Abstract
Objectives
To determine the association of HIV, immunologic, and inflammatory factors on coronary artery calcium (CAC), a marker of subclinical atherosclerosis.
Methods
Cross-sectional study comparing baseline data of males from Hawaii Aging with HIV –Cardiovascular Study (HAHCS) with the Multi-Ethnic Study of Atherosclerosis (MESA) cohort. The cohorts were pooled to determine effects of HIV on CAC and explore immunologic and inflammatory factors that may explain development of CAC in HIV. Multivariable regression models compared CAC prevalence in HAHCS with MESA adjusting for coronary heart disease (CHD) risk profiles.
Results
We studied 100 men from HAHCS and 2733 men from MESA. Positive CAC was seen in 58% HAHCS participants and 57% MESA participants. Mean CAC was 260.8 in HAHCS and 306.5 in MESA. Using relative risk (RR) regression, HAHCS participants had a greater risk (RR=1.20, P<0.05) of having positive CAC than MESA when adjusting for age, smoking status, diabetes, antihypertensive therapy, BMI, systolic blood pressure, total cholesterol, and HDL cholesterol. Among participants with positive CAC, HIV infection was not associated with larger amounts of CAC. Among HAHCS participants, current HIV viral load, CD4, length of HIV, interleukin 6 (IL-6), fibrinogen, C-reactive protein (CRP), and D-dimer were not associated with the presence or amount of CAC.
Discussion
HIV was independently associated with a positive CAC in men with increased likelihood occurring between 45 and 50 years of age. Current HIV viral load, CD4 count, length of HIV, and inflammatory markers were unrelated to either presence or amount of CAC.
Keywords: Antiviral therapy, Coronary artery calcium, MESA, Framingham Risk Score
Introduction
Coronary heart disease (CHD) is a leading cause of morbidity and mortality in HIV-infected patients.1 This increased burden of CHD may be attributed to chronic infection and inflammation, effects of highly active antiretroviral medications, and/or higher rates of smoking in the HIV population. In addition to traditional risk factors such as smoking, hypertension, dyslipidemia, and diabetes, length of HIV infection, current CD4 count, CD4 nadir, and duration of antiretroviral therapy (ART) treatment have also been suggested as predictors of increased CHD.2 Although certain classes of ART have been associated with cardiovascular risk and dyslipidemia, composition of ART has not been consistently associated with CHD.3,4
Accurate and timely risk assessment for CHD is an important part of primary care. Identification of modifiable lifestyle risk factors and consideration of cost-effective interventions is critical to preventing progression of disease.5 One of the most common tools used today in assessing 10-year cardiovascular risk is the Framingham Risk Score (FRS). Although useful, the FRS has been reported to overestimate risk in the general population and underestimate risk in the HIV-infected population.6,7 Coronary artery calcification (CAC) scoring by fast computed tomography is proving to be one of the more common and validated methods of risk stratification. Presence of CAC has been shown to predict CHD risk independently of FRS. Absolute CAC has been shown to be superior to age–sex–race/ethnicity percentiles in predicting cardiac events.8 The objective of this study was to provide a descriptive analysis of CAC in a HIV population, comparing this to the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, a study of the characteristics of subclinical cardiovascular disease involving more than 6000 men and women from six communities in the United States. The cohorts were pooled to determine the association between HIV and CAC and explore clinical, immunologic, and inflammatory factors that may explain the development of CAC among those with HIV.
Methods
Study population
The present study is a cross-sectional study comparing baseline data of males from Hawaii Aging with HIV – Cardiovascular Study (HAHCS) with the MESA cohort. Hawaii Aging with HIV – Cardiovascular Study is a longitudinal study of the role of oxidative stress and inflammation in HIV cardiovascular risk.9 Multi-Ethnic Study of Atherosclerosis is a prospective study on the prevalence, risk factors, and progression of subclinical CHD. The study design and methods for both cohorts have been previously published.9,10 Comparing the cohorts, MESA had a larger proportion of female participants than the HAHCS did. Due to the predominance of male subjects in the HAHCS cohort, we restricted this study to males only. Analysis was also restricted to participants aged 45–74, representing the overlapping age range of the two cohorts. Medical and medication history was obtained through chart review and self-report. Current and past smoking status was taken from patient report as yes or no responses. Signed informed consent was obtained from all participants and the study protocol was approved by the institutional review boards at each site.
HAHCS cohort
Hawaii Aging with HIV – Cardiovascular Study enrolled HIV-infected volunteer adults ≥40 years old, recruited from the state of Hawaii. Although the cohorts are geographically distinct, the health statistics of Hawaii are similar to the general population. The prevalence of tobacco smoking, acute myocardial infarction, and stroke in Hawaii is comparable to the general US population [15.6, 2.8, and 2.4% in Hawaii compared to 12.1, 3.2, and 2.7% in the general US population; Centers for Disease Control and Prevention Division for Heart Disease and Stroke Prevention: Data Trends & Maps http://nccd.cdc.gov/DHDSP_DTM/LocationSummary.aspx?state=Hawaii] Additionally, a large portion of the HIV-infected patients in the HAHCS cohort lived in the continental US prior to residing in Hawaii. Inclusion criteria for participation were documented HIV-positive status and having been on stable ART for at least 3 months. Height, weight, body mass index (BMI), systolic and diastolic blood pressure, waist-hip ratio, and EKG were measured. Patients had blood drawn for fasting lipids, and an oral glucose tolerance test was administered. Participants were accepted into the original study whether or not they had a history of CHD or diabetes, but those with CHD were excluded from the current analysis. Patients with CHD were excluded from the analysis to make it comparable to the MESA study design. This study contains data from 100 male participants from HAHCS who had no missing data on the measures used in this study, except where noted.
MESA cohort
Multi-Ethnic Study of Atherosclerosis enrolled 6814 participants aged 45–84 years free of clinical CHD from six US Communities (Los Angeles County, CA, USA; Chicago, IL, USA; Baltimore City and Baltimore County, MD, USA; St. Paul, MN, USA; Forsyth County, NC, USA; Northern Manhattan and the Bronx, NY, USA). This report contains 2733 male participants from MESA who had no missing data on the measures used in this analysis. The study design and methods have been previously published.10
CAC measurements
Baseline imaging for this paper included measurement of CAC in both studies. Both cohorts had CAC measured by the same cardiac computerized tomography (CT) core laboratory. Carr et al. have reported details of the methods used by MESA for CT scanning and interpretation, and the same methodology was used for the HAHCS cohort.11 Computerized tomography examinations for CAC were performed following previously published methods, on a dual source CT (DSCT) scanner for HAHCS participants.11 For MESA, each of the six centers measured CAC with either a cardiac-gated electron-beam CT scanner (Chicago, Los Angeles, New York), or a multidetector CT (Baltimore, Forsyth County, St. Paul). All participants were scanned twice, with mean CAC (Agatston) used for all analyses. Images were interpreted at the MESA CT reading center (Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles, Torrance, CA, USA), where a radiologist or cardiologist blinded to clinical data quantified CAC using an interactive scoring system to calculate Agatston score, with any Agatston score >0 defining the presence of CAC.12 Multi-Ethnic Study of Atherosclerosis utilized an exponential survival model to estimate the arterial age as a function of CAC.13 The same research group has published a method of calculating CAC by age- and gender-matched percentile rank.
Statistical analysis
Variables of interest in this study were arterial age, traditional cardiovascular risk factors, and HIV-related variables. Arterial age was captured from the North American MESA study table.2,7,14–16 Because the MESA equation has been validated for people over the age of 45 years, those younger than 45 were excluded from the analysis. The global FRS was calculated using the equation-based method.17 Participants with missing values on any of the measures used for the risk score calculation or in the multivariable models were excluded from the analysis, leaving a pooled sample size of 100 men for the HAHCS cohort and 2733 men for the MESA cohort.
Multi-Ethnic Study of Atherosclerosis was designed as a multi-ethnic cohort with large samples of European, Hispanic, African, and Chinese descent racial/ethnic groups. Despite this diversity, the only way to compare categories between MESA and HAHCS was to dichotomize race/ethnicity as white and non-white. For example, MESA did not collect other Asian groups except Chinese. This limitation precluded a case-control design because there were no clearly comparable matches for some participants. Rather than age-matching participants from MESA, we used the entire cohort with available CAC measurements in this age range and adjusted for age in years as a continuous covariate.
To evaluate study differences, descriptive statistics were compared and a dichotomous indicator for cohort was included in bivariate RR and linear regression models predicting the presence of and amount of CAC when present. Locally weighted scatterplot smoothing (LOWESS) was used to show differences in the probabilities of a positive CAC by study and chronological age.
For each study, variables are described for groups with zero (below the clinical threshold) CAC and for people with CAC present. The presence of CAC was predicted for HAHCS participants using a RR regression model with a Gaussian distribution and log link.18 When detectable, the amount of CAC was predicted using linear regression on the natural log transformation of CAC. HIV-specific variables (detectable viral load, CD4 nadir, CD4 absolute, and length of HIV) and markers of inflammation [Interleukin 6, Fibrinogen, C-reactive protein (CRP), and D-dimer] were the predictors of interest. Models including age (years) and race (white or non-white), and models including age, race, and traditional cardiovascular risk factors as covariates are shown for each HIV-related variable in inflammation marker. Each variable was tested independently of other covariates of interest. Stata 12.1 was used for all analyses.
Results
This cross-sectional study compared 100 male participants from HAHCS with 2733 participants from MESA. Table 1 shows descriptive statistics for the HAHCS and MESA studies. Hawaii Aging with HIV Cardiovascular Study had the following characteristics: mean age of 53.9 years, BMI 26.73 kg/m2, 63% current or former smokers, 9% diabetic, and 27% on anti-hypertensive therapy. Among HIV-infected subjects from HAHCS, 84% had HIV RNA <50 copies with a mean CD4 count of 497 cells/mm and mean CD4 nadir of 149 cells/mm. Participants from MESA had a mean age of 59.6 years, BMI 28.0 kg/m2, 60% current or former smokers, 14% diabetic, and 33% on anti-hypertensive therapy.
Table 1.
Descriptive statistics.
| N (%) | HAHCS
|
MESA
|
P-value HAHCS and MESA totals | ||||
|---|---|---|---|---|---|---|---|
| Coronary artery calcium (CAC)=0
|
CAC>0
|
Total
|
CAC=0
|
CAC>0
|
Total
|
||
| 42 (42) | 58 (58) | (N=100) | 1176 (43) | 1557 (57) | 2733 (100) | ||
| Race/ethnicity* | |||||||
| African-American | 4 (9.52) | 1 (1.72) | 5 (5.00) | 376 (31.97) | 341 (21.90) | 717 (26.23) | n/a |
| Asian | 4 (9.52) | 5 (8.62) | 9 (9.00) | n/a | n/a | n/a | n/a |
| Caucasian | 22 (52.38) | 34 (58.62) | 56 (56.00) | 354 (30.10) | 708 (45.47) | 1062 (38.86) | n/a |
| Chinese | n/a | n/a | n/a | 145 (12.33) | 184 (11.82) | 329 (12.04) | n/a |
| Hispanic | 6 (14.29) | 4 (6.90) | 10 (10.00) | 301 (25.60) | 324 (20.81) | 625 (22.87) | n/a |
| Native Hawaiian | 4 (9.52) | 6 (10.34) | 10 (10.00) | n/a | n/a | n/a | n/a |
| Mixed or unknown ethnicity | 2 (4.76) | 8 (13.79) | 10 (10.00) | n/a | n/a | n/a | n/a |
| Former smoker | 15 (35.71) | 24 (41.38) | 39 (39.00) | 451 (38.35) | 745 (47.85) | 1196 (43.76) | 0.095 |
| Current smoker | 10 (23.81) | 14 (24.14) | 24 (24.00) | 191 (16.24) | 243 (15.61) | 434 (15.88) | |
| Diabetes mellitus | 4 (9.52) | 5 (8.62) | 9 (9.00) | 110 (9.35) | 263 (16.89) | 373 (13.65) | 0.181 |
| Anti-hypertensive medication | 9 (21.43) | 18 (31.03) | 27 (27.00) | 287 (24.40) | 622 (39.95) | 909 (33.26) | 0.191 |
| Lipid-lowering medication | 16 (38.10) | 22 (37.93) | 38 (38.00) | 122 (10.37) | 312 (20.04) | 434 (15.88) | <0.001 |
| Viral load (RNA) undetectable, <48 copies/ml | 35 (83.33) | 49 (84.48) | 84 (84.00) | – | – | – | n/a |
| Nucleoside reverse transcriptase inhibitors | 39 (92.87) | 50 (86.20) | 89 (89.00) | – | – | – | n/a |
| Non-nucleoside reverse transcriptase inhibitors | 20 (47.62) | 31 (53.44) | 51 (51.00) | – | – | – | n/a |
| Protease inhibitors | 33 (78.57) | 45 (77.44) | 78 (78.00) | – | – | – | n/a |
| Mean (standard deviation) | |||||||
| Age, years | 51.45 (5.45) | 55.71 (7.00) | 53.92 (6.71) | 56.09 (7.93) | 62.19 (7.88) | 59.56 (8.46) | <0.001 |
| Body mass index (BMI), kg/m2 | 26.27 (5.45) | 27.06 (3.86) | 26.73 (4.59) | 27.67 (4.31) | 28.30 (4.58) | 28.03 (4.47) | 0.004 |
| Systolic blood pressure, mm Hg | 121.19 (16.23) | 128.81 (16.64) | 125.61 (16.82) | 121.00 (17.10) | 127.59 (19.24) | 124.75 (18.64) | 0.650 |
| Total cholesterol, mg/dl | 171.00 (38.49) | 182.53 (37.17) | 177.69 (37.97) | 187.13 (33.71) | 190.4 (36.40) | 189.00 (35.30) | 0.002 |
| HDL cholesterol, mg/dl | 43.71 (14.75) | 42.79 (18.97) | 43.18 (17.25) | 44.82 (11.02) | 44.58 (11.90) | 44.69 (11.52) | 0.209 |
| CAC, Agatston | – | 260.77 (484.97) | 151.25 (390.06) | – | 306.52 (571.57) | 174.62 (457.28) | 0.614 |
| CD4 nadir1 | 147.70 (138.68) | 150.29 (162.64) | 149.26 (152.78) | – | – | – | n/a |
| CD4 absolute, cell/ml | 475.00 (237.12) | 513.28 (264.36) | 497.20 (252.75) | – | – | – | n/a |
| Length of HIV, months | 196.31 (89.43) | 191.86 (93.55) | 193.73 (91.41) | – | – | – | n/a |
| Interleukin 6, pg/ml | 3.68 (13.40) | 2.21 (3.38) | 2.83 (9.02) | – | – | – | n/a |
| Fibrinogen, mg/dl | 326.79 (66.70) | 350.34 (78.16) | 340.45 (74.14) | – | – | – | n/a |
| C-reactive protein, mg/dl | 1.52 (1.90) | 3.43 (6.95) | 2.63 (5.50) | – | – | – | n/a |
| D-dimer, ug/ml | 0.15 (0.18) | 0.16 (0.13) | 0.15 (0.15) | – | – | – | n/a |
| FRS, global CHD | 0.12 (0.07) | 0.19 (0.11) | 0.16 (0.10) | 0.15 (0.11) | 0.24 (0.15) | 0.20 (0.14) | <0.001 |
| FRS, arterial age | 0.05 (0.03) | 0.31 (0.18) | 0.20 (0.19) | 0.05 (0.03) | 0.34 (0.21) | 0.22 (0.21) | 0.580 |
CHD: coronary heart disease; FRS: Framingham Risk Score; HAHCS: Hawaii Aging with HIV Cardiovascular Study; HDL: high-density lipoprotein; MESA: Multi-Ethnic Study of Atherosclerosis (MESA); n/a: not applicable.
Comparison among race/ethnicity not comparable. Hawaii Aging with HIV Cardiovascular Study population was based out of Hawaii and consisted of Caucasians and a mixed group of Asians classified as non-Caucasian. The MESA study population consisted primarily of Caucasians, African-Americans, Hispanics, and Chinese.
N=37 for CAC=0; N=58 for CAC>0; N=93 for total.
The HAHCS and MESA studies used dissimilar measures of race and ethnicity. The most comparable measure between studies that allowed for adequate sample sizes in each race/ethnicity category was to identify whether participants were Caucasian. In the HAHCS, 47.6% of participants with zero CAC were non-Caucasians and 41.4% of those with positive CAC present were non-Caucasians. In the MESA sample, 69.9% of participants with zero CAC were non-Caucasians and 54.5% of those with positive CAC were non-Caucasian. We controlled for Caucasian race/ethnicity in all models.
Participants in the HAHCS were, on average, 5 years younger than people included in the MESA study. The mean age among those with CAC present in the HAHCS was 56 years old, whereas the average age was 62 years among the MESA participants. For those with zero CAC, the average age was 51 years among the HAHCS and 56 years in the MESA study. We adjusted for age in all models.
Additional risk factors for CHD were also compared between the studies. Participants in the HAHCS were more likely to be currently smoking or have formerly smoked cigarettes than MESA study participants. Diabetes and anti-hypertensive medication use was higher among MESA participants than observed among those in the HAHCS. Participants in the MESA study had slightly higher BMI and total cholesterol than HAHCS. Framingham Risk Score distributions were similar between HAHCS and MESA groups. Calculations of FRS using arterial age showed similar changes in FRS regardless of presence or absence of CAC among the two groups.
For the HAHCS study participants, HIV-specific measurements showed relatively unremarkable differences between those with zero CAC and people with positive CAC. Viral load was undetectable in 83% of participants for people without CAC present and 84% of participant with positive CAC. The mean absolute CD4 was 475 for participants with zero CAC and 513 for those with positive CAC. The number of months participants reported to have had HIV was also similar between the groups; 196 months for those with zero CAC and 192 months for participants with a positive CAC.
Among participants with a positive CAC, the mean CAC score was 260.8 in HAHCS and 306.5 in MESA. Using a RR regression model, having HIV infection showed a RR of 1.20 (95% CI [1.04–1.40]; P<0.05) in predicting the RR of a positive CAC. Adjusting for age, Caucasian ethnicity, and risk factor differences between the studies, HAHCS participants were significantly more likely to have CAC present than participants in the MESA study, as shown in Table 2.
Table 2.
Regression models for presence and amount of CAC for pooled cohort of HAHCS and MESA participants.
| Variables | Relative risk (RR) regression for non-zero CAC in all participants (N=2833)
|
Linear regression for amount of CAC in participants with CAC>0 (N=1615)1
|
||
|---|---|---|---|---|
| RR [95% CI] | P-value | b-Coefficient [95% CI] | P-value | |
| Models including age and race2 | ||||
| HAHCS | 1.16 [1.00–1.36] | 0.058 | 0.15 [−0.31–0.60] | 0.533 |
| MESA (reference) | ||||
| Models including age, race, and traditional cardiovascular risk factors3 | ||||
| HAHCS | 1.20 [1.04–1.40] | 0.015 | 0.18 [−0.27–0.63] | 0.438 |
| MESA (reference) | ||||
CAC: coronary artery calcium; HAHCS: Hawaii Aging with HIV Cardiovascular study; MESA: Multi-Ethnic Study of Atherosclerosis.
CAC was transformed by natural log to account for skewed distribution.
Age in years; race defined as white or non-white.
Risk factor variables included cigarette smoking (never smoker compared to former or current), diabetes (yes/no), anti-hypertensive medication use (yes/no), body mass index (BMI), systolic blood pressure (mm Hg), total cholesterol (mg/dl), and high-density lipoprotein cholesterol (mg/dl).
Figure 1 shows the probability of a positive CAC plotted against age for each study. Locally weighted scatterplot smoothing estimated the overall trend lines. The solid line is for the HAHCS and the dashed line shows the MESA study. At the youngest ages, the probability of a positive CAC was similar between the studies. The probability rose sharply between ages 45 and 49 (N=35) years among the HIV patients and progressed in a somewhat linear fashion thereafter. The probability of a positive CAC increased linearly between ages 45 and 75 years among the MESA participants. Among participants with a positive CAC, there were no differences in the amount of CAC between studies. When other coronary risk factors were included in the regression model, no significant differences were noted between the studies. Age was a strong predictor of CAC in both studies (RR=1.04 and P<0.001 in HAHCS; RR=1.03 and P<0.001 in MESA) in minimally adjusted models, but the interaction between age and study was not significant (P=0.547; results not shown). This suggests that the pattern shown in Table 1 may be due to small sample variability.
Figure 1.
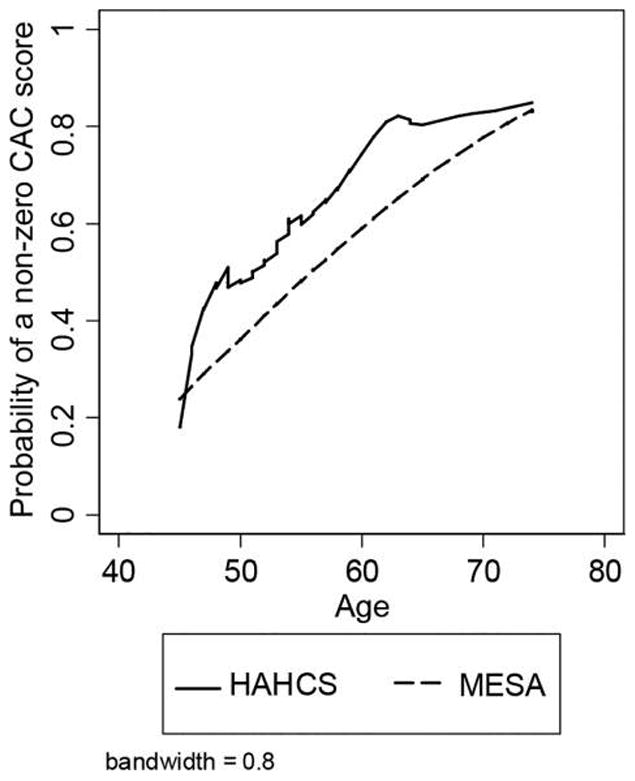
Locally weighted scatterplot smoothing (LOWESS) of positive coronary artery calcium (CAC) scores by study (N = 2833). Figure shows a locally weighted regression of CAC presence on age, by study. The smoothing process determines each smoothed data point by the neighboring data points. Coronary artery calcium presence is predicted by age using a weighted linear least squares regression. Thirty-five HAHCS participants were between ages 45 and 49, 44 participants were between ages 50 and 59, and 21 were aged 60 or above.
HIV-specific variables were not found to be significant predictors of participants having a positive CAC or the amount of CAC among participants with a positive CAC. A RR regression model predicting the probability of a positive CAC using HIV-specific variables for the HAHCS study is shown in Table 3. Lack of significance was noted whether these variables were tested alone or in combination (results not shown), and whether tested in the demographic or risk factor adjusted models.
Table 3.
Regression models for presence and amount of coronary artery calcium (CAC) for Hawaii aging with HIV – cardiovascular study (HAHCS) participants.
| Variables | Relative risk (RR) regression for non-zero CAC in all HAHCS participants (N=100)
|
Linear regression for amount of CAC in HAHCS participants with CAC>0 (N=58)1
|
||
|---|---|---|---|---|
| RR [95% CI] | p-value | b-Coefficient [95% CI] | p-value | |
| Models including age and race2 | ||||
| Viral load (RNA) undetectable | 1.01 [0.65–1.57] | 0.958 | −0.39 [−1.66–0.87] | 0.535 |
| CD4 nadir (per SD3)5 | 1.00 [0.87–1.15] | 0.994 | −0.28 [−0.60–0.24] | 0.189 |
| CD4 absolute (per SD3) | 1.05 [0.93–1.18] | 0.426 | −0.31 [−0.72–0.11] | 0.081 |
| Length of HIV (per SD3, months) | 0.95 [0.84–1.07] | 0.363 | 0.03 [−0.39–0.44] | 0.901 |
| IL-6 (log, per SD3, pg/ml) | 1.00 [0.89–1.13] | 0.963 | −0.36 [−0.84–0.11] | 0.131 |
| Fibrinogen (log, per SD3, mg/dl) | 1.05 [0.85–1.29] | 0.652 | −0.20 [−0.68–0.29] | 0.419 |
| CRP (log, per SD3, ug/ml) | 1.10 [0.96–1.26] | 0.156 | −0.24 [−0.66–0.18] | 0.261 |
| D-dimer (log, per SD3, ug/ml) | 1.09 [0.97–1.22] | 0.172 | −0.23 [−0.64–0.18] | 0.267 |
| Models including age, race, and traditional cardiovascular risk factors4 | ||||
| Viral load (RNA) undetectable | 1.12 [0.78–1.60] | 0.550 | −0.22 [−1.43–0.99] | 0.716 |
| CD4 nadir (SD3)5 | 1.02 [0.90–1.16] | 0.772 | −0.38 [−0.78–0.01] | 0.058 |
| CD4 absolute (SD3) | 1.06 [0.96–1.17] | 0.242 | −0.34 [−0.72–0.03] | 0.074 |
| Length of HIV (SD3, months) | 0.93 [0.81–1.07] | 0.335 | 0.14 [−0.27–0.54] | 0.492 |
| IL-6 (log, SD3, pg/ml) | 1.04 [0.92–1.17] | 0.525 | −0.47 [−0.90–0.04] | 0.034 |
| Fibrinogen (log, SD3, mg/dl) | 1.09 [0.92–1.30] | 0.331 | −0.27 [−0.71–0.17] | 0.225 |
| CRP (log, SD3, ug/ml) | 1.09 [0.96–1.25] | 0.198 | −0.26 [−0.65–0.13] | 0.192 |
| D-dimer (log, SD3, ug/ml) | 1.13 [1.01–1.27] | 0.037 | −0.12 [−0.50–0.27] | 0.548 |
CAC: coronary artery calcium; CRP: C-reactive protein; HAHCS: Hawaii Aging with HIV Cardiovascular Study; IL-6: interleukin 6; MESA: multi-Ethnic Study of Atherosclerosis; SD: standard deviation.
CAC was transformed by natural log.
Age in years; race defined as white or non-white.
Continuous measures are presented in standard deviation (SD) units; the estimates reflect a 1-SD increase in the exposure.
Risk factor variables included cigarette smoking (never smoker compared to former or current), diabetes (yes/no), anti-hypertensive medication use (yes/no), body mass index (BMI), systolic blood pressure (mm Hg), total cholesterol (mg/dl), and high-density lipoprotein cholesterol (mg/dl).
N=93 for RR regression model; N=56 for linear regression model.
Markers of inflammation including interleukin 6 (IL-6), fibrinogen, CRP, and D-dimer were obtained and compared between groups with zero CAC and positive CAC. Although fibrinogen was increased in the positive CAC group, after adjusting for age-race and risk factors, the RRs were not statistically significant. C-reactive protein was also found to be elevated in the positive CAC group with a mean of 3.43 mg/dl compared to the zero CAC group at 1.52 mg/dl and after adjusting for age-race and risk factors, the RRs were not significant, 1.10 [0.96–1.26] and 1.09 [0.96–1.25], respectively. Interleukin-6 was found to be higher in those with zero CAC group with a mean of 3.68 pg/ml compared to those with positive CAC with a mean of 2.21 pg/ml. D-dimer was found to be nearly the same in those with zero CAC group and positive CAC group at 0.15 and 0.16 ug/dl, respectively. All four inflammatory markers were unrelated to either the presence or amount of CAC.
Discussion
This study found over half of all HIV-infected participants to have a positive CAC. There was a significant increased RR of 1.20 associated with HIV infection and the probability of CAC, with a steep incline of CAC between 45 and 50 years of age. While HIV participants were more likely to have positive CAC, the amount of CAC when present was similar between studies (mean=260.5 for HAHCS and 306.5 for MESA) considering that MESA participants were an average of 5 years older. Our study findings are consistent with other studies in that HIV infection was independently associated with a positive CAC.19 A review by Stein et al. suggested the increased use of non-invasive imaging studies including carotid ultrasound, CAC, arterial inflammatory imaging using PET, and brachial artery reactivity testing for monitoring of CHD risk in HIV-infected patients.20 Mechanisms by which HIV increases cardiovascular risk include: ART metabolic dysfunction caused by ART,3 and a chronic inflammatory state, particularly in the vasculature, caused by HIV. Individuals with HIV are also more likely to be smokers or have smoked cigarettes, and have higher rates of other traditional risk factors such as dyslipidemia and insulin resistance.
Increased vascular age has been reported in HIV-infected patients on ART as measured by CAC.14 Even though certain ART, in particular protease inhibitors, have demonstrated an increased risk of CHD events,3,4 most studies have demonstrated that CAC was detectable in individuals with HIV regardless of whether or not the individual was on ART,21 and the CAC scores did not significantly differ between those on ART and those not on ART. Composition and duration of ART have not been associated with CAC.
HIV has been hypothesized to cause a chronic inflammatory state, particularly in the vasculature, which leads to progressive atherosclerosis. Certain markers of inflammation (CRP, IL-6, D-dimer, and fibrinogen) have been used to demonstrate increased risk of CHD events and mortality.22,23 In the Strategies for Management of Antiretroviral Therapy (SMART) study, interruption of ART was associated with higher levels of inflammatory markers, including IL-6 and D-dimer, and greater CHD events and mortality.24 It is suggested that therapeutic strategies to reduce inflammation, in addition to ART, may reduce CHD risk in HIV.25 Despite previous findings, studies connecting CAC and inflammatory markers have been conflicting. A study by Folsom et al. demonstrated progression of CAC unrelated to inflammatory markers.26 Our study did not find any relationship between the presence and amount of CAC and inflammatory markers even after adjusting for age, ethnicity, and traditional risk factors. HIV patients may represent a non-homogeneous population that overall displays an increased “social and economic vulnerability” that cannot be “adjusted” by traditional risk factors for CHD.
Length of HIV infection, current CD4 count and CD4 nadir have been predictors of increased CAC.5,6 Conversely, ART duration has been shown to have an inverse relationship with CAC.2 However, our study did not find any association between current HIV viral load, CD4 count, and length of HIV to either the presence or amount of CAC. Antilipid medication use (statin, fibrates, and niacin) was higher in the HAHCS group. When antilipid medication use was adjusted along with the other risk factors, the RR was reduced slightly 1.16 [1.00–1.35] but the overall effect size remained the same. Overall, antilipid medications did not have a modifying effect on the presence or amount of CAC in HIV.
Traditional cardiovascular risk factors (age, gender, diabetes, hypercholesterolemia, and cigarette smoking) have been shown to accelerate CAC.27 HIV infection increases the rate of progression of CAC28 and accelerates the development of CAC at younger ages.29 Our study found that younger HAHCS participants were more likely to have CAC than MESA participants, particularly between the ages of 45 and 50 years. However, the age of onset of detectable CAC and the degree of CAC between the two groups did not differ significantly. Traditional risk factors such as smoking tobacco likely contributed more to cardiovascular risk stratification in those infected with HIV. Tobacco use was higher among the HAHCS group. However, the higher predicted probability of CAC remained significant after controlling for tobacco use. The steep rise in prevalence of CAC between the ages of 45 and 50 in those infected with HIV may be of particular interest for increased CHD awareness and need for more aggressive preventative CHD interventions at an earlier age.
Limitations
Limitations of our study include the use of cross-sectional data for both risk factors and CAC. Ideally, the cohorts would have been identical except for the presence of HIV infection. Given the retrospective nature of this analysis, this was not possible. Multi-Ethnic Study of Atherosclerosis did not explicitly exclude individuals with HIV infection but the number of HIV-infected individuals in this cohort would be quite small and unlikely to influence the overall means. Multi-Ethnic Study of Atherosclerosis has been used as a control comparison to other HIV cohorts.30 Another limitation of our study is the small number of females enrolled in the HAHCS group, limiting our comparisons to male subjects only. Although we adjusted for age, race, and risk factors, it is important to note the original MESA study population included more than 50% female subjects. Our study was additionally limited by divergent measures of race and ethnicity. Our study population was based out of Hawaii and consisted of Caucasians and a mixed group of Asians here classified as “non-Caucasian.” The MESA study population consisted primarily of Caucasians, African-Americans, Hispanics, and Chinese. The age, gender, and ethnicity restrictions reduced the analysis to two-thirds of the original HAHCS cohort but this allowed for a focused analysis of HIV-infected males with no prior CHD receiving stable ART. Due to the observational nature of the study, unmeasured confounding and biases could potentially explain the study findings. However, a strength of using these cohorts is that both were set up to address the issue of cardiovascular disease, and therefore both contain data on traditional cardiovascular risk factors, including smoking, diabetes, and cholesterol, which could be adjusted for in the analysis. The 20% increased RR of CAC in people with HIV compared to people who are HIV negative must be explored in other cohorts before any clinical recommendations can be made.
The regression models did not adjust for ART use and specific ART since none of the non-HIV subjects were treated with ART. Our ability to find a relationship between inflammatory markers and CAC may also be limited by sample size. Additionally, systemic biomarkers may not accurately reflect inflammation in the vascular wall.
Conclusion
Our study found that HIV was independently associated with a positive CAC in men, with increased likelihood of CAC between 45 and 50 years of age. Current HIV viral load, CD4 count, length of HIV, and inflammatory markers were unrelated to either presence or amount of CAC. The pathophysiologic mechanism of CHD in HIV needs to be further investigated.
Acknowledgments
We thank our study participants and community physicians for their roles in this study.
Funding
This work was supported by NIH grants U54RR026136, U54MD007584, R01HL095135 (CMS), K23 HL088981 (DC), and N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR.
Footnotes
Contributors
All authors were involved in study conception or design, or acquisition of data or analysis, and interpretation of data. All authors participated in revising the manuscript critically for important intellectual content, and approved the final version for publication.
Conflicts of interest
None of the authors have any conflicts of interest in respect to this article.
Ethics approval
The study protocol was approved by the institutional review board or independent ethics committee at each study center. Written informed consent was obtained from all participants. The studies were performed in accordance with the Declaration of Helsinki and all International Conference on Harmonisation Good Clinical Practice guidelines and applicable local regulatory requirements and laws.
References
- 1.Carballo D, Delhumeau C, Carballo S, Bahler C, Radovanovic D, Hirschel B. Increased mortality after a first myocardial infarction in human immunodeficiency virus-infected patients; a nested cohort study. AIDS Res Ther. 2015;12:4. doi: 10.1186/s12981-015-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingsley LA, Cuervo-Rojas J, Muñoz A, Palella FJ, Post W, Witt MD. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: multicenter AIDS cohort study. AIDS. 2008;22:1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DAD Study Group. Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte Ad, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 4.Iloeje UH, Yuan Y, L’Italien G, Mauskopf J, Holmberg SD, Moorman AC. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med. 2005;6:37–44. doi: 10.1111/j.1468-1293.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 5.Law MG, Friis-Moller N, El-Sadr WM, Weber R, Reiss P, D’Arminio Monforte A. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D study. HIV Med. 2006;7:218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 6.Parra S, Coll B, Aragones G, Marsillach J, Beltran R, Rull A. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–231. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 7.Falcone EL, Mangili A, Skinner S, Alam A, Polak JF, Wanke CA. Framingham risk score and early markers of atherosclerosis in a cohort of adults infected with HIV. Antivir Ther. 2011;16:1–8. doi: 10.3851/IMP1682. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2009;53:345–352. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shikuma CM, Seto T, Liang CY, Bennett K, DeGruttola V, Gerschenson M. Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res Hum Retroviruses. 2012;28:793–797. doi: 10.1089/aid.2011.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR., Jr Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standarized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Disk Development in Young Adults (CARDIA) study. Radiology. 2005;234:34–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte MJ, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.Park KJ, Singer W, Sletten DM, Low PA, Bharucha AE. Gastric emptying in postural tachycardia syndrome: a preliminary report. Clin Auton Res. 2013;23(4):163–167. doi: 10.1007/s10286-013-0193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G. Coronary aging in HIV-infected patients. Clin Infect Dis. 2009;49:1756–1762. doi: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- 15.Medina S, Wessman D, Krause D, Stephenosky J, Boswell G, Crum-Cianflone N. Coronary aging in HIV-infected patients. Clin Infect Dis. 2010;51:990. doi: 10.1086/656442. [DOI] [PubMed] [Google Scholar]
- 16.Guaraldi G, Zona S, Orlando G, Carli F, Ligabue G, Fiocchi F. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2012;28:935–941. doi: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]
- 17.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 18.Lumley T, Kronmal R, Ma S. Relative Risk Regression in Medical Research: Models, Contrasts, Estimators, and Algorithms. UW Biostatistics Working Paper Series. 2006 Jul; Working Paper 293. http://biostats.bepress.com/uwbiostat/paper293.
- 19.Post WS, Budoff M, Kingsley LA, Palella FJ, Witt MD, Li X. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–468. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein JH, Currier JS, Hsue PY. Arterial disease in patients with human immunodeficiency virus infection. JACC Cardiovasc Imaging. 2014;7:515–525. doi: 10.1016/j.jcmg.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangili A, Gerrior J, Tang AM, O-Leary DH, Polak JK, Schaefer E. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis. 2006;43:1482–1489. doi: 10.1086/509575. [DOI] [PubMed] [Google Scholar]
- 22.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 23.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality A population-based, prospective study. Thromb Haemostasis. 2006;95:511–518. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 24.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 25.Stein JH, Hsue PY. Inflammation, immune activation and CVD risk in individuals with HIV infection. J Am Med Assoc. 2012;308:405–406. doi: 10.1001/jama.2012.8488. [DOI] [PubMed] [Google Scholar]
- 26.Folsom AR, Evans GW, Carr JJ, Stillman AE. Association of traditional and nontraditional cardiovascular risk factors with coronary artery calcification. Angiology. 2004;55:613–623. doi: 10.1177/00033197040550i602. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann N, Mohlenkamp S, Mahabadi A, Schmermund A, Roggenbuck U, Seibel R. Effect of smoking and other traditional risk factors on the onset of coronary artery calcification: results of the Heinz Nixdorf recall study. Atherosclerosis. 2014;232(2):339–345. doi: 10.1016/j.atherosclerosis.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 28.Mangili A, Polak J, Skinner SC, Gerrior J, Sheehan H, Harington A. HIV infection and progression of carotid and coronary atherosclerosis: the CARE study. J Acquir Immune Defic Syndr. 2011;58:148–153. doi: 10.1097/QAI.0b013e31822d4993. [DOI] [PubMed] [Google Scholar]
- 29.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA. Increased prevalence of subclinical coronary atherosclerosis detected by coronary Computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]


