ER stress response and protect cells from ER stress-induced apoptosis. Cell Death Differ. 2011;18:133–144. doi: 10.1038/cdd.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Nakajima S, Saito Y, Takahashi S, Katoh R, Kitamura M. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ. 2012;19:310–320. doi: 10.1038/cdd.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kimball SR. Eukaryotic initiation factor eIF2. The international journal of biochemistry & cell biology. 1999;31:25–29. doi: 10.1016/s1357-2725(98)00128-9. [DOI] [PubMed] [Google Scholar]
- Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Molecular and cellular biology. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Kubota H, Obata T, Ota K, Sasaki T, Ito T. Rapamycin-induced translational derepression of GCN4 mRNA involves a novel mechanism for activation of the eIF2 alpha kinase GCN2. The Journal of biological chemistry. 2003;278:20457–20460. doi: 10.1074/jbc.C300133200. [DOI] [PubMed] [Google Scholar]
- Leitman J, Barak B, Benyair R, Shenkman M, Ashery U, Hartl FU, Lederkremer GZ. ER stress-induced eIF2-alpha phosphorylation underlies sensitivity of striatal neurons to pathogenic huntingtin. PloS one. 2014;9:e90803. doi: 10.1371/journal.pone.0090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu J, Song J, Wang X, Weiss HL, Townsend CM, Jr, Gao T, Evers BM. mTORC1 inhibition increases neurotensin secretion and gene expression through activation of the MEK/ERK/c-Jun pathway in the human endocrine cell line BON. American journal of physiology. Cell physiology. 2011;301:C213–C226. doi: 10.1152/ajpcell.00067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Molecular cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. The Journal of cell biology. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, Cavener DR, Klann E. Suppression of eIF2alpha kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nature neuroscience. 2013;16:1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. J Biol Chem. 2004;279:13792–13799. doi: 10.1074/jbc.M313724200. [DOI] [PubMed] [Google Scholar]
- Matsuo R, Kubota H, Obata T, Kito K, Ota K, Kitazono T, Ibayashi S, Sasaki T, Iida M, Ito T. The yeast eIF4E-associated protein Eap1p attenuates GCN4 translation upon TOR-inactivation. FEBS letters. 2005;579:2433–2438. doi: 10.1016/j.febslet.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Mounir Z, Krishnamoorthy JL, Robertson GP, Scheuner D, Kaufman RJ, Georgescu MM, Koromilas AE. Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Science signaling. 2009;2:ra85. doi: 10.1126/scisignal.2000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WM, Biagioli M, Shahani N, Swarnkar S, Huang WC, Page DT, MacDonald ME, Subramaniam S. Huntingtin promotes mTORC1 signaling in the pathogenesis of Huntington's disease. Science signaling. 2014;7:ra103. doi: 10.1126/scisignal.2005633. [DOI] [PubMed] [Google Scholar]
- Schneider A, Younis RH, Gutkind JS. Hypoxia-induced energy stress inhibits the mTOR pathway by activating an AMPK/REDD1 signaling axis in head and neck squamous cell carcinoma. Neoplasia. 2008;10:1295–1302. doi: 10.1593/neo.08586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahani N, Pryor W, Swarnkar S, Kholodilov N, Thinakaran G, Burke RE, Subramaniam S. Rheb GTPase Regulates beta-Secretase Levels and Amyloid beta Generation. J Biol Chem. 2013 doi: 10.1074/jbc.M113.532713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–2791. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Mealer RG, Sixt KM, Barrow RK, Usiello A, Snyder SH. Rhes, a physiologic regulator of sumoylation, enhances cross-sumoylation between the basic sumoylation enzymes E1 and Ubc9. J Biol Chem. 2010;285:20428–20432. doi: 10.1074/jbc.C110.127191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee AR, Proud CG. Staurosporine inhibits phosphorylation of translational regulators linked to mTOR. Cell death and differentiation. 2001;8:841–849. doi: 10.1038/sj.cdd.4400876. [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang KY, Chan D, Bateman JF, Cheah KS. In vivo cellular adaptation to ER stress: survival strategies with double-edged consequences. J Cell Sci. 2010;123:2145–2154. doi: 10.1242/jcs.068833. [DOI] [PubMed] [Google Scholar]
- Tyagi R, Shenoy AR, Visweswariah SS. Characterization of an evolutionarily conserved metallophosphoesterase that is expressed in the fetal brain and associated with the WAGR syndrome. J Biol Chem. 2009;284:5217–5228. doi: 10.1074/jbc.M805996200. [DOI] [PubMed] [Google Scholar]
- Valbuena N, Rozalen AE, Moreno S. Fission yeast TORC1 prevents eIF2alpha phosphorylation in response to nitrogen and amino acids via Gcn2 kinase. Journal of cell science. 2012;125:5955–5959. doi: 10.1242/jcs.105395. [DOI] [PubMed] [Google Scholar]
- Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology. 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang BP, Luciani DS, Wang X, Johnson JD, Proud CG. Rheb activates protein synthesis and growth in adult rat ventricular cardiomyocytes. Journal of molecular and cellular cardiology. 2008;45:812–820. doi: 10.1016/j.yjmcc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochemical Society transactions. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. The Journal of clinical investigation. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Wang YH, Wu XN, Wu SQ, Lu BJ, Dong MQ, Zhang H, Sun P, Lin SC, Guan KL, et al. Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol. 2011;13:263–272. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.